Abstract
The genus Aeromonas is widely distributed in aquatic environments and is recognized as a potential human pathogen. Some Aeromonas species are able to cause a wide spectrum of diseases, mainly gastroenteritis, skin and soft-tissue infections, bacteremia, and sepsis. Currently, untreated river water is used for irrigation and recreational purposes. In this study, the Aeromonas spp. present in a river recreational environment was investigated by quantifying its presence in water, soil, and vegetation using three techniques: qPCR, plate counting in selective ADA medium, and Most Probable Number, in parallel. The presence of clones in the three types of samples was elucidated through genotyping with the ERIC-PCR technique, whereas the identification of the isolated Aeromonas was carried out by sequencing the rpoD gene. Finally, the pathogenic potential of some of the strains was explored by studying the presence and expression of virulence genes characteristic of the genus, their antimicrobial susceptibility profile, as well as the quantification of their cell damage and intracellular survival in an in vitro macrophages infection model. The results showed the presence of Aeromonas in all samples with the three quantification methods, with Aeromonas popoffii being the most prevalent species. The presence of strains with the same genotype (ERIC-PCR) was also confirmed in different samples. Some of the strains showed a high level of cell damage and intracellular bacterial survival, as well as the presence of various virulence factors. Furthermore, these strains showed resistance to some of the antibiotics tested and used therapeutically in both humans and animals. These results indicate that the presence of Aeromonas in this environment may represent a biosanitary risk that could be a public health problem.
1. Introduction
The genus Aeromonas comprises a group of Gram-negative bacteria autochthonous to aquatic environments and widely distributed in numerous ecosystems, including groundwater, drinking water, bottled water, river water, seawater, irrigation water, and reclaimed wastewater [1,2,3,4,5,6,7,8,9,10]. However, these bacteria have also been recovered from soil, animals, food products, and humans infections in immunocompromised and immunocompetent patients [11,12]. Cases of severe Aeromonas infections have been reported, mainly due to contact with contaminated waters in rivers and lakes [13,14,15,16,17,18]. The most frequent Aeromonas infections are gastroenteritis and skin and soft-tissue infections, followed by bacteremia and sepsis, as well as other infections that affect the hepatobiliary system, respiratory tract, bones, and joints [12,19].
Water is a limited resource, so currently waters from different sources, such as reclaimed water or untreated river water, are used for irrigation [8,10]. Previous literature has demonstrated the presence of the same Aeromonas strains (clones) in vegetables and in the irrigation water used, as well as in areas close to these water sources [8], which may represent a health problem [8,20,21]. In addition, a very severe case of Aeromonas necrotizing fasciitis in a young healthy girl was reported after she fell into a river [17,18]. The progression of the infection led to the amputation of a large part of her four limbs.
The pathogenesis of Aeromonas infections is complex and considered multifactorial. Several genes encoding for virulence factors link to the capacity of Aeromonas to evade the host immune response and contribute to the infectious process [19,22]. These include cell structural components; extracellular proteins like aerolysins, hemolysins, lipases, cytolytic and cytotonic enterotoxins; secretion systems; and metal-associated proteins [22,23]. Another concerning characteristic of Aeromonas is its increasing resistance to antibiotics, resulting in treatment failure in human and animal infections [11,24]. A wide diversity of genetic elements responsible for antimicrobial resistance in Aeromonas has been described, which can be encoded in the chromosome, in mobile genetic elements, or integrons [25,26,27,28].
In the natural area “El Clot de la Mare de Déu” (Burriana, Spain), which belongs to the fluvial area of the Mijares river, untreated and uncontrolled waters are frequently used for agricultural irrigation and for recreational purposes. The present study quantifies the presence and diversity of Aeromonas species in the river, soil, and vegetation of the recreational area and characterizes the presence and expression of virulence genes, the antimicrobial susceptibility profile, as well as the survival and infective capacity of the Aeromonas strains in macrophages, in order to evaluate the potential risk to human health associated with the use of this water environment.
2. Material and Methods
2.1. Sample Collection and Processing
Three types of samples were analyzed from “El Clot de la Mare de Déu” (Burriana, Spain): water, soil, and vegetation. The water sample was collected in a 2 L polypropylene bottle, while the soil and vegetation samples were collected in polyethylene bags. All of them were refrigerated and transported to the laboratory and processed on the same day as collection. The water sample was serial diluted, while the soil and vegetation samples were mixed with distilled water, vortexed, and a serial dilution was performed.
2.2. Aeromonas Quantification by Plate Counting
Aliquots of 100 µL of each sample were plated on the surface of Ampicillin Dextrin Agar (ADA, HiMedia, Mumbai, India). After incubation of the plates at 37 °C for 24 h, the colony-forming units (CFU) were counted [8,12].
2.3. Aeromonas Quantification by qPCR
DNA was extracted from all samples using the Easy-DNATM kit (Invitrogen, Carlsbad, CA, USA). Quantitative real-time PCR was performed with purified DNA using the DNA TargetSpecies dtec-qPCR kit for Aeromonas species (Genetic PCR solutions, Orihuela, Spain) and the SteponePlusTM Real-Time PCR system (Applied Biosystems, Waltham, MA, USA). The number of copies was calculated based on the standard line and the corresponding amplification cycle threshold (Ct).
2.4. Aeromonas Quantification by Most Probable Number (MPN)
All samples were quantified via a 3-tube MPN method, inoculating 500 μL of the serial dilutions by triplicate in tubes with 2.5 mL of Alkaline Peptone Water supplemented with ampicillin at a concentration of 10 mg/L. After incubating the tubes for 24 h at 37 °C, the number of positives (tubes that presented yellow/orange turbidity) was counted and the most probable number was obtained with the “Most Probable Number Calculator” computer tool (https://mostprobablenumbercalculator.epa.gov/mpnForm, accessed on 1 March 2021). Later, the tubes were plated in ADA as a control to demonstrate the presence of Aeromonas [8,12].
2.5. Bacterial Strains Maintenance and Culture Conditions
From the ADA plates, 24 colonies were selected based on the typical morphology of Aeromonas, i.e., yellow colonies grown on this medium. Bacteria were subcultured on DifcoTM Tryptic Soy Agar (TSA; Becton Dickinson and Company, Sparks, MD, USA), performing successive passages to obtain pure cultures. For conservation, the strains were maintained in DifcoTM Tryptic Soy Broth (TSB; Becton Dickinson and Company) plus glycerol (15%) at −80 °C. Before experiments, bacteria were routinely grown in TSA at 37 °C for 24 h.
2.6. DNA Extraction and Genus-Level Identification Based on GCAT Gene
The genomic DNA of the bacterial strains was extracted from pure cultures grown in TSA using the InstaGeneTM DNA purification matrix (Bio-Rad, Hercules, CA, USA) and following the manufacturer’s instructions. The 24 strains were identified as Aeromonas or not via the detection of the GCAT gene, which encodes a glycerophospholipid cholesterol acyl transferase specific to this genus, using the primers and conditions described by Chacón et al. [29] (Table 1). The PCR products with the expected amplicons size of 237 bp [29] were verified in a 1% agarose gel electrophoresis. Gels were stained using RedSafeTM nucleic acid staining solution (iNtRON biotechnology, Seongnam, Korea) and visualized using a transilluminator (Molecular Imager® Gel DocTM XRT) and the Image LabTM Software, both from Bio-Rad.

