The Innate Defense in the Zika-Infected Placenta
Abstract
1. Introduction
2. The Zika Virus
3. Zika, Transmission and Clinical Manifestations
4. The Placenta
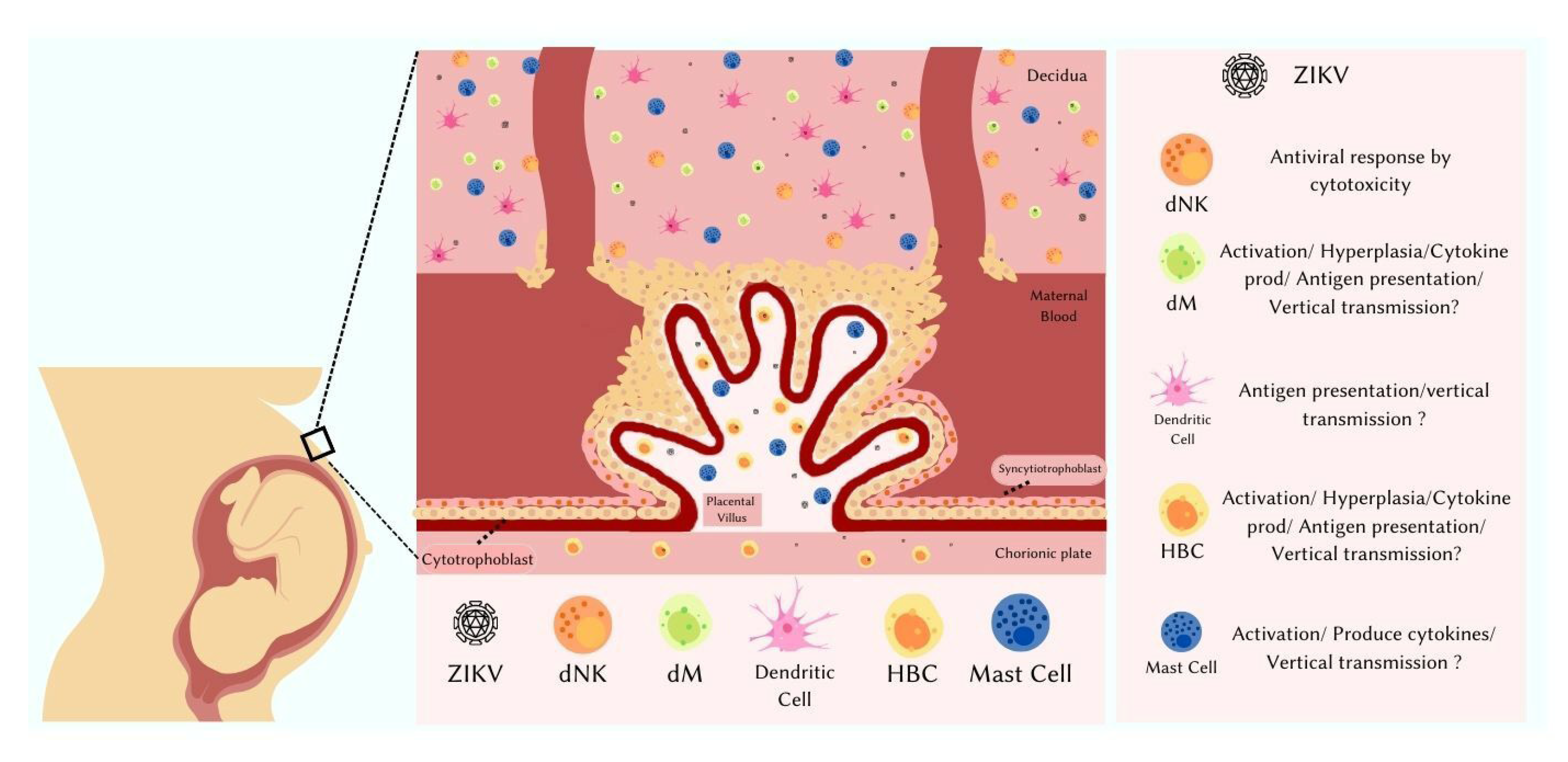
5. Placental Immune Cells
6. Placenta and Vertical Transmission
7. Mastocytes and ZIKV Infection
8. Dendritic Cells in ZIKV Placental Infection
9. The Importance of Macrophages and Hofbauer Cells in ZIKV Placental Infection
10. Natural Killer Cells in ZIKV Placental Infection
11. IFN-I Response in ZIKV Placental Infection
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dick, G.W.A. Zika Virus (I). Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- McCrae, A.W.R.; Kirya, B.G. Yellow Fever and Zika Virus Epizootics and Enzootics in Uganda. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.L.; Fischer, M.; Petersen, L.R. Epidemiology of Zika Virus Infection. J. Infect. Dis. 2017, 216, S868–S874. [Google Scholar] [CrossRef]
- Villinger, F.; de Noronha, L.; Nunes Duarte dos Santos, C.; Ld, N.; Zanluca, C.; Burger, M.; Akemi Suzukawa, A.; Azevedo, M.; Rebutini, P.Z.; Maria Novadzki, I.; et al. Zika Virus Infection at Different Pregnancy Stages: Anatomopathological Findings, Target Cells and Viral Persistence in Placental Tissues. Front. Microbiol. 2018, 9, 2266. [Google Scholar] [CrossRef]
- Faria, N.R.; Quick, J.; Claro, I.M.; Thézé, J.; de Jesus, J.G.; Giovanetti, M.; Kraemer, M.U.G.; Hill, S.C.; Black, A.; da Costa, A.C.; et al. Establishment and Cryptic Transmission of Zika Virus in Brazil and the Americas. Nature 2017, 546, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First Report of Autochthonous Transmission of Zika Virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- BRAZIL. Brazil’s Ministry of Health. Health Surveillance Department. Monitoring of Arbovirus Cases until Epidemiological Week 35 of 2022. Epidemiological Bulletin. ISSN 9352–7864. Vol 53, No.34.2022. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2022/boletim-epidemiologico-vol-53-no34#:~:text=Situa%C3%A7%C3%A3o%20epidemiol%C3%B3gica%20de%202022&text=At%C3%A9%20a%20SE%2035%20de,per%C3%ADodo%20analisado%20(Figura%201) (accessed on 9 September 2021).
