Comparison of Three Diagnostic Methods to Detect the Occurrence of Fasciola Species in Communally Grazed Cattle in the North West Province, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
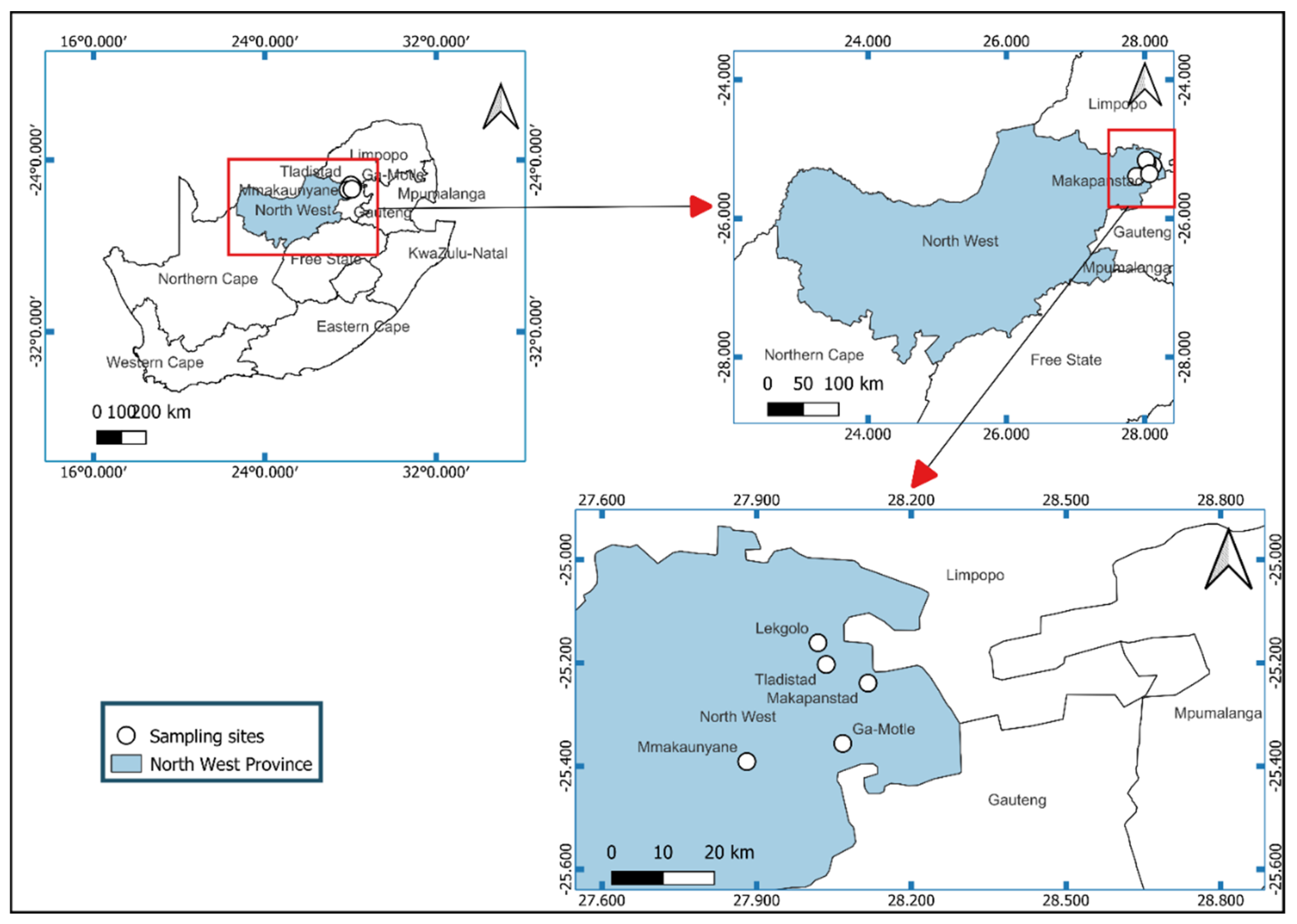
2.2. Study Area
2.3. Study Design and Sample Size
2.4. Collection of Faecal Samples and Animal Records
2.5. Sedimentation Technique to Identify Fluke Eggs
2.6. DNA Extraction
2.7. Quantitative Real-Time PCR for Fasciola Species
2.8. Coproantigen ELISA (coproELISA)
2.9. Data Analysis
3. Results
3.1. Description of the Sampled Cattle
3.2. Presence of Fasciola Eggs in Faecal Samples Using the Sedimentation Technique
3.3. Occurrence of Fasciola Species in Faecal Samples Using qPCR
3.4. Association between Faecal Consistency and Presence of Fasciola DNA in Faeces
3.5. Comparison of Detection Rate of Fasciola spp. between Sedimentation and qPCR
3.6. Detection of Fasciola spp. Using coproELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol. 2005, 35, 1255–1278. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fascioliasis. In Digenetic Trematodes. Advances in Experimental Medicine and Biology; Toledo, R., Fried, B., Eds.; Springer: Cham, Switzerland, 2019; Volume 1154. [Google Scholar]
- Le, T.H.; De, N.V.; Agatsuma, T.; Blair, D.; Vercruysse, J.; Dorny, P.; McManus, D.P. Molecular confirmation that Fasciola gigantica can undertake aberrant migrations in human hosts. J. Clin. Microbiol. 2007, 45, 648–650. [Google Scholar] [CrossRef]
- Admassu, B.; Shite, A.; Kinfe, G. A review on bovine fasciolosis. Eur. J. Biol. Sci. 2015, 7, 139–146. [Google Scholar]
- Haridwal, S.; Malatji, M.P.; Mukaratirwa, S. Morphological and molecular characterization of Fasciola hepatica and Fasciola gigantica phenotypes from co-endemic localities in Mpumalanga and KwaZulu-Natal provinces of South Africa. Food Waterborne Parasitol. 2021, 22, e00114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.G.T.; Van De, N.; Vercruysse, J.; Dorny, P.; Le, T.H. Genotypic characterization and species identification of Fasciola spp. with implications regarding the isolates infecting goats in Vietnam. Exp. Parasitol. 2009, 123, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Van De, N.; Agatsuma, T.; Nguyen, T.G.T.; Nguyen, Q.D.; McManus, D.P.; Blair, D. Human fascioliasis and the presence of hybrid/introgressed forms of Fasciola hepatica and Fasciola gigantica in Vietnam. Int. J. Parasitol. 2008, 38, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.V.; Valero, M.A.; El Sayed, M.; Ashrafi, K.; El Wakeel, A.; Mohamed, M.Y.; Mas-Coma, S. First phenotypic description of Fasciola hepatica/Fasciola gigantica intermediate forms from the human endemic area of the Nile Delta, Egypt. Infect. Genet. Evol. 2008, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mahulu, A.; Clewing, C.; Stelbrink, B.; Chibwana, F.D.; Tumwebaze, I.; Russell Stothard, J.; Albrecht, C. Cryptic intermediate snail host of the liver fluke Fasciola hepatica in Africa. Parasit. Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Kock, K.N.; Wolmarans, C.T. The geographical distribution and habitats of three liver fluke intermediate hosts in South-Africa and the health implications involved. Suid-Afrik. Tydskr. Nat. Tegnol. 2008, 27, 1–16. [Google Scholar] [CrossRef][Green Version]