Table 1.
Primers used in this study.
2.7. Genotyping of Aeromonas Strains
All the Aeromonas strains were genotyped using the Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction (ERIC-PCR) technique, using the primers described by Versalovic et al. [30] (Table 1) and conditions described by Houf et al. [37]. Amplicons of different sizes generated during amplification of genomic DNA were separated using 2% agarose gels, and both gel staining and band visualization were performed in the same manner as described above. Patterns with at least one band difference were considered as different genotypes [8].
2.8. Identification of the Aeromonas Species Based on the rpoD Gene
All the Aeromonas strains positive for the GCAT gene were identified by sequencing the rpoD gene, using the primers and conditions described by Soler et al. [31] (Table 1). The PCR products were sequenced and subsequently aligned with the rpoD sequences of the type strains of 36 described Aeromonas species using the ClustalW algorithm [38] in MEGA v6.0 [39]. The phylogenetic analysis was performed with the neighbor-joining (NJ) algorithm in MEGA v6.0.
2.9. Detection of Virulence Genes
For the subsequent experiments, six strains (A6 and A7, isolated from water; T8 and T10, isolated from soil; and MV8 and MV11, isolated from vegetation) were selected. The presence of five virulence genes (lafA, alt, ast, stx1, and ascF-G) was studied in the six selected strains by PCR using the primers (Table 1) and conditions described by Lee et al. [34].
2.10. Macrophages Growth Conditions and Infection
The cell line J744A.1 from mouse BALB/C monocyte/macrophage was used for the infection experiments [40]. The cells were maintained in adhesion in Dulbecco’s Modified Eagle’s Medium (DMEM; PAA Laboratories GmbH, Munich, Germany) (pH = 8) supplemented with 10% fetal bovine serum (FBS; PAA Laboratories GmbH) plus 1% P/S solution (penicillin-streptomycin stock; PAA Laboratories GmbH) at 37 °C and 5% CO2. Prior to infection, cells were seeded in tissue culture plates (1 × 106 cells/mL) containing serum and antibiotic-free DMEM (serum-starvation conditions) for 24 h to form confluent monolayers [41]. Then, macrophages were infected with the six Aeromonas strains, from overnight cultures, at MOI (multiplicity of infection) 10. Two strains from a previous study, Aeromonas veronii 123384 and Aeomonas jandaei AE214, were used as controls [42].
2.11. Analysis of the Expression of ascF-G and ast Genes after Infecting Macrophages
The expression of two different genes implicated in the virulence, ascF-G (associated with the Type III secretion system) and ast (cytotonic enterotoxin), was studied after infection of the macrophage cell line J744A.1 with each Aeromonas strain. The primers used to evaluate the expression of the selected genes are shown in Table 1. After 3 h of infection at MOI 10, total RNA was isolated from Aeromonas cultures using TRIzol® Reagent (Invitrogen) as previously described [43]. RNA quality and integrity were confirmed using NanoDrop 2000. The cDNA was transcribed from RNA using iScript cDNA Synthesis Kit (Bio-Rad) following the manufacturer’s instructions. Quantitative Real-Time PCR was performed in triplicate using Real-Power SYBR® green PCR Mastermix (Applied Biosystems) on a StepOnePlus™ Real-Time PCR System (Applied Biosystems). Threshold cycle (CT) values were obtained to establish the relative RNA levels of the tested genes, using the 16S rRNA gene as a housekeeping gene, and then calculated with the 2−ΔΔCt method.
2.12. Quantification of Cell Damage in Macrophages
Co-cultures of bacteria-macrophages were incubated at 37 °C and 5% CO2 for 1 h. Then, the medium was removed and fresh medium was added to the wells and incubated at 37 °C and 5% CO2 for 5 h. To determine cell damage, the amount of lactate dehydrogenase (LDH) enzyme in the supernatant was quantified using the commercial CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit (Promega, Madison, WI, USA), following the protocol specified by the manufacturer. Recombinant bovine LDH was used to generate a standard curve and sample values were extrapolated from there [42].
2.13. Intracellular Bacterial Survival of Aeromonas Strains in Macrophages
The quantitative determination of bacteria present within the macrophages was determined by the gentamicin exclusion assay [44]. Briefly, co-cultures of bacteria-macrophages were incubated at 37 °C and 5% CO2 for 1 h. Then, gentamicin (50 μg/mL) was added to wells for killing extracellular bacteria. After 45 min, the medium was removed, and fresh medium was added to the wells and incubated at 37 °C and 5% CO2 for 3 h. To determine the number of bacteria, serial dilution, followed by culturing on TSA plates, was carried out. The percentage of intracellular bacterial survival was calculated with the number of colony-forming units at time 0 h in relation to the initial dose of infection [42].
2.14. Antimicrobial Susceptibility Profile
The in vitro antimicrobial susceptibility profile of the six Aeromonas strains against five antibiotics (ampicillin, cefuroxime, ceftriaxone, tetracycline, and piperacillin/tazobactam) was determined by the Kirby–Bauer disk diffusion method according to the procedure described by Clinical and Laboratory Standards Institute [45], using antibiotic BD BBL™ Sensi-Disc™ disks (Becton Dickinson and Company), DifcoTM Mueller–Hinton agar medium plates (Becton Dickinson and Company), and incubation at 37 °C for 16–18 h. CLSI breakpoints were used to categorize an isolate as susceptible, intermediate, or antibiotic resistant.
2.15. Statistical Analysis
All experiments were performed in triplicate and significant differences were determined using Student’s two-tailed t-test and two-way ANOVA calculated on Graph Pad Prism 6.0 (GraphPad Prism Software Inc., San Diego, CA, USA). p-values < 0.05 were considered statistically significant (*).
3. Results
3.1. Aeromonas Quantification
The results of the quantification of the presence of Aeromonas in the samples are shown in Figure 1. Although the soil samples showed a greater number of Aeromonas compared to the other samples, no significant differences were observed between samples. The technique that quantified a greater quantity of Aeromonas was qPCR, with an average of the three samples of 9.15 × 107 CFU/100 mL, followed by the Plate counting in ADA medium with 7.11 × 105 CFU/100 mL and the MPN of 2.2 × 104 CFU/100 mL. In the case of water and soil samples, a significant difference was shown between the qPCR and the Plate Counting method and the MPN (p < 0.05). In vegetation samples, only significant differences were observed between MPN and qPCR (p < 0.05).
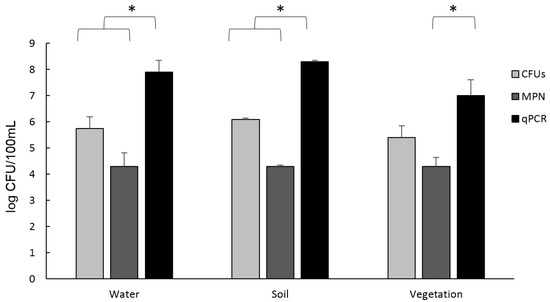
Figure 1.