- BRAZIL. Brazil’s Ministry of Health. Health Surveillance Department. Monitoring of Arbovirus Cases until Epidemiological Week 1–51 of 2021. Vol 52—No. 48. 2021. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2021/boletim-epidemiologico-vol-52-no-48.pdf/@@download/file/Boletim%20Epidemiol%C3%B3gico%20Vol%2052%20-%20N%C2%BA%2048.pdf (accessed on 9 September 2021).
- Brazil’s Ministry of Health Brazil. Ministry of Health. Secretary of Health Surveillance. Portaria n.° 1.813, 11 November 2015. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2015/prt1813_11_11_2015.html (accessed on 9 September 2021).
- World Health Organization. WHO Statement on the First Meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations. 2016. Available online: https://www.paho.org/annual-report-2016/index.html (accessed on 9 September 2021).
- Dyer Montreal, O. Zika Virus Spreads across Americas as Concerns Mount over Birth Defects. BMJ 2015, 351, h6983. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Feitosa, I.M.L.; Ribeiro, E.M.; Horovitz, D.D.G.; Pessoa, A.L.S.; França, G.V.A.; García-Alix, A.; Doriqui, M.J.R.; Wanderley, H.Y.C.; Sanseverino, M.V.T.; et al. The Phenotypic Spectrum of Congenital Zika Syndrome. Am. J. Med. Genet. A 2017, 173, 841–857. [Google Scholar] [CrossRef]
- Brazil’s Ministry of Health. Epidemiological Situation of Congenital Syndrome Associated with Zika Virus Infection: Brazil, 2015 to 2022, by SE 31. Epidemiological Bulletin. Vol 53, No. 35. 2022. Available online: http://plataforma.saude.gov.br/anomalias-congenitas/boletim-epidemiologico-SVS-35–2022.pdf (accessed on 9 September 2021).
- Faye, O.; Freire, C.C.M.; Iamarino, A.; Faye, O.; de Oliveira, J.V.C.; Diallo, M.; Zanotto, P.M.A.; Sall, A.A. Molecular Evolution of Zika Virus during Its Emergence in the 20th Century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic Characterization of Zika Virus Strains: Geographic Expansion of the Asian Lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B. Zika Virus outside Africa. Emerg. Infect. Dis. 2009, 15, 1347–1350. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.P. Fundamentals of Viruses and Their Proteases. In Viral Proteases and Their Inhibitors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–24. ISBN 9780128097120. [Google Scholar]
- Sevvana, M.; Rogers, T.F.; Miller, A.S.; Long, F.; Klose, T.; Beutler, N.; Lai, Y.C.; Parren, M.; Walker, L.M.; Buda, G.; et al. Structural Basis of Zika Virus Specific Neutralization in Subsequent Flavivirus Infections. Viruses 2020, 12, 1346. [Google Scholar] [CrossRef]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus Genome Organization, Expression and Replication. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Filgueirasid, I.S.; Torrentes de Carvalho, A.; Cunha, D.P.; Mathias da Fonseca, D.L.; el Khawanky, N.; Freire, P.P.; Cabral-Miranda, G.; Schimke, L.F.; Camara, N.O.S.; Ochs, H.D.; et al. The Clinical Spectrum and Immunopathological Mechanisms Underlying Zikv-Induced Neurological Manifestations. PLoS Negl. Trop. Dis. 2021, 15, e0009575. [Google Scholar]
- Badolato-Corr, J.; Camilo anchez-Arcila, J.S.; Manuele Alves de Souza, T.; Santos Barbosa, L.; Conrado Guerra Nunes, P.; da Rocha Queiroz Lima, M.; Gandini, M.; Maria Bispo de Filippis, A.; Ven, R.; da Cunha, A.; et al. Human T Cell Responses to Dengue and Zika Virus Infection Compared to Dengue/Zika Coinfection. Immun. Inflamm. Dis. 2018, 6, 194–206. [Google Scholar] [CrossRef]
- Grard, G.; Caron, L.; Mombo, I.M.; Nkoghe, D.; Ondo, S.M.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika Virus in Gabon (Central Africa)-2007: A New Threat from Aedes Albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef] [PubMed]
- Gatherer, D.; Kohl, A. Zika Virus: A Previously Slow Pandemic Spreads Rapidly through the Americas. J. Gen. Virol. 2016, 97, 269–273. [Google Scholar] [CrossRef]
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An Update on Zika Virus Infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef]
- Baud, D.; Musso, D.; Vouga, M.; Alves, M.P.; Vulliemoz, N. Zika Virus: A New Threat to Human Reproduction. Am. J. Reprod. Immunol. 2017, 77, e12614. [Google Scholar] [CrossRef]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika Virus-Spread, Epidemiology, Genome, Transmission Cycle, Clinical Manifestation, Associated Challenges, Vaccine and Antiviral Drug Development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Brugada, J.; Albenque, J.P. Evidence for Transmission of Zika Virus by Platelet Transfusion. N. Engl. J. Med. 2016, 375, 1099–1101. [Google Scholar] [CrossRef]