- Moema, E.B.E.; King, P.H.; Baker, C. Cercariae developing in Lymnaea natalensis Krauss, 1848 collected in the vicinity of Pretoria, Gauteng Province, South Africa. Onderstepoort J. Vet. Res. 2008, 75, 215–223. [Google Scholar] [CrossRef]
- Malatji, M.P.; Mukaratirwa, S. Molecular detection of natural infection of Lymnaea (Pseudosuccinea) columella (Gastropoda: Lymnaeidae) with Fasciola gigantica (Digenea: Fasciolidae) from two provinces of South Africa. J. Helminthol. 2020, 94, e38. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. 2009, 69, 41–146. [Google Scholar] [PubMed]
- Ibrahim, N. Fascioliasis: Systematic review. Adv. Biol. Res. 2017, 11, 278–285. [Google Scholar]
- Mungube, E.O.; Bauni, S.M.; Tenhagen, B.A.; Wamae, L.W.; Nginyi, J.M.; Mugambi, J.M. The prevalence and economic significance of Fasciola gigantica and Stilesia hepatica in slaughtered animals in the semi-arid coastal Kenya. Trop. Anim. Health Prod. 2006, 38, 475–483. [Google Scholar] [CrossRef]
- Mehmood, K.; Zhang, H.; Sabir, A.J.; Abbas, R.Z.; Ijaz, M.; Durrani, A.Z.; Li, J. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb. Pathog. 2017, 109, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Jaja, I.F.; Mushonga, B.; Green, E.; Muchenje, V. Financial loss estimation of bovine fasciolosis in slaughtered cattle in South Africa. Parasite Epidemiol. Control. 2017, 2, 27–34. [Google Scholar] [CrossRef]
- Ndlovu, T.; Chimonyo, M.; Muchenje, V. Monthly changes in body condition scores and internal parasite prevalence in Nguni, Bonsmara and Angus steers raised on sweetveld. Trop. Anim. Health Prod. 2009, 41, 1169–1177. [Google Scholar] [CrossRef]
- Mpisana, Z.; Jaja, I.F.; Byaruhanga, C.; Marufu, M.C. Body condition scores, fluke intensity, liver pathology, and carcass quality of different dairy cattle genotypes infected with Fasciola species at high throughput abattoirs in South Africa. Parasitol. Res. 2022, 121, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Brockwell, Y.M.; Spithill, T.W.; Anderson, G.R.; Grillo, V.; Sangster, N.C. Comparative kinetics of serological and coproantigen ELISA and faecal egg count in cattle experimentally infected with Fasciola hepatica and following treatment with triclabendazole. Vet. Parasitol. 2013, 196, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sernandez, V.; Orbegozo-Medina, R.A.; Gonzalez-Warleta, M.; Mezo, M.; Ubeira, F.M. Rapid enhanced MM3-COPRO ELISA for detection of Fasciola coproantigens. PLoS Negl. Trop. Dis. 2016, 10, e0004872. [Google Scholar] [CrossRef]