Aeromonas quantification by Plate Counting in ADA medium (CFUs), qPCR, and MPN for the 3 types of samples. Results are means ± SD from three independent experiments. * Significant differences p < 0.05.
3.2. Genus-Level Identification Based on the GCAT Gene
The presence of the GCAT gene was detected in 19 of the 24 (79%) selected isolates grown in ADA medium. Of the 19 presumptive Aeromonas isolates nine were from water (A1, A2, A4, A5, A6, A7, A9, A14, A18), seven from soil (T3, T4, T5, T6, T8, T9, T10), and three from vegetation (MV8, MV11, and MV18).
3.3. Genotyping of Aeromonas Isolates
The ERIC-PCR analysis showed some clonal relations of the different isolates (Figure 2). These relations corresponded to isolates of water (isolated A1 and A2; A5 and A14), soil (T5, T8 and T9; T6 and T10), and vegetation (MV11 and MV18). In addition, some of the water and soil isolates were found to be identical, e.g., A6 and T3, as well as isolates A1 and A2 and T5, T8, and T9 soil isolates.
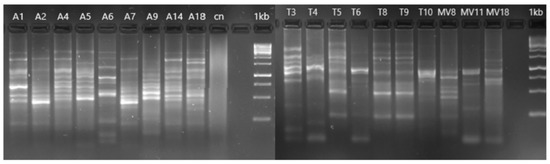
Figure 2.
ERIC-PCR profile of the 19 Aeromonas strains isolated in the present study. The ERIC-PCR analysis was used to determine the genetic similarity of isolates from water (A1, A2, A4, A5, A6, A7, A9, A14, and A18), soil (T3, T4, T5, T6, T8, T9, and T10) and vegetation (MV8, MV11 and MV18). Identical band patterns between isolates indicated a clonal relation.
3.4. Species-Level Identification Based on the rpoD Gene
Phylogenetic analysis of the rpoD gene confirmed that the 19 strains belonged to the genus Aeromonas (Figure 3). The results showed that nine of the strains were identified as A. popoffii (T5, T8, T9, A1, A2, A4, A5, A14, A18), one strain as A. hydrophila (A9), four strains as A. media (A6, T3, T6, T10), one as A. rivipollensis (T4), and one as A. jandaei (MV8). Two strains, MV11 and MV18, were included in the clade of A. media and A. rivipollensis and clustered with Aeromonas sp. genomospecies paramedia. The A7 strain was identified as Aeromonas sp., forming a different clade from its closest species. These results were in agreement with the genotyping results, describing some of the isolates as clones.
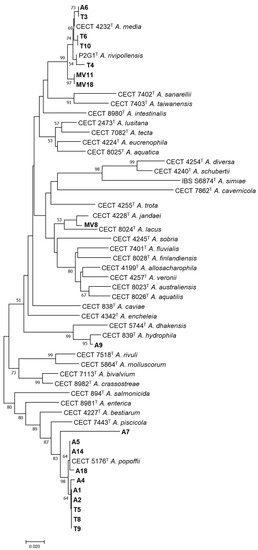
Figure 3.
Neighbor-joining phylogenetic tree constructed with the rpoD gene sequences of the type strains of all known Aeromonas species and with the sequences of the 19 isolates recovered from water (A1, A2, A4, A5, A6, A7, A9, A14, and A18), soil (T3, T4, T5, T6, T8, T9, and T10) and vegetation (MV8, MV11 and MV18). Bootstrap percentages of more than 50% based on 1000 replications are shown at branch nodes. Bar, 0.02 substitutions per nucleotide position.
3.5. Identification of Virulence Genes
The presence/absence of the five virulence genes studied in the selected six strains (A6 and A7 from water; T8 and T10 from soil; and MV8 and MV11 from vegetation) is shown in Table 2. The results showed that the six strains (100%) possess the laf gene that encodes for the lateral flagellum, while the other genes were not present in all strains. Cytotonic enterotoxins alt and ast were found in four (66.6%) and five (83.3%) of the strains, respectively. The ascF-G gene, associated with the Type III secretion system, was present in four of the strains (66.6%). Finally, the stx1 gene, encoding for Shiga toxin type 1 was not found in any of the strains.

Table 2.
Presence of virulence genes in 6 Aeromonas strains isolated from the natural area “El Clot de la Mare de Dèu”.
3.6. Virulence-Associated Gene Expression
Expression of ast and ascF-G genes is shown in Figure 4. The results showed a higher expression of both genes in the strains recovered from water and vegetation A7 (Aeromonas sp.) and MV8 (A. jandaei), respectively, showing significant differences with the other strains and the control strain A. veronii 123384, after 3 h of infection (p < 0.05). In addition, all strains studied showed a higher expression of both genes in comparison with the other control strain, A. jandaei AE214 (p < 0.05).
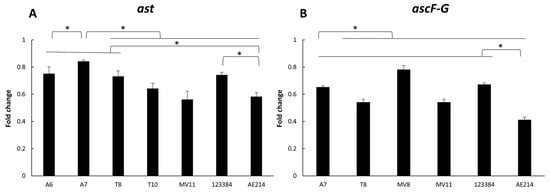
Figure 4.
Gene expression of ast (A) and ascF-G (B) genes in the six selected Aeromonas strains (A6 and A7 from water; T8 and T10 from soil; and MV8 and MV11 from vegetation) and in Aeromonas veronii 123384 and Aeromonas jandaei AE214, after macrophage J744A.1 infection. Results are expressed as the mean of qPCR values. * Significant differences compared with non-infected cells p < 0.05.
3.7. Quantification of Cell Damage in Macrophages
The six strains caused a significantly higher level of cell damage (p < 0.05) after infection of the macrophages at MOI 10 compared with the non-infected cells, as measured by the LDH released. The strains A7 (Aeromonas sp.) and T8 (A. popoffii) caused a higher level of cell damage than the other strains, similar to the one caused by the control A. veronii 123384 (Figure 5).
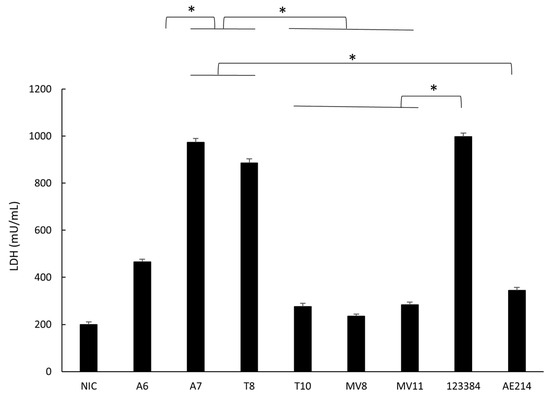
Figure 5.
Determination of the cell damage to macrophages (J744A.1) induced by the six selected Aeromonas strains (A6 and A7 from water; T8 and T10 from soil; and MV8 and MV11 from vegetation) and by Aeromonas veronii 123384 and Aeromonas jandaei AE214, at MOI 10 after 3 h of incubation. Cell damage was evaluated measuring the release of lactate dehydrogenase (LDH). * Significant differences compared with non-infected cells (NIC) p < 0.05. Results are means ± SD from at least three independent experiments.