- Colt, S.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Finkelstein, J.L.; Rayco-Solon, P.; Weise Prinzo, Z.C.; Mehta, S. Transmission of Zika Virus through Breast Milk and Other Breastfeeding-Related Bodily-Fluids: A Systematic Review. PLoS Negl. Trop. Dis. 2017, 11, e0005528. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Sarno, M.; Khouri, R.; de Paula Freitas, B.; Siqueira, I.; Ribeiro, G.S.; Ribeiro, H.C.; Campos, G.S.; Alcântara, L.C.; Reis, M.G.; et al. Emergence of Congenital Zika Syndrome: Viewpoint from the Front Lines HHS Public Access. Ann. Intern. Med. 2016, 164, 689–691. [Google Scholar] [CrossRef] [PubMed]
- de Barros Miranda-Filho, D.; Turchi Martelli, C.M.; Arraes De Alencar Ximenes, R.; Velho, T.; Araújo, B.; Angela, M.; Rocha, W.; Coeli, R.; Ramos, F.; Dhalia, R.; et al. Initial Description of the Presumed Congenital Zika Syndrome. Public Health 2016, 106, 598–600. [Google Scholar] [CrossRef]
- Victora, C.G.; Schuler-Faccini, L.; Matijasevich, A.; Ribeiro, E.; Pessoa, A.; Barros, F.C. Microcephaly in Brazil: How to Interpret Reported Numbers? Lancet 2016, 387, 621–624. [Google Scholar] [CrossRef]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T.D.A.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef]
- Marbán-Castro, E.; Goncé, A.; Fumadó, V.; Romero-Acevedo, L.; Bardají, A. Zika Virus Infection in Pregnant Women and Their Children: A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.G.; Quicke, K.M.; O’Neal, J.T.; Arora, N.; Machiah, D.; Priyamvada, L.; Kauffman, R.C.; Register, E.; Adekunle, O.; Swieboda, D.; et al. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24, 731–742.e6. [Google Scholar] [CrossRef] [PubMed]
- Driggers, R.W.; Ho, C.-Y.; Korhonen, E.M.; Kuivanen, S.; Jaaskelainen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and Sequencing of Zika Virus from Amniotic Fluid of Fetuses with Microcephaly in Brazil: A Case Study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Sisman, J.; Jaleel, M.A.; Moreno, W.; Rajaram, V.; Collins, R.R.J.; Savani, R.C.; Rakheja, D.; Evans, A.S. Intrauterine Transmission of SARS-COV-2 Infection in a Preterm Infant. Pediatr. Infect. Dis. J. 2020, 265–267. [Google Scholar] [CrossRef]
- Rice, M.E.; Galang, R.R.; Roth, N.M.; Ellington, S.R.; Moore, C.A.; Valencia-Prado, M.; Ellis, E.M.; John Tufa, A.; Taulung, L.A.; Alfred, J.M.; et al. Morbidity and Mortality Weekly Report Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection-U.S. Territories and Freely Associated States, 2018. Morb. Mortal. Wkly. Rep. 2018, 67, 858. [Google Scholar]
- Rabelo, K.; Souza, L.J.; Salomão, N.G.; Oliveira, E.R.A.; Sentinelli, L.d.P.; Lacerda, M.S.; Saraquino, P.B.; Rosman, F.C.; Basílio-de-Oliveira, R.; Carvalho, J.J.; et al. Placental Inflammation and Fetal Injury in a Rare Zika Case Associated with Guillain-Barré Syndrome and Abortion. Front. Microbiol. 2018, 9, 1018. [Google Scholar] [CrossRef]
- Song, B.H.; Yun, S.I.; Woolley, M.; Lee, Y.M. Zika Virus: History, Epidemiology, Transmission, and Clinical Presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef]
- Grosmark, A.D.; Mizuseki, K.; Pastalkova, E.; Diba, K.; Buzsáki, G. Zika Virus Impairs Growth in Human Neurospheres and Brain Organoids. Electroencephalogr. Clin. Neurophysiol. 2004, 44, 1301–1315. [Google Scholar] [CrossRef]
- Uncini, A.; Shahrizaila, N.; Kuwabara, S. Zika Virus Infection and Guillain-Barré Syndrome: A Review Focused on Clinical and Electrophysiological Subtypes. J. Neurol. Neurosurg. Psychiatry 2017, 88, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.G.; Fortier, A.L.; Cross, J.C. Diverse Subtypes and Developmental Origins of Trophoblast Giant Cells in the Mouse Placenta. Dev. Biol. 2007, 304, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.D.; Cross, J.C. Development of Structures and Transport Functions in the Mouse Placenta. Physiology 2005, 20, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.C. How to Make a Placenta: Mechanisms of Trophoblast Cell Differentiation in Mice—A Review. Placenta 2005, 26, S3–S9. [Google Scholar] [CrossRef]
- el Costa, H.; Gouilly, J.; Mansuy, J.M.; Chen, Q.; Levy, C.; Cartron, G.; Veas, F.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N. ZIKA Virus Reveals Broad Tissue and Cell T, ropism during the First Trimester of Pregnancy. Sci. Rep. 2016, 6, 35296. [Google Scholar] [CrossRef] [PubMed]
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of Human Placental Development by Oxygen Tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.H.; Pawlina, W. Histology: Text and Atlas, 5th ed.; Guanabara Koogan: Buenos Aires, Argentina, 2006. [Google Scholar]