- Calvani, N.E.D.; Windsor, P.A.; Bush, R.D.; Šlapeta, J. Scrambled eggs: A highly sensitive molecular diagnostic workflow for Fasciola species specific detection from faecal samples. PLoS Negl. Trop. Dis. 2017, 11, e0005931. [Google Scholar] [CrossRef]
- Martínez-Pérez, J.M.; Robles-Pérez, D.; Rojo-Vázquez, F.A.; Martínez-Valladares, M. Comparison of three different techniques to diagnose Fasciola hepatica infection in experimentally and naturally infected sheep. Vet. Parasitol. 2012, 190, 80–86. [Google Scholar] [CrossRef]
- Robles-Pérez, D.; Martínez-Pérez, J.M.; Rojo-Vázquez, F.A.; Martínez-Valladares, M. The diagnosis of fasciolosis in feces of sheep by means of a PCR and its application in the detection of anthelmintic resistance in sheep flocks naturally infected. Vet. Parasitol. 2013, 197, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, S.L.; Viljoen, A.; Marais, D.; Wenhold, F.; McIntyre, A.; Ngidi, M.; Stewart, D. The Current Rain-Fed and Irrigated Production of Food Crops and Its Potential to Meet the Year-Round Nutritional Requirements of Rural Poor People in North West, Limpopo, Kwazulu-Natal and the Eastern Cape: Report to the Water Research Commission and Department of Agriculture, Forestry & Fisheries; Water Research Commission: Pretoria, South Africa, 2016. [Google Scholar]
- Letsoalo, S.S.; Krecek, R.C.; Botha, C.A.J.; Ngetu, X. Animal husbandry in Moretele 1 of North-West Province: Implications for veterinary training and research. J. S. Afr. Vet. Assoc. 2000, 71, 92–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Makina, S.O.; Whitacre, L.K.; Decker, J.E.; Taylor, J.F.; MacNeil, M.D.; Scholtz, M.M.; Maiwashe, A. Insight into the genetic composition of South African Sanga cattle using SNP data from cattle breeds worldwide. Genet. Sel. Evol. 2016, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sojl, Z.; Mabusela, S.P.; Muchenje, V. Associations between animal traits, carcass traits and carcass classification in a selected abattoir in the Eastern Cape Province, South Africa. S. Afr. J. Anim. Sci. 2015, 45, 278–288. [Google Scholar] [CrossRef]
- Renaud, D.L.; Buss, L.; Wilms, J.N.; Steele, M.A. Is fecal consistency scoring an accurate measure of fecal dry matter in dairy calves? J. Dairy Sci. 2020, 103, 10709–10714. [Google Scholar] [CrossRef]
- Happich, F.A.; Boray, J.C. QUANTITATIVE DIAGNOSIS OF CHRONIC FASCIOLOSIS: 1. Comparative Studies on Quantitative Faecal Examinations for Chronic Fasciola hepatica Infection in Sheep. Aust. Vet. J. 1969, 45, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Alasaad, S.; Soriguer, R.C.; Abu-Madi, M.; El Behairy, A.; Jowers, M.J.; Baños, P.D.; Zhu, X.Q. A TaqMan real-time PCR-based assay for the identification of Fasciola spp. Vet. Parasitol. 2011, 179, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 11 June 2022).