3.8. Intracellular Bacterial Survival of Aeromonas Strains
The percentage of intracellular survival of the six Aeromonas strains at MOI 10 after 3 h of incubation was calculated after serial dilution and plating and it is shown in Figure 6. Significant differences were observed when comparing the survival of A7 (Aeromonas sp.) and T8 (A. popoffii) strains to the rest, with survival rates of 60% and 50% (p < 0.05), respectively. With the rest of the strains, no significant differences were observed between them, with intracellular survival at 3 h being approximately between 20–30%.
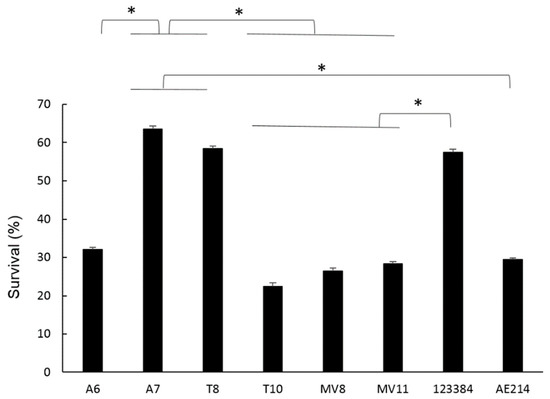
Figure 6.
Percentages of intracellular survival of six Aeromonas strains (A6 and A7 from water; T8 and T10 from soil; and MV8 and MV11 from vegetation) and of Aeromonas veronii 123384 and Aeromonas jandaei AE214, in macrophages (J744A.1) at MOI 10 after 3 h of incubation. Percentages were calculated with respect to non-infected cells. * Significant differences compared with non-infected cells p < 0.05. Results are means ± SD from at least three independent experiments.
3.9. Antimicrobial Susceptibility Profile
The results of the antimicrobial susceptibility test are showed in Table 3. All strains were categorized as resistant to ampicillin, and none of them showed in vitro resistance to tetracycline. For the rest of the antibiotics tested, the percentage of resistant strains was variable: 50% for cefuroxime and 33.3% for both ceftriaxone and piperacillin/tazobactam.

Table 3.
Antimicrobial susceptibility profile of six Aeromonas strains from water (A6, A7) soil (T8, T10) and vegetation (MV8, MV11) isolated from the natural area “El Clot de la Mare de Dèu”.
4. Discussion
Our work focused on the presence of Aeromonas in the natural area “El Clot de la Mare de Déu”, which belongs to the fluvial area of the Mijares river. The waters of this area are supplied by an underground spring and flow into the Mediterranean Sea. These waters are used for human recreation, especially in the warmer months of the year, and to irrigate the adjacent crop fields without being subjected to any type of depuration treatment. The aim was to determine the incidence of Aeromonas in this recreational environment, the potential virulence of the strains isolated, and their antimicrobial susceptibility, considering the fact that cases of wound infections can evolve as severe cases of necrotizing fasciitis [17,18,46,47], which is a life-threatening infection if proper antibiotic treatment is delayed. On this basis, some authors consider Aeromonas a flesh-eating bacteria and an emerging aggressive pathogen [17,18,48]. An impacting case occurred in a healthy immunocompetent woman, who fell into a river while practicing a zip line and an open wound got contaminated with Aeromonas, generating a fast-evolving necrotizing fasciitis that required progressive amputations of large parts of her limbs to survive [17,18].
In our study, the presence of Aeromonas was confirmed at high concentrations in samples of water, soil, and vegetation with the techniques used (qPCR, MPN, and Plate Counting in ADA medium). In general, our results showed that there was a relatively high concentration of Aeromonas in all of the samples, in line with the concentration normally found in rivers, lakes, and other natural reservoirs (up to 3.4 × 104 and 6.9 × 103 CFU/100 mL) [9], as well as in waters used for irrigation (from 7.0 × 102 CFU/100 mL to 2.45 × 104 CFU/100 mL) [8].
As expected, the technique that showed a higher concentration of Aeromonas compared to the other techniques was the qPCR, due to the high degree of sensitivity and because it is not able to discriminate between live and dead bacteria [49,50]. The quantification technique that showed a lower concentration of Aeromonas was MPN, because it provides an approximate result. In the case of plate counting in ADA medium, it was observed that there was an intermediate value of concentration between the values obtained with qPCR and with the MPN. It has been observed that in ADA medium, other bacteria are able to grow, as proven in previous studies [51]. For this reason, there is a need to perform the three methods for a more reliable Aeromonas quantification.
To study the diversity of Aeromonas spp. in the samples, 24 colonies that showed the typical morphology described for Aeromonas grown in the ADA culture media plates were selected. Of the selected 24 isolates, only 19 (79%) belonged presumptively to Aeromonas on the basis of the presence of the GCAT gene, which is considered specific for the genus [29,52], indicating that the ADA generated, in this case, 21% of false positives.
The phylogenetic analysis constructed with the sequences of the rpoD gene of the 19 isolates identified them as belonging to seven known Aeromonas species and one as a potential new species. These results corroborate that the GCAT and the rpoD genes are excellent tools for the identification of the members of the genus Aeromonas and for recognizing known and potentially new species [12,29,31,52]. The latter seems to be the case for strain A7, isolated from water, which clustered in the phylogenetic tree at a significant distance from all the Aeromonas species known so far. Further studies that sequence either the genome of this strain or additional housekeeping genes would be necessary to confirm this finding [53]. It was also observed that two of the strains isolated from vegetation clustered with the species A. media and A. rivipollensis, but phylogenetically are close to the not-yet-described genomic candidate species “A. paramedia”. The clonal relationships between strains was confirmed on the basis of their identical rpoD sequences and the identical ERIC-PCR patterns, demonstrating that water and soil samples are colonized by the same bacteria. Previous studies showed that water acts as a vector for the transmission of Aeromonas to other substrates [8], as our results also corroborate.
The pathogenic potential of six Aeromonas strains was evaluated by the presence of five virulence genes related to the flagellum mobility (laf), the Type III secretion system (ascF-G), and toxins (alt, ast and stx1). There was a great variability between strains, as shown in previous studies [12,22]. All the strains were positive for the presence of the laf gene, encoding for structural protein of the lateral flagellum [32]. The presence of lateral flagellum gives the bacteria a fast or “swarming” type of mobility, which allows them to move on solid surfaces and form biofilms expressed during bacterial growth on viscous surfaces [22,23,32,54,55,56]. The presence of alt and ast genes, encoding for thermolabile and thermostable cytotonic enterotoxins, and the expression level of ast were variable between strains. The cytotonic enterotoxins have a similar mechanisms of action of the choleric toxin, increasing the cyclic adenosine monophosphate (cAMP) levels and prostaglandins in the intestinal epithelial cells [22]. On the other hand, the stx1 gene, encoding for a Shiga-like toxin [57], was not present in any of the strains studied. Previous reports have detected Shiga-like toxins in clinical strains of Aeromonas [58] and some strains recovered from food [12]. The Shiga toxin inactivates ribosomes (arrest of protein synthesis) of vascular endothelial cells, leading to cell death [57]. Finally, the presence of the ascF-G gene, which encodes for Type III Secretion System (T3SS) [36], was detected in four of the strains and the expression level was different between the strains. The T3SS has been previously observed as having a great role in the virulence of Aeromonas [36,59,60]. This is one of the secretion systems by which proteins can be injected directly from the bacterial cell protoplasm to the cytoplasm of the target cell or to the extracellular space [61,62].