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Jabrane-Ferrat, N.; Siewiera, J. The up Side of Decidual Natural Killer Cells: New Developments in Immunology of Pregnancy. Immunology 2014, 141, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Gnainsky, Y.; Granot, I.; Aldo, P.; Barash, A.; Or, Y.; Mor, G.; Dekel, N. Biopsy-Induced Inflammatory Conditions Improve Endometrial Receptivity: The Mechanism. Reproduction 2015, 149, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK Cells Regulate Key Developmental Processes at the Human Fetal-Maternal Interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Ramhorst, R.; Fraccaroli, L.; Aldo, P.; Alvero, A.B.; Cardenas, I.; Leirós, C.P.; Mor, G. Modulation and Recruitment of Inducible Regulatory T Cells by First Trimester Trophoblast Cells. Am. J. Reprod. Immunol. 2012, 67, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Mor, G. Placental Inflammatory Response to Zika Virus May Affect Fetal Brain Development. Am. J. Reprod. Immunol. 2016, 75, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.K.; Tay, C.S.; Erlebacher, A. Dendritic Cell Entrapment within the Pregnant Uterus Inhibits Immune Surveillance of the Maternal/Fetal Interface in Mice. J. Clin. Investig. 2009, 119, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.; Mayorquin Galvan, E.E.; Zavala Trujillo, I.G.; Zavala-Cerna, M.G. Congenital Zika Syndrome: Pitfalls in the Placental Barrier. Rev. Med. Virol. 2018, 28, e1985. [Google Scholar] [CrossRef]
- Rowe, J.H.; Ertelt, J.M.; Xin, L.; Way, S.S. Pregnancy Imprints Regulatory Memory That Sustains Anergy to Fetal Antigen. Nature 2012, 490, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, B.; González, Á. Chapter 2: Innate Immune System. In Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, K.; de Souza, L.J.; Salomão, N.G.; Machado, L.N.; Pereira, P.G.; Portari, E.A.; Basílio-de-Oliveira, R.; dos Santos, F.B.; Neves, L.D.; Morgade, L.F.; et al. Zika Induces Human Placental Damage and Inflammation. Front. Immunol. 2020, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, K.; de Souza Campos Fernandes; de Souza, L.J.; de Souza, T.L.; dos Santos, F.B.; Nunes, P.C.G.; de Azeredo, E.L.; Salomão, N.G.; Trindade, G.F.; Basílio-de-Oliveira, C.A.; et al. Placental Histopathology and Clinical Presentation of Severe Congenital Zika Syndrome in a Human Immunodeficiency Virus-Exposed Uninfected Infant. Front. Immunol. 2017, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- de Noronha, L.; Zanluca, C.; Azevedo, M.L.V.; Luz, K.G.; dos Santos, C.N.D. Zika Virus Damages the Human Placental Barrier and Presents Marked Fetal Neurotropism. Mem. Inst. Oswaldo Cruz 2016, 111, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Brasil Martines, R.; Bhatnagar, J.; Kelly Keating, M.; Silva-Flannery, L.; Muehlenbachs, A.; Gary, J. Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 159–160. [Google Scholar] [CrossRef]
- Rabelo, K.; Gonçalves, A.J.d.S.; Souza, L.J.d.; Sales, A.P.; Lima, S.M.B.d.; Trindade, G.F.; Ciambarella, B.T.; Amorim Tasmo, N.R.; Diaz, B.L.; Carvalho, J.J.d.; et al. Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes. Cells 2020, 9, 975. [Google Scholar] [CrossRef]
- Derbala, Y.; Elazzamy, H.; Bilal, M.; Reed, R.; Salazar Garcia, M.D.; Skariah, A.; Dambaeva, S.; Fernandez, E.; Germain, A.; Gilman-Sachs, A.; et al. Mast Cell–Induced Immunopathology in Recurrent Pregnancy Losses. Am. J. Reprod. Immunol. 2019, 82, e13128. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Toyoshima, S.; Sakamoto-Sasaki, T.; Kashiwakura, J.; Matsuda, A.; Watanabe, Y.; Azuma, H.; Kawana, K.; Yamamoto, T.; Okayama, Y. Characterization of Human Decidual Mast Cells and Establishment of a Culture System. Allergol. Int. 2018, 67, S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Munjal, A.; Khandia, R.; Dhama, K.; Sachan, S.; Karthik, K.; Tiwari, R.; Malik, Y.S.; Kumar, D.; Singh, R.K.; Iqbal, H.M.N.; et al. Advances in Developing Therapies to Combat Zika Virus: Current Knowledge and Future Perspectives. Front. Microbiol. 2017, 8, 1469. [Google Scholar] [CrossRef] [PubMed]
- Woidacki, K.; Jensen, F.; Zenclussen, A.C. Mast Cells as Novel Mediators of Reproductive Processes. Front. Immunol. 2013, 4, 29. [Google Scholar] [CrossRef]
- Coish, J.M.; Crozier, R.W.E.; Schieffelin, J.S.; Coorssen, J.R.; Hunter, F.F.; MacNeil, A.J. Zika Virus Replication in a Mast Cell Model Is Augmented by Dengue Virus Antibody-Dependent Enhancement and Features a Selective Immune Mediator Secretory Profile. Microbiol. Spectr. 2022, 10, e01772–22. [Google Scholar] [CrossRef] [PubMed]
- Jamali Moghadam, S.R.; Bayrami, S.; Jamali Moghadam, S.; Golrokhi, R.; Golsoorat Pahlaviani, F.; SeyedAlinaghi, S.A. Zika Virus: A Review of Literature. Asian Pac. J. Trop. Biomed. 2016, 6, 989–994. [Google Scholar] [CrossRef]
- Rodrigues de Sousa, J.; Azevedo, R.d.S.d.S.; Quaresma, J.A.S.; Vasconcelos, P.F.d.C. The Innate Immune Response in Zika Virus Infection. Rev. Med. Virol. 2021, 31, e2166. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.; Moffett, A. Dendritic Cells in the Human Decidua. Biol. Reprod. 2003, 69, 1438–1446. [Google Scholar] [CrossRef]