- Calvani, N.E.D.; George, S.D.; Windsor, P.A.; Bush, R.D.; Šlapeta, J. Comparison of early detection of Fasciola hepatica in experimentally infected Merino sheep by real-time PCR, coproantigen ELISA and sedimentation. Vet. Parasitol. 2018, 251, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Calvani, N.E.D.; Ichikawa-Seki, M.; Bush, R.D.; Khounsy, S.; Šlapeta, J. Which species is in the faeces at a time of global livestock movements: Single nucleotide polymorphism genotyping assays for the differentiation of Fasciola spp. Int. J. Parasitol. 2020, 50, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Reigate, C.; Williams, H.W.; Denwood, M.J.; Morphew, R.M.; Thomas, E.R.; Brophy, P.M. Evaluation of two Fasciola hepatica faecal egg counting protocols in sheep and cattle. Vet. Parasitol. 2021, 294, 109435. [Google Scholar] [CrossRef]
- Arifin, M.I.; Höglund, J.; Novobilský, A. Comparison of molecular and conventional methods for the diagnosis of Fasciola hepatica infection in the field. Vet. Parasitol. 2016, 232, 8–11. [Google Scholar] [CrossRef]
- Mazeri, S.; Sargison, N.; Kelly, R.F.; Bronsvoort, B.M.D.; Handel, I. Evaluation of the performance of five diagnostic tests for Fasciola hepatica infection in naturally infected cattle using a Bayesian no gold standard approach. PLoS ONE 2016, 11, e0161621. [Google Scholar] [CrossRef]
- Paras, K.L.; George, M.M.; Vidyashankar, A.N.; Kaplan, R.M. Comparison of fecal egg counting methods in four livestock species. Vet. Parasitol. 2018, 257, 21–27. [Google Scholar] [CrossRef]
- Mezo, M.; González-Warleta, M.; Carro, C.; Ubeira, F.M. An ultrasensitive capture ELISA for detection of Fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (MM3). J. Parasitol. 2004, 90, 845–852. [Google Scholar] [CrossRef]
- Kajugu, P.E.; Hanna, R.E.B.; Edgar, H.W.; McMahon, C.; Cooper, M.; Gordon, A.; Fairweather, I. Fasciola hepatica: Specificity of a coproantigen ELISA test for diagnosis of fasciolosis in faecal samples from cattle and sheep concurrently infected with gastrointestinal nematodes, coccidians and/or rumen flukes (paramphistomes), under field conditions. Vet. Parasitol. 2015, 212, 181–187. [Google Scholar] [CrossRef]
- Gordon, D.; Zadoks, R.; Skuce, P.; Sargison, N. Confirmation of triclabendazole resistance in liver fluke in the UK. Vet. Rec. 2012, 171, 159. [Google Scholar] [CrossRef]
- French, A.S.; Zadoks, R.N.; Skuce, P.J.; Mitchell, G.; Gordon-Gibbs, D.K.; Craine, A.; Taggart, M.A. Prevalence of liver fluke (Fasciola hepatica) in wild red deer (Cervus elaphus): Coproantigen ELISA is a practicable alternative to faecal egg counting for surveillance in remote populations. PLoS ONE 2016, 11, e0162420. [Google Scholar] [CrossRef]
- Novobilský, A.; Averpil, H.B.; Höglund, J. The field evaluation of albendazole and triclabendazole efficacy against Fasciola hepatica by coproantigen ELISA in naturally infected sheep. Vet Parasitol. 2012, 190, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Mochankana, M.E.; Robertson, I.D. Cross-sectional prevalence of Fasciola gigantica infections in beef cattle in Botswana. Trop. Anim. Health Prod. 2018, 50, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Opio, L.G.; Abdelfattah, E.M.; Terry, J.; Odongo, S.; Okello, E. Prevalence of fascioliasis and associated economic losses in cattle slaughtered at lira municipality abattoir in northern Uganda. Animals 2021, 11, 681. [Google Scholar] [CrossRef]