In the in vitro infection assay, two parameters were studied—the cell damage caused in the macrophage cell line J774A.1 and the intracellular survival of the bacteria within the macrophages. These parameters were used as indicators of the ability of Aeromonas strains to induce an infection. The measuring of cell damage in animal cell models through quantification of the enzyme LDH, released during apoptosis or pyroptosis, is a well-established method [63]. It was observed that the strains A7 from river water and T8 from soil caused a greater cell damage in the macrophages than the other strains. Some Aeromonas infection studies have used this method to assess the pathogenicity of the strains tested, like Epple et al. [64], in which colon epithelial cells HT-29/B6 were infected with A. hydrophila and A. veronii strains isolated from stool. Regarding the obtained percentages of intracellular bacterial survival, these were higher for the strains A7 and T8 compared to the other strains after 3 h of infection. Studies with Aeromonas environmental strains have shown that these are also capable, like clinical strains, of invading and surviving within different cell lines. For example, Couto et al. [65] demonstrated that A. hydrophila and A. caviae strains isolated from human diarrheic feces, vegetables, and water were able to adhere and invade different intestinal epithelial cell lines and produce cytotoxic and cytopathic effects. In another study, dos Santos et al. [66] demonstrated that eight Aeromonas spp. strains isolated from human feces, food, and water were able to invade intestinal (T-84, Caco-2) and epithelial (HEp-2) cell lines cultivated in vitro. Dias et al. [67] proved that A. salmonicida isolated from wild animal feces exhibited the highest ability to internalize and survive in Caco-2 cells under simulated human gastrointestinal conditions among other bacteria tested.
Aeromonas, like other Gram-negative bacilli, are intrinsically resistant to benzylpenicillin, glycopeptides, lipoglycopeptides, fusidic acid, macrolides, lincosamides, streptogramins, rifampicin, and oxazolidinones, such that, these drugs should not be considered for either therapy or clinical susceptibility testing [68]. Previous literature has shown that these bacteria present a high level of resistance to antibiotics, such as aminopenicillins and their beta-lactamase inhibitor combinations, and first-generation cephalosporins, with few exceptions [12,68,69]. In contrast, antimicrobial agents, such as third- and fourth-generation cephalosporins, carbapenems, monobactams, piperacillin-tazobactam, aminoglycosides, fluoroquinolones, and cotrimoxazole show in vitro activity against these bacteria [11,12,70,71]. However, the resistance of Aeromonas to these drugs has increased in recent decades, both in clinical and environmental isolates [12,71,72]. The use of these antibiotics for prophylactic and therapeutic purposes, both in humans and animals, mainly in fish, has probably influenced the increase in this acquired resistance.
In our work, antimicrobial susceptibility profiles of six Aeromonas strains against five antimicrobial agents (ampicillin, cefuroxime, ceftriaxone, tetracycline, and piperacillin/tazobactam), which are frequently used as empirical treatments for a wide range of bacterial infections, were analyzed. In this assay, as could be expected, all strains were resistant to ampicillin. Our results are in accordance with previous published studies describing Aeromonas trota as the only species of the genus susceptible to this aminopenicillin [73,74,75,76,77,78]. Variable resistances to other beta-lactam antibiotics tested, including piperacillin/tazobactam, cefuroxime, and ceftriaxone, were observed. The resistance to these drugs of some strains was probably due to the production of beta-lactamase enzymes, such as extended-spectrum beta-lactamases (ESBLs), metallo-beta-lactamases, cephalosporinases, and penicillinases, which confer resistance to most beta-lactam antibiotics, including penicillin, cephalosporins, carbapenems, and the monobactam aztreonam [11,79,80,81]. In the study of Piotrowska et al. [82], it was observed that the different genes encoding beta-lactamases present in Aeromonas species isolated from sewage were mainly found in plasmids. This suggested that Aeromonas resistance to this group of antimicrobials could spread from water residues to other substrates, thus increasing the biosanitary risk involved considering that third- and fourth-generation cephalosporin regimes have been used to treat systemic infections in humans [69]. Finally, in regard to tetracycline, we did not detect any strain resistant to this drug. The main mechanisms of bacterial resistance to the tetracycline group are the ribosomal protection and the efflux pump, both generally associated with the presence of tet genes. The tet genes encode different cytoplasmic proteins (Tet) that can interact with ribosomes, preventing tetracycline from binding to its target, and can interact with tetracycline, behaving as active exporters of the drug to out of the cell [83]. In general, higher percentages of tetracycline resistance have been described in clinical strains than in environmental strains, although an increase in such resistance has also been detected in the latter [84,85].
5. Conclusions
This work constitutes a preliminary study that will continue with a more exhaustive investigation to determine the risk assessment associated with the presence of pathogenic Aeromonas spp. in these waters. With the techniques used here, high concentrations of Aeromonas spp. were found in water, soil, and vegetation samples of this natural recreational environment. Considering the pathogenic potential of some of the isolated strains, the presence of this bacteria may represent a threat in the case of exposed open wounds. Furthermore, the antimicrobial susceptibility profile of the strains studied confirm the increased resistance to antibiotics of these bacteria, endangering the ability to treat infections. The presence of clones among the different samples supports the hypothesis that water can act as a transmission vector of Aeromonas to other substrates. This study confirmed once more that the use of the sequences of the rpoD enables the identification and recognition of known and potential new species of Aeromonas.