- Barrientos, G.; Tirado-González, I.; Klapp, B.F.; Karimi, K.; Arck, P.C.; Garcia, M.G.; Blois, S.M. The Impact of Dendritic Cells on Angiogenic Responses at the Fetal-Maternal Interface. J. Reprod. Immunol. 2009, 83, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef]
- Narayanan, R. Zika Virus Therapeutic Lead Compounds Discovery Using Chemoinformatics Approaches. MOJ Proteom. Bioinform. 2016, 3, 00084. [Google Scholar] [CrossRef][Green Version]
- Vielle, N.J.; Zumkehr, B.; García-Nicolás, O.; Blank, F.; Stojanov, M.; Musso, D.; Baud, D.; Summerfield, A.; Alves, M.P. Silent Infection of Human Dendritic Cells by African and Asian Strains of Zika Virus. Sci. Rep. 2018, 8, 5440. [Google Scholar] [CrossRef]
- Österlund, P.; Jiang, M.; Westenius, V.; Kuivanen, S.; Järvi, R.; Kakkola, L.; Lundberg, R.; Melén, K.; Korva, M.; Avšič-Županc, T.; et al. Asian and African Lineage Zika Viruses Show Differential Replication and Innate Immune Responses in Human Dendritic Cells and Macrophages. Sci. Rep. 2019, 9, 15710. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.R.; Quicke, K.M.; Maddur, M.S.; O’Neal, J.T.; McDonald, C.E.; Fedorova, N.B.; Puri, V.; Shabman, R.S.; Pulendran, B.; Suthar, M.S. Zika Virus Antagonizes Type I Interferon Responses during Infection of Human Dendritic Cells. PLoS Pathog. 2017, 13, e1006164. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Harris, E.; Pereira, L. Zika Virus Replicates in Proliferating Cells in Explants from First-Trimester Human Placentas, Potential Sites for Dissemination of Infection. J. Infect. Dis. 2018, 217, 1202–1213. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Innate Immune Cells in the Placental Bed in Healthy Pregnancy and Preeclampsia. Placenta 2018, 69, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Tagliani, E.; Erlebacher, A. Dendritic Cell Function at the Maternal-Fetal Interface. Expert Rev. Clin. Immunol. 2011, 7, 593–602. [Google Scholar] [CrossRef]
- Tong, M.; Abrahams, V.M. Immunology of the Placenta. Obs. Gynecol. Clin. N. Am. 2020, 47, 49–63. [Google Scholar] [CrossRef]
- Hirsch, A.J.; Roberts, V.H.J.; Grigsby, P.L.; Haese, N.; Schabel, M.C.; Wang, X.; Lo, J.O.; Liu, Z.; Kroenke, C.D.; Smith, J.L.; et al. Zika Virus Infection in Pregnant Rhesus Macaques Causes Placental Dysfunction and Immunopathology. Nat. Commun. 2018, 9, 263. [Google Scholar] [CrossRef]
- Shaily, S.; Upadhya, A. Zika Virus: Molecular Responses and Tissue Tropism in the Mammalian Host. Rev. Med. Virol. 2019, 29, e2050. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Chu, H.; Byrareddy, S.N.; Spearman, P.; Chakraborty, R. Placental Hofbauer Cells Assemble and Sequester HIV-1 in Tetraspanin-Positive Compartments That Are Accessible to Broadly Neutralizing Antibodies. J. Int. AIDS Soc. 2015, 18, 19385. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, V.M.; Tang, Z.; Mor, G.; Guller, S. NLRP3 Inflammasome Function and Pyroptotic Cell Death in Human Placental Hofbauer Cells. J. Reprod. Immunol. 2020, 142, 103214. [Google Scholar] [CrossRef] [PubMed]
- Zulu, M.Z.; Martinez, F.O.; Gordon, S.; Gray, C.M. The Elusive Role of Placental Macrophages: The Hofbauer Cell Journal of Innate Immunity. J. Innate Immun. 2019, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Hyg, M.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Bulfamante, G.; Cheng, K.; Collins, R.R.; Debelenko, L.; ele De Luca, D.; et al. Hofbauer Cells and COVID-19 in Pregnancy Molecular Pathology Analysis of Villous Macrophages, Endothelial Cells, and Placental Findings from 22 Placentas Infected by SARS-CoV-2 with and Without Fetal Transmission. Arch. Pathol. Lab. Med. 2021, 145, 1328–1340. [Google Scholar] [CrossRef]
- Lee, J.K.; Oh, S.-J.; Park, H.; Shin, O.S. Recent Updates on Research Models and Tools to Study Virus-Host Interactions at the Placenta. Viruses 2019, 12, 5. [Google Scholar] [CrossRef]
- Reyes, L.; Golos, T.G. Hofbauer Cells: Their Role in Healthy and Complicated Pregnancy. Front. Immunol. 2018, 9, 2628. [Google Scholar] [CrossRef]
- Khan, S.; Katabuchi, H.; Araki, M.; Nishimura, R.; Okamura, H. Human Villous Macrophage-Conditioned Media Enhance Human Trophoblast Growth and Differentiation In Vitro. Biol. Reprod. 2000, 62, 1075–1083. [Google Scholar] [CrossRef]
- Castellucci, M.; Kaufmann, P. Basic Structure of the Villous Trees; Pathology of the Human Placenta; Springer: Berlin, Germany, 2012. [Google Scholar]