- Kouadio, J.N.; Giovanoli Evack, J.; Achi, L.Y.; Fritsche, D.; Ouattara, M.; Silué, K.D.; N’Goran, E.K. Prevalence and distribution of livestock schistosomiasis and fascioliasis in Côte d’Ivoire: Results from a cross-sectional survey. BMC Vet. Res. 2020, 16, 1–13. [Google Scholar] [CrossRef]
- Phiri, A.M.; Phiri, I.K.; Sikasunge, C.S.; Monrad, J. Prevalence of fasciolosis in Zambian cattle observed at selected abattoirs with emphasis on age, sex and origin. J. Vet. Med. B 2005, 52, 414–416. [Google Scholar] [CrossRef]
| Variable (Category) | Number of Positive Cattle (%) | 95% CI | p-Value |
|---|---|---|---|
| Location | |||
| Makanpastad (n = 47) | 26 (55.3) | 40.12, 69.83 | <0.001 |
| Legkolo (n = 50) | 11 (22.0) | 11.53, 35.96 | |
| Makayauna (n = 65) | 11 (16.9) | 8.76, 28.27 | |
| GaMotle (n = 60) | 8 (13.3) | 5.94, 24.59 | |
| Tladistad (n = 55) | 17 (30.9) | 19.14, 44.81 | |
| Breed | |||
| Afrikaner (n = 32) | 3 (9.4) | 1.98, 25.02 | 0.065 |
| Brahman (n = 90) | 24 (26.7) | 17.89, 37.03 | |
| Crossbreed (n = 143) | 42 (29.4) | 22.06, 37.56 | |
| Sex | |||
| Female (n = 246) | 62 (25.2) | 19.90, 31.11 | 0.221 |
| Male (n = 31) | 11 (35.5) | 19.23, 54.63 | |
| Age | |||
| 2 to 4 years (n = 153) | 33 (21.6) | 15.34, 28.94 | 0.045 |
| >4 years (n = 124) | 40 (32.3) | 24.15, 41.24 |
| Faecal Consistency | No. of Positive Samples | % of Positive Samples | Odds Ratio | p-Value |
|---|---|---|---|---|
| Good (n = 41) (reference) | 7 | 17.1 | ||
| Average (n = 206) | 57 | 27.7 | 1.86 | 0.162 |
| Poor (n = 30) | 9 | 30.0 | 2.08 | 0.203 |
| qPCR n (%) | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Sedimentation n (%) | Positive | 9 (3.2) | 27 (9.7) | 36 (13.0) |
| Negative | 64 (23.1) | 177 (63.9) | 241 (87.0) | |
| Total | 73(26.4) | 204 (73.6) | 277 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaogun, S.C.; Byaruhanga, C.; Ochai, S.O.; Fosgate, G.T.; Marufu, M.C. Comparison of Three Diagnostic Methods to Detect the Occurrence of Fasciola Species in Communally Grazed Cattle in the North West Province, South Africa. Pathogens 2022, 11, 1398. https://doi.org/10.3390/pathogens11121398
Olaogun SC, Byaruhanga C, Ochai SO, Fosgate GT, Marufu MC. Comparison of Three Diagnostic Methods to Detect the Occurrence of Fasciola Species in Communally Grazed Cattle in the North West Province, South Africa. Pathogens. 2022; 11(12):1398. https://doi.org/10.3390/pathogens11121398
Chicago/Turabian StyleOlaogun, Sunday C., Charles Byaruhanga, Sunday O. Ochai, Geoffrey T. Fosgate, and Munyaradzi C. Marufu. 2022. "Comparison of Three Diagnostic Methods to Detect the Occurrence of Fasciola Species in Communally Grazed Cattle in the North West Province, South Africa" Pathogens 11, no. 12: 1398. https://doi.org/10.3390/pathogens11121398
APA StyleOlaogun, S. C., Byaruhanga, C., Ochai, S. O., Fosgate, G. T., & Marufu, M. C. (2022). Comparison of Three Diagnostic Methods to Detect the Occurrence of Fasciola Species in Communally Grazed Cattle in the North West Province, South Africa. Pathogens, 11(12), 1398. https://doi.org/10.3390/pathogens11121398