Author Contributions
Conceptualization, I.P.-B. and A.F.-B.; methodology, R.M.G., F.D.M. and A.F.-B.; software, F.D.M.; investigation, R.M.G., F.D.M. and A.F.-B.; data curation, R.M.G., A.F.-B. and M.J.F.; writing-original draft preparation, R.M.G. and A.F.-B.; writing-review and editing R.M.G., M.J.F., I.P.-B. and A.F.-B.; supervision, M.J.F., I.P.-B. and A.F.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
R.M.G. is indebted to University Rovira i Virgili for a Martí-Franqués doctoral grant. The authors thank the staff in the Microbiology Unit at the University Rovira i Virgili, and support from Carme Sanmartí Solé and Núria Pilas López.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Araujo, R.M.; Pares, R.; Lucena, F. The effect of terrestrial effluents on the incidence of Aeromonas spp. in coastal waters. J. Appl. Bacteriol. 1990, 69, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M.; Coote, B.G.; Ashbolt, N.J.; Stevenson, I.M. Relationships between indicators, pathogens and water quality in an estuarine system. Water Res. 1996, 30, 2045–2054. [Google Scholar] [CrossRef]
- Borrell, N.; Figueras, M.J.; Guarro, J. Phenotypic identification of Aeromonas genomospecies from clinical and environmental sources. Can. J. Microbiol. 1998, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Figueras, M.J.; Suarez-Franquet, A.; Chacón, M.R.; Soler, L.; Navarro, M.; Alejandre, C.; Grasa, B.; Martínez-Murcia, A.J.; Guarro, J. First record of the rare species Aeromonas culicicola from a drinking water supply. Appl. Environ. Microbiol. 2005, 71, 538–541. [Google Scholar] [CrossRef] [PubMed]
- McLellan, S.L.; Huse, S.M.; Mueller-Spitz, S.R.; Andreishcheva, E.N.; Sogin, M.L. Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ. Microbiol. 2010, 12, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.; Remacha, M.A.; Rodríguez-Calleja, J.M.; Santos, J.A.; Otero, A.; García-López, M.L. Identity, virulence genes, and clonal relatedness of Aeromonas isolates from patients with diarrhea and drinking water. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Aravena-Román, M.; Beaz-Hidalgo, R.; Inglis, T.J.J.; Riley, T.V.; Martínez-Murcia, A.J.; Chang, B.J.; Figueras, M.J. Aeromonas australiensis sp. nov., isolated from irrigation water. Int. J. Syst. Evol. Microbiol. 2013, 63, 2270–2276. [Google Scholar] [CrossRef]
- Latif-Eugenín, F.; Beaz-Hidalgo, R.; Silvera-Simón, C.; Fernandez-Cassi, X.; Figueras, M.J. Chlorinated and ultraviolet radiation -treated reclaimed irrigation water is the source of Aeromonas found in vegetables used for human consumption. Environ. Res. 2017, 154, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Figueras Salvat, M.J.; Ashbolt, N. Aeromonas. Water Sanit. 21st Century Health Microbiol. Asp. Excreta Wastewater Manag. Glob. Water Pathog. Proj. 2019. [Google Scholar] [CrossRef]
- Rusiñol, M.; Hundesa, A.; Cárdenas-Youngs, Y.; Fernández-Bravo, A.; Pérez-Cataluña, A.; Moreno-Mesonero, L.; Moreno, Y.; Calvo, M.; Alonso, J.L.; Figueras, M.J.; et al. Microbiological contamination of conventional and reclaimed irrigation water: Evaluation and management measures. Sci. Total Environ. 2020, 710, 136298. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Figueras, M.J. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.W.; Carnahan, A.M.; Brayton, P.R.; Fanning, G.R.; Almazan, R.; Drabick, C.; Trudo, E.W.; Colwell, R.R. Aeromonas jandaei and Aeromonas veronii dual infection of a human wound following aquatic exposure. J. Clin. Microbiol. 1991, 29, 565–569. [Google Scholar] [CrossRef]
- Goncalves, J.R.; Brum, G.; Fernandes, A.; Biscaia, I.; Salvado Correia, M.J.; Bastardo, J. Aeromonas hydrophila fulminant pneumonia in a fit young man. Thorax 1992, 47, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Vally, H.; Whittle, A.; Cameron, S.; Dowse, G.K.; Watson, T. Outbreak of Aeromonas hydrophila wound infections associated with mud football. Clin. Infect. Dis. 2004, 38, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, R.C.; Winn, R.E.; Jeter, R.M.; Warren, W.J.; Huddleston, J.R.; Zak, J.C. Aeromonas infection from river and playa lake waters in west texas and southeastern New Mexico. Southwest Respir. Crit. Care Chronicles 2016, 4, 19. [Google Scholar] [CrossRef]
- Grim, C.J.; Kozlova, E.V.; Ponnusamy, D.; Fitts, E.C.; Sha, J.; Kirtley, M.L.; van Lier, C.J.; Tiner, B.L.; Erova, T.E.; Joseph, S.J.; et al. Functional genomic characterization of virulence factors from necrotizing fasciitis-causing strains of Aeromonas hydrophila. Appl. Environ. Microbiol. 2014, 80, 4162–4183. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, D.; Kozlova, E.V.; Sha, J.; Erova, T.E.; Azar, S.R.; Fitts, E.C.; Kirtley, M.L.; Tiner, B.L.; Andersson, J.A.; Grim, C.J.; et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc. Natl. Acad. Sci. USA. 2016, 113, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Figueras, M.J.; Beaz-Hidalgo, R. Aeromonas infections in humans. In Aeromonas; Academic Press: Norfolk, UK, 2015; pp. 65–108. [Google Scholar]
- Pianetti, A.; Sabatini, L.; Bruscolini, F.; Chiaverini, F.; Cecchetti, G. Faecal contamination indicators, Salmonella, Vibrio and Aeromonas in water used for the irrigation of agricultural products. Epidemiol. Infect. 2004, 132, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Al-Jassim, N.; Ansari, M.I.; Harb, M.; Hong, P.Y. Removal of bacterial contaminants and antibiotic resistance genes by conventional wastewater treatment processes in Saudi Arabia: Is the treated wastewater safe to reuse for agricultural irrigation? Water Res. 2015, 73, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Tomás, J.M. The main Aeromonas pathogenic factors. ISRN Microbiol. 2012, 2012, 256261. [Google Scholar] [CrossRef] [PubMed]