- Loegl, J.; Hiden, U.; Nussbaumer, E.; Schliefsteiner, C.; Cvitic, S.; Lang, I.; Wadsack, C.; Huppertz, B.; Desoye, G. Hofbauer Cells of M2a, M2b and M2c Polarization May Regulate Feto-Placental Angiogenesis. Reproduction 2016, 152, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L.; Barron, D.H.; Lenz, L.L.; Hutchinson, A.T. M1/M2 Macrophage Polarity in Normal and Complicated Pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; Flores-Espinosa, P.; Mancilla-Herrera, I.; Vega-Sánchez, R.; Díaz, L.; Zaga-Clavellina, V. Innate Immune Cells and Toll-like Receptor-Dependent Responses at the Maternal-Fetal Interface. Int. J. Mol. Sci. 2019, 20, 3564. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Chakraborty, R. Placental Hofbauer Cells Limit HIV-1 Replication and Potentially Offset Mother to Child Transmission (MTCT) by Induction of Immunoregulatory Cytokines. Retrovirology 2012, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Geffers, R.; Berg, G.; Ernerudh, J.; Svensson, J.; Jenmalm, M.C.; Matussek, A. Macrophages at the Fetal–Maternal Interface Express Markers of Alternative Activation and Are Induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef]
- Morotti, D.; Cadamuro, M.; Rigoli, E.; Sonzogni, A.; Gianatti, A.; Parolin, C.; Patanè, L.; Schwartz, D.A. Molecular Pathology Analysis of SARS-CoV-2 in Syncytiotrophoblast and Hofbauer Cells in Placenta from a Pregnant Woman and Fetus with COVID-19. Pathogens 2021, 10, 479. [Google Scholar] [CrossRef]
- Schliefsteiner, C.; Ibesich, S.; Wadsack, C. Placental Hofbauer Cell Polarization Resists Inflammatory Cues In Vitro. Int. J. Mol. Sci. 2020, 21, 736. [Google Scholar] [CrossRef]
- Young, O.M.; Tang, Z.; Niven-Fairchild, T.; Tadesse, S.; Krikun, G.; Norwitz, E.R.; Mor, G.; Abrahams, V.M.; Guller, S. Toll-like Receptor-Mediated Responses by Placental Hofbauer Cells (HBCs): A Potential Pro-Inflammatory Role for Fetal M2 Macrophages. Am. J. Reprod. Immunol. 2015, 73, 22–35. [Google Scholar] [CrossRef]
- Takeuchi, H.; Tanaka, M.; Tanaka, A.; Tsunemi, A.; Yamamoto, H. Predominance of M2-polarized Macrophages in Bladder Cancer Affects Angiogenesis, Tumor Grade and Invasiveness. Oncol. Lett. 2016, 11, 3403–3408. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.K.; Jurado, K.A.; Abrahams, V.M.; Fikrig, E.; Guller, S. Zika Virus Infection of Hofbauer Cells. Am. J. Reprod. Immunol. 2017, 77, e12613. [Google Scholar] [CrossRef] [PubMed]
- Jurado, K.A.; Simoni, M.K.; Tang, Z.; Uraki, R.; Hwang, J.; Householder, S.; Wu, M.; Lindenbach, B.D.; Abrahams, V.M.; Guller, S.; et al. Zika Virus Productively Infects Primary Human Placenta-Specific Macrophages. Ref. Inf. JCI Insight 2016, 1, 88461. [Google Scholar] [CrossRef] [PubMed]
- Amb Uhl, L.M.M.; Leonhard, A.K.; Zakhary, C.W.; Jørgensen, A.; Blaakaer, J.; Dybkaer, K.; Baandrup, U.; Uldbjerg, N.; Sørensen, S. Human Papillomavirus Infects Placental Trophoblast and Hofbauer Cells, but Appears Not to Play a Causal Role in Miscarriage and Preterm Labor. Acta Obstet. Gynecol. Scand. 2017, 96, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Moström, M.J.; Scheef, E.A.; Sprehe, L.M.; Szeltner, D.; Tran, D.; Hennebold, J.D.; Roberts, V.H.J.; Maness, N.J.; Fahlberg, M.; Kaur, A. Immune Profile of the Normal Maternal-Fetal Interface in Rhesus Macaques and Its Alteration Following Zika Virus Infection. Front. Immunol. 2021, 12, 2906. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.; Harris, L.K.; Innes, B.A.; Lash, G.E.; Aljunaidy, M.M.; Aplin, J.D.; Baker, P.N.; Robson, S.C.; Bulmer, J.N. Uterine Natural Killer Cells Initiate Spiral Artery Remodeling in Human Pregnancy; Uterine Natural Killer Cells Initiate Spiral Artery Remodeling in Human Pregnancy. FASEB J. Res. Commun. 2012, 26, 4876–4885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Crespo, Â.C.; Strominger, J.L.; Tilburgs, T.; Altfeld, M.; Yokoyama, W.M. Expression of KIR2DS1 by Decidual Natural Killer Cells Increases Their Ability to Control Placental HCMV Infection. Proc. Natl. Acad. Sci. USA 2016, 113, 15072–15077. [Google Scholar] [CrossRef]
- Zheng, Q.; Jin, L.; Yang, F. Dynamic Function and Composition Changes of Immune Cells during Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef]
- Caligiuri, M.A.; Vacca, P.; Gesù, B.; Moffett, A.; Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and During Viral Infection. Front. Immunol. 2019, 1, 1397. [Google Scholar] [CrossRef]
- de Mendonça Vieira, R.; Meagher, A.; Crespo, A.; Kshirsagar, S.K.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Human Term Pregnancy Decidual NK Cells Generate Distinct Cytotoxic Responses. J. Immunol. 2020, 204, 3149–3159. [Google Scholar] [CrossRef]