- Beaz-Hidalgo, R.; Figueras, M.J. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J. Fish Dis. 2013, 36, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Figueira, V.; Vaz-Moreira, I.; Silva, M.; Manaia, C.M. Diversity and antibiotic resistance of Aeromonas spp. in drinking and waste water treatment plants. Water Res. 2011, 45, 5599–5611. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Shih, D.Y.C.; Wang, J.Y.; Yang, S.S. Molecular characterization of class 1 integrons and antimicrobial resistance in Aeromonas strains from foodborne outbreak-suspect samples and environmental sources in Taiwan. Diagn. Microbiol. Infect. Dis. 2007, 59, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, K.; von Czapiewski, E.; Kaspar, H.; Wallmann, J.; Michael, G.B.; Steinacker, U.; Schwarz, S. Molecular basis of sulfonamide and trimethoprim resistance in fish-pathogenic Aeromonas isolates. Appl. Environ. Microbiol. 2011, 77, 7147–7150. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Henriques, I.; Ribeiro, R.; Correia, A. Prevalence and Characterization of integrons from bacteria isolated from a slaughterhouse wastewater treatment plant. J. Antimicrob. Chemother. 2007, 60, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Martínez-Murcia, A.; Esteves, A.C.; Correia, A.; Saavedra, M.J. Phylogenetic Diversity, antibiotic resistance and virulence traits of Aeromonas spp. from untreated waters for human consumption. Int. J. Food Microbiol. 2012, 159, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Chacón, M.R.; Castro-Escarpulli, G.; Soler, L.; Guarro, J.; Figueras, M.J. A DNA probe specific for Aeromonas colonies. Diagn. Microbiol. Infect. Dis. 2002, 44, 221–225. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, R. Distribution of repetitive dna sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Yáñez, M.A.; Chacon, M.R.; Aguilera-Arreola, M.G.; Catalán, V.; Figueras, M.J.; Martínez-Murcia, A.J. Phylogenetic Analysis of the genus Aeromonas based on two housekeeping genes. Int. J. Syst. Evol. Microbiol. 2004, 54, 1511–1519. [Google Scholar] [CrossRef]
- Merino, S.; Gavín, R.; Vilches, S.; Shaw, J.G.; Tomás, J.M. A Colonization factor (production of lateral flagella) of mesophilic Aeromonas spp. is inactive in Aeromonas salmonicida strains. Appl. Environ. Microbiol. 2003, 69, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.K.; Peterson, J.W.; Xu, X.J.; Coppenhaver, D.H.; Houston, C.W. Molecular and biochemical characterization of a heat-labile cytotonic enterotoxin from Aeromonas hydrophila. Microb. Pathog. 1996, 21, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hoel, S.; Lunestad, B.T.; Lerfall, J.; Jakobsen, A.N. Aeromonas spp. Isolated from ready-to-eat seafood on the norwegian market: Prevalence, putative virulence factors and antimicrobial resistance. J. Appl. Microbiol. 2021, 130, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Clark, C.G.; Rodgerst, F.G. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 shiga toxin family by multiplex PCR. J. Clin. Microbiol. 2002, 40, 3613–3619. [Google Scholar] [CrossRef] [PubMed]
- Chacón, M.R.; Soler, L.; Groisman, E.A.; Guarro, J.; Figueras, M.J. Type III Secretion System Genes in Clinical Aeromonas Isolates. J. Clin. Microbiol. 2004, 42, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Houf, K.; De Zutter, L.; Van Hoof, J.; Vandamme, P. Assessment of the genetic diversity among Arcobacters isolated from poultry products by using two PCR-based typing methods. Appl. Environ. Microbiol. 2002, 68, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Rama, D.; Esendagli, G.; Guc, D. Expression of chemokine-like receptor 1 (CMKLR1) on J744A.1 macrophages co-cultured with fibroblast and/or tumor cells: Modeling the influence of microenvironment. Cell. Immunol. 2011, 271, 134–140. [Google Scholar] [CrossRef]
- Murciano, C.; Hor, L.I.; Amaro, C. Host-pathogen interactions in Vibrio vulnificus: Responses of monocytes and vascular endothelial cells to live bacteria. Future Microbiol. 2015, 10, 471–487. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. Immune response of the monocytic cell line THP-1 against six Aeromonas spp. Front. Immunol. 2022, 13, 875689. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; López-Fernández, L.; Figueras, M.J. The metallochaperone encoding gene HypA Is widely distributed among pathogenic Aeromonas spp. and its expression is increased under acidic pH and within macrophages. Microorganisms 2019, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.A.; Burke, V.; Chang, B.J. Invasion of HEp-2 cells by fecal isolates of Aeromonas hydrophila. Infect. Immun. 1985, 47, 680–683. [Google Scholar] [CrossRef]
- CLSI Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Mohanty, S.; Ali, S.M.; Singh, P.K. Necrotizing fasciitis and gas gangrene due to Aeromonas hydrophila in an immunocompetent host: A rare entity. IDCases 2022, 28, e01508. [Google Scholar] [CrossRef]
- Hasan, O.; Khan, W.; Jessar, M.; Pathan, A.Z.; Lakdawala, R.H. Bone graft donor site infection with a rare organism, Aeromonas hydrophila. a typical location, presentation and organism with 2 years follow-up. case report. Int. J. Surg. Case Rep. 2018, 51, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Kilgore, P.B.; Andersson, J.A.; Blears, E.; Figueras, M.J.; Hasan, N.A.; Colwell, R.R.; Sha, J.; Chopra, A.K. T6SS and ExoA of flesh-eating Aeromonas hydrophila in peritonitis and necrotizing fasciitis during mono- and polymicrobial infections. Proc. Natl. Acad. Sci. USA 2019, 116, 24084–24092. [Google Scholar] [CrossRef]
- Botes, M.; De Kwaadsteniet, M.; Cloete, T.E. Application of quantitative PCR for the detection of microorganisms in water. Anal. Bioanal. Chem. 2013, 405, 91–108. [Google Scholar] [CrossRef]
- Loozen, G.; Boon, N.; Pauwels, M.; Quirynen, M.; Teughels, W. Live/dead real-time polymerase chain reaction to assess new therapies against dental plaque-related pathologies. Mol. Oral Microbiol. 2011, 26, 253–261. [Google Scholar] [CrossRef]
- Latif-Eugenín, F.; Beaz-Hidalgo, R.; Figueras, M.J. Evaluation of different conditions and culture media for the recovery of Aeromonas spp. from water and shellfish samples. J. Appl. Microbiol. 2016, 121, 883–891. [Google Scholar] [CrossRef]
- Latif-Eugenín, F.; Beaz-Hidalgo, R.; Figueras, M.J. A Culture Independent method for the detection of Aeromonas sp. from water samples. Ital. J. food Saf. 2016, 5, 11–14. [Google Scholar] [CrossRef][Green Version]
- Figueras, M.J.; Beaz Hidalgo, R.; Collado, L.; Martínez-Murcias, A. Recommendations for a new bacterial species description based on analyses of the unrelated genera Aeromonas and Arcobacter. Bull. BISMiS 2011, 2, 1–16. [Google Scholar]
- Gavín, R.; Rabaan, A.A.; Merino, S.; Tomás, J.M.; Gryllos, I.; Shaw, J.G. Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Mol. Microbiol. 2002, 43, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.G.; Santos, P.A.; Bello, A.R.; Freitas-Almeida, A.C. Association of Aeromonas caviae polar and lateral flagella with biofilm formation. Lett. Appl. Microbiol. 2011, 52, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Aravena-Román, M.; Inglis, T.J.J.; Riley, T.V.; Chang, B.J. Distribution of 13 virulence genes among clinical and environmental Aeromonas spp. in western australia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1889–1895. [Google Scholar] [CrossRef]
- Alperi, A.; Figueras, M.J. Human isolates of Aeromonas possess shiga toxin genes (stx1 and stx2) highly similar to the most virulent gene variants of Escherichia coli. Clin. Microbiol. Infect. 2010, 16, 1563–1567. [Google Scholar] [CrossRef]
- Palma-Martínez, I.; Guerrero-Mandujano, A.; Ruiz-Ruiz, M.J.; Hernández-Cortez, C.; Molina-López, J.; Bocanegra-García, V.; Castro-Escarpulli, G. Active shiga-like toxin produced by some Aeromonas spp., isolated in mexico city. Front. Microbiol. 2016, 7, 1522. [Google Scholar] [CrossRef]
- Vilches, S.; Urgell, C.; Merino, S.; Chacón, M.R.; Soler, L.; Castro-Escarpulli, G.; Figueras, M.J.; Tomás, J.M. Complete type III secretion system of a mesophilic Aeromonas hydrophila strain. Appl. Environ. Microbiol. 2004, 70, 6914–6919. [Google Scholar] [CrossRef]
- Frey, J.; Origgi, F.C. Type III secretion system of Aeromonas salmonicida undermining the host’s immune response. Front. Mar. Sci. 2016, 3, 130. [Google Scholar] [CrossRef]
- Tseng, T.T.; Tyler, B.M.; Setubal, J.C. Protein secretion systems in bacterial-host associations, and their description in the gene ontology. BMC Microbiol. 2009, 9, S2. [Google Scholar] [CrossRef]
- Rangel, L.T.; Marden, J.; Colston, S.; Setubal, J.C.; Graf, J.; Gogarten, J.P. Identification and characterization of putative Aeromonas spp. t3ss effectors. PLoS ONE 2019, 14, e0214035. [Google Scholar] [CrossRef]
- Madara, J.L.; Stafford, J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 1989, 83, 724–727. [Google Scholar] [CrossRef]
- Epple, H.J.; Mankertz, J.; Ignatius, R.; Liesenfeld, O.; Fromm, M.; Zeitz, M.; Chakraborty, T.; Schulzke, J.D. Aeromonas hydrophila beta-hemolysin induces active chloride secretion in colon epithelial cells (HT-29/B6). Infect. Immun. 2004, 72, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.R.A.; Oliveira, S.S.; Queiroz, M.L.P.; Freitas-Almeida, A.C. Interactions of clinical and environmental Aeromonas isolates with Caco-2 and HT29 intestinal epithelial cells. Lett. Appl. Microbiol. 2007, 45, 405–410. [Google Scholar] [CrossRef]
- Dos Santos, P.A.; Pereira, A.C.M.; Ferreira, A.F.; de Mattos Alves, M.A.; Rosa, A.C.P.; Freitas-Almeida, A.C. Adhesion, invasion, intracellular survival and cytotoxic activity of strains of Aeromonas spp. in HEp-2, Caco-2 and T-84 cell lines. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Ribeiro, M.; Correia-Branco, A.; Domínguez-Perles, R.; Martel, F.; Saavedra, M.J.; Simões, M. Virulence, attachment and invasion of Caco-2 cells by multidrug-resistant bacteria isolated from wild animals. Microb. Pathog. 2019, 128, 230–235. [Google Scholar] [CrossRef] [PubMed]
- EUCAST Expert Rules Version 3.2. 20 February 2020. Available online: https://www.eucast.org/expert_rules_and_expected_phenotypes (accessed on 5 October 2022).
- Lamy, B.; Kodjo, A.; Laurent, F. Prospective nationwide study of Aeromonas infections in France. J. Clin. Microbiol. 2009, 47, 1234–1237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ndi, O.L.; Barton, M.D. Incidence of class 1 integron and other antibiotic resistance determinants in Aeromonas spp. from rainbow trout farms in Australia. J. Fish Dis. 2011, 34, 589–599. [Google Scholar] [CrossRef]
- Aravena-Román, M.; Inglis, T.J.J.; Henderson, B.; Riley, T.V.; Chang, B.J. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob. Agents Chemother. 2012, 56, 1110–1112. [Google Scholar] [CrossRef]
- Scarano, C.; Piras, F.; Virdis, S.; Ziino, G.; Nuvoloni, R.; Dalmasso, A.; De Santis, E.P.L.; Spanu, C. Antibiotic resistance of Aeromonas ssp. strains isolated from Sparus aurata reared in italian mariculture farms. Int. J. Food Microbiol. 2018, 284, 91–97. [Google Scholar] [CrossRef]
- Carnahan, A.M.; Chakraborty, T.; Fanning, G.R.; Verma, D.; Ali, A.; Janda, J.M.; Joseph, S.W. Aeromonas trota sp. nov., an ampicillin-susceptible species isolated from clinical specimens. J. Clin. Microbiol. 1991, 29, 1206–1210. [Google Scholar] [CrossRef]
- Overman, T.L.; Janda, J.M. Antimicrobial susceptibility patterns of Aeromonas jandaei, A. schubertii, A. trota, and A. veronii biotype veronii. J. Clin. Microbiol. 1999, 37, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Huys, G.; Denys, R.; Swings, J. DNA-DNA reassociation and phenotypic data indicate synonymy between Aeromonas enteropelogenes Schubert et al. 1990 and Aeromonas trota Carnahan et al. 1991. Int. J. Syst. Evol. Microbiol. 2002, 52, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.L.; Cheung, W.K.W.; Janda, J.M. The genus Aeromonas: Biochemical characteristics, atypical reactions, and phenotypic identification schemes. J. Clin. Microbiol. 2003, 41, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Ding, L.W.; Hsueh, P.R. Wound infection and septic shock due to Aeromonas trota in a patient with liver cirrhosis. Clin. Infect. Dis. 2007, 44, 1523–1524. [Google Scholar] [CrossRef] [PubMed]
- Dallagassa, C.B.; Surek, M.; Vizzotto, B.S.; Prediger, K.C.; Moriel, B.; Wolf, S.; Weiss, V.; Cruz, L.M.; Assis, F.E.A.; Paludo, K.S.; et al. Characteristics of an Aeromonas trota strain isolated from cerebrospinal fluid. Microb. Pathog. 2018, 116, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.Y.; Jung, D.S.; Peck, K.R. Clinical and therapeutic implications of Aeromonas bacteremia: 14 years nation-wide experiences in Korea. Infect. Chemother. 2016, 48, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, Y.; Jiang, N.; Liu, W.; Shi, Y.; Zhao, J.; Zeng, L. Molecular characteristics and virulence analysis of eight Aeromonas hydrophila isolates obtained from diseased amur sturgeon acipenser schrenckii brandt, 1869. J. Vet. Med. Sci. 2018, 80, 421–426. [Google Scholar] [CrossRef]
- Chen, P.L.; Ko, W.C.; Wu, C.J. Complexity of β-lactamases among clinical Aeromonas isolates and its clinical implications. J. Microbiol. Immunol. Infect. 2012, 45, 398–403. [Google Scholar] [CrossRef]
- Piotrowska, M.; Przygodzinska, D.; Matyjewicz, K.; Popowska, M. Occurrence and variety of β lactamase genes among Aeromonas spp. isolated from urban wastewater treatment plant. Front. Microbiol. 2017, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, D.d.S.C.M.; Guedes, G.M.d.M.; Brilhante, R.S.N.; Rocha, M.F.G.; Sidrim, J.J.C.; Moreira, J.L.B.; Cordeiro, R.d.A.; Sales, J.A.; Riello, G.B.; De Alencar, L.P.; et al. Virulence and antimicrobial susceptibility of clinical and environmental strains of Aeromonas spp. from northeastern Brazil. Can. J. Microbiol. 2015, 61, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Dhanapala, P.M.; Kalupahana, R.S.; Kalupahana, A.W.; Wijesekera, D.P.H.; Kottawatta, S.A.; Jayasekera, N.K.; Silva-Fletcher, A.; Jagoda, S.S.S.S. Characterization and antimicrobial resistance of environmental and clinical Aeromonas species isolated from fresh water ornamental fish and associated farming environment in Sri Lanka. Microorganisms 2021, 9, 2106. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).