- Lash, G.E. Expression of Angiogenic Growth Factors by Uterine Natural Killer Cells during Early Pregnancy. J. Leukoc. Biol. 2006, 80, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Crespo, Â.C.; Mulik, S.; Dotiwala, F.; Ansara, J.A.; sen Santara, S.; Ingersoll, K.; Ovies, C.; Junqueira, C.; Tilburgs, T.; Strominger, J.L.; et al. Decidual NK Cells Transfer Granulysin to Selectively Kill Bacteria in Trophoblasts. Cell 2020, 182, 1125–1139.e18. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Gentili, V.; Rotola, A.; Cultrera, R.; Marci, R.; di Luca, D.; Rizzo, R. HHV-6A Infection of Endometrial Epithelial Cells Affects Immune Profile and Trophoblast Invasion. Am. J. Reprod. Immunol. 2019, 82, e13174. [Google Scholar] [CrossRef]
- Carlino, C.; Stabile, H.; Morrone, S.; Bulla, R.; Soriani, A.; Agostinis, C.; Bossi, F.; Mocci, C.; Sarazani, F.; Tedesco, F.; et al. Recruitment of Circulating NK Cells through Decidual Tissues: A Possible Mechanism Controlling NK Cell Accumulation in the Uterus during Early Pregnancy. Blood 2008, 111, 3108–3115. [Google Scholar] [CrossRef]
- Ikumi, N.M.; Matjila, M. Preterm Birth in Women with HIV: The Role of the Placenta. Front. Glob. Womens Health 2022, 3, 820759. [Google Scholar] [CrossRef]
- Parker, E.L.; Silverstein, R.B.; Verma, S.; Mysorekar, I.U. Viral-Immune Cell Interactions at the Maternal-Fetal Interface in Human Pregnancy. Front. Immunol. 2022, 11, 522047. [Google Scholar] [CrossRef]
- Juttukonda, L.J.; Wachman, E.M.; Boateng, J.; Jain, M.; Benarroch, Y.; Taglauer, E.S. Decidual Immune Response Following COVID-19 during Pregnancy Varies by Timing of Maternal SARS-CoV-2 Infection. J. Reprod. Immunol. 2022, 151, 490–497. [Google Scholar] [CrossRef]
- Johnson, E.L.; Swieboda, D.; Olivier, A.; Enninga, E.A.L.; Chakraborty, R. Robust Innate Immune Responses at the Placenta during Early Gestation May Limit in Utero HIV Transmission. PLoS Pathog. 2021, 17, e1009860. [Google Scholar] [CrossRef]
- Rizzuto, G.; Tagliani, E.; Manandhar, P.; Erlebacher, A.; Bakardjiev, A.I. Limited Colonization Undermined by Inadequate Early Immune Responses Defines the Dynamics of Decidual Listeriosis. Infect. Immun. 2017, 85, e00153–17. [Google Scholar] [CrossRef]
- Xu, X.; Fu, Q.; Zhang, Q.; Zhao, M.; Gao, Z.; Liu, X.; Liu, Y.; Hu, X. Changes of Human Decidual Natural Killer Cells Cocultured with YFP-Toxoplasma Gondii: Implications for Abnormal Pregnancy. Fertil. Steril. 2013, 99, 427–432. [Google Scholar] [CrossRef]
- sen Santara, S.; Crespo, Â.C.; Mulik, S.; Ovies, C.; Boulenouar, S.; Strominger, J.L.; Lieberman, J. Decidual NK Cells Kill Zika Virus–Infected Trophoblasts. Proc. Natl. Acad. Sci. USA 2021, 118, e2115410118. [Google Scholar] [CrossRef]
- Glasner, A.; Oiknine-Djian, E.; Weisblum, Y.; Diab, M.; Panet, A.; Wolf, D.G.; Mandelboim, O. Zika Virus Escapes NK Cell Detection by Upregulating Major Histocompatibility Complex Class I Molecules. J. Virol. 2017, 91, e00785–17. [Google Scholar] [CrossRef]
- Wells, A.I.; Coyne, C.B. Type III Interferons in Antiviral Defenses at Barrier Surfaces HHS Public Access. Trends Immunol. 2018, 39, 848–858. [Google Scholar] [CrossRef]
- Koyama, S.; Ishii, K.J.; Coban, C.; Akira, S. Innate Immune Response to Viral Infection. Cytokine 2008, 43, 336–341. [Google Scholar] [CrossRef]
- Raftery, N.; Stevenson, N.J. Advances in Anti-Viral Immune Defence: Revealing the Importance of the IFN JAK/STAT Pathway. Cell. Mol. Life Sci. 2017, 74, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Trus, I.; Udenze, D.; Cox, B.; Berube, N.; Nordquist, R.E.; Van Der Staay, F.J.; Huang, Y.; Kobinger, G.; Safronetz, D.; Gerdts, V.; et al. Subclinical in Utero Zika Virus Infection Is Associated with Interferon Alpha Sequelae and Sex-Specific Molecular Brain Pathology in Asymptomatic Porcine Offspring. PLoS Pathog. 2019, 15, e1008038. [Google Scholar] [CrossRef]
- MacMicking, J.D. Interferon-Inducible Effector Mechanisms in Cell-Autonomous Immunity. Nat. Rev. Immunol. 2012, 12, 367–382. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type i Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Coldbeck-Shackley, R.C.; Eyre, N.S.; Beard, M.R. The Molecular Interactions of ZIKV and DENV with the Type-I IFN Response. Vaccines 2020, 8, 530. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of Type i Interferon Responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, S.; Farr, D.; Kumar, A. Interferon-Stimulated Gene 15 (ISG15) Restricts Zika Virus Replication in Primary Human Corneal Epithelial Cells. Ocul. Surf. 2019, 17, 551–559. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R. Interferon-Inducible Antiviral Effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef]
- Kwon, J.-Y.; Aldo, P.; You, Y.; Ding, J.; Racicot, K.; Dong, X.; Murphy, J.; Glukshtad, G.; Silasi, M.; Peng, J.; et al. Relevance of Placental Type I Interferon Beta Regulation for Pregnancy Success. Cell. Mol. Immunol. 2018, 15, 1010–1026. [Google Scholar] [CrossRef]
- Yockey, L.J.; Jurado, K.A.; Arora, N.; Millet, A.; Rakib, T.; Milano, K.M.; Hastings, A.K.; Fikrig, E.; Kong, Y.; Horvath, T.L.; et al. Type I Interferons Instigate Fetal Demise after Zika Virus Infection. Sci. Immunol. 2018, 3, eaao1680. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Rice, C.M. Interferon-Stimulated Genes and Their Antiviral Effector Functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef]
- Cappelletti, M.; Presicce, P.; Lawson, M.J.; Chaturvedi, V.; Stankiewicz, T.E.; Vanoni, S.; Harley, I.T.W.; Mcalees, J.W.; Giles, D.A.; Moreno-Fernandez, M.E.; et al. Type I Interferons Regulate Susceptibility to Inflammation-Induced Preterm Birth. JCI Insight 2017, 2, e91288. [Google Scholar] [CrossRef]
- Racicot, K.; Aldo, P.; El-Guindy, A.; Kwon, J.-Y.; Romero, R.; Mor, G. Cutting Edge: Fetal/Placental Type I IFN Can Affect Maternal Survival and Fetal Viral Load during Viral Infection. J. Immunol. 2017, 198, 3029–3032. [Google Scholar] [CrossRef]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; Schinazi, R.F.; Chakraborty, R.; Suthar, M.S.; Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Haese, N.N.; Smith, H.; Onwuzu, K.; Kreklywich, C.N.; Smith, J.L.; Denton, M.; Kreklywich, N.; Streblow, A.D.; Frias, A.E.; Morgan, T.K.; et al. Differential Type 1 IFN Gene Expression in CD14+ Placenta Cells Elicited by Zika Virus Infection During Pregnancy. Front. Virol. 2021, 1, 783407. [Google Scholar] [CrossRef]
- Weisblum, Y.; Oiknine-Djian, E.; Vorontsov, O.M.; Haimov-Kochman, R.; Zakay-Rones, Z.; Meir, K.; Shveiky, D.; Elgavish, S.; Nevo, Y.; Roseman, M.; et al. Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface. J. Virol. 2017, 91, e01905–16. [Google Scholar] [CrossRef]
- Quanquin, N.M.; Barres, L.G.; Aliyari, S.R.; Day, N.T.; Gerami, H.; Fisher, S.J.; Kakuru, A.; Kamya, M.R.; Havlir, D.V.; Feeney, M.; et al. Gravidity-Dependent Associations between Interferon Response and Birth Weight in Placental Malaria. Malar. J. 2020, 19, 280. [Google Scholar] [CrossRef]
- Tripathi, S.; Balasubramaniam, V.R.M.T.; Brown, J.A.; Mena, I.; Grant, A.; Bardina, S.V.; Maringer, K.; Schwarz, M.C.; Maestre, A.M.; Sourisseau, M.; et al. A Novel Zika Virus Mouse Model Reveals Strain Specific Differences in Virus Pathogenesis and Host Inflammatory Immune Responses. PLoS Pathog. 2017, 13, e1006258. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Miner, J.; Cao, B.; Govero, J.; Smith, A.; Fernandez, E.; Cabrera, O.; Garber, C.; Noll, M.; Klein, R.; Noguchi, K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Duarte, L.; Pandya, Y.; Neres, R.; Penha-Gonçalves, C. Fetal and Maternal Innate Immunity Receptors Have Opposing Effects on the Severity of Experimental Malaria in Pregnancy: Beneficial Roles for Fetus-Derived Toll-like Receptor 4 and Type I Interferon Receptor 1. Infect. Immun. 2018, 86, e00708–17. [Google Scholar] [CrossRef]
- Kwon, J.-Y.; Romero, R.; Mor, G. New Insights into the Relationship between Viral Infection and Pregnancy Complications. Am. J. Reprod. Immunol. 2014, 71, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Szaba, F.M.; Tighe, M.; Kummer, L.W.; Lanzer, K.G.; Ward, J.M.; Lanthier, P.; Kim, I.J.; Kuki, A.; Blackman, M.A.; Thomas, S.J.; et al. Zika Virus Infection in Immunocompetent Pregnant Mice Causes Fetal Damage and Placental Pathology in the Absence of Fetal Infection. PLoS Pathog. 2018, 14, e1006994. [Google Scholar] [CrossRef] [PubMed]
- Azamor, T.; Prado Cunha, D.; Marques Vieira da Silva, A.; Cavalcanti de Lima Bezerra, O.; Ribeiro-Alves, M.; Leal Calvo, T.; de Souza Gomes Kehdy, F.; Saloum de Neves Manta, F.; Gomes de Toledo Pinto, T.; Pereira Ferreira, L.; et al. Congenital Zika Syndrome Is Associated With Interferon Alfa Receptor 1. Front. Immunol. 2021, 12, 764746. [Google Scholar] [CrossRef]
- Harding, A.T.; Goff, M.A.; Froggatt, H.M.; Lim, J.K.; Heaton, N.S. GPER1 Is Required to Protect Fetal Health from Maternal Inflammation. Science 2021, 371, 271–276. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, L.V.; Salomão, N.G.; Alves, F.d.A.V.; Rabelo, K. The Innate Defense in the Zika-Infected Placenta. Pathogens 2022, 11, 1410. https://doi.org/10.3390/pathogens11121410
Arruda LV, Salomão NG, Alves FdAV, Rabelo K. The Innate Defense in the Zika-Infected Placenta. Pathogens. 2022; 11(12):1410. https://doi.org/10.3390/pathogens11121410
Chicago/Turabian StyleArruda, Laíza Vianna, Natália Gedeão Salomão, Felipe de Andrade Vieira Alves, and Kíssila Rabelo. 2022. "The Innate Defense in the Zika-Infected Placenta" Pathogens 11, no. 12: 1410. https://doi.org/10.3390/pathogens11121410
APA StyleArruda, L. V., Salomão, N. G., Alves, F. d. A. V., & Rabelo, K. (2022). The Innate Defense in the Zika-Infected Placenta. Pathogens, 11(12), 1410. https://doi.org/10.3390/pathogens11121410






