In Vivo Inhibitory Assessment of Potential Antifungal Agents on Nosema ceranae Proliferation in Honey Bees
Abstract
1. Introduction
2. Results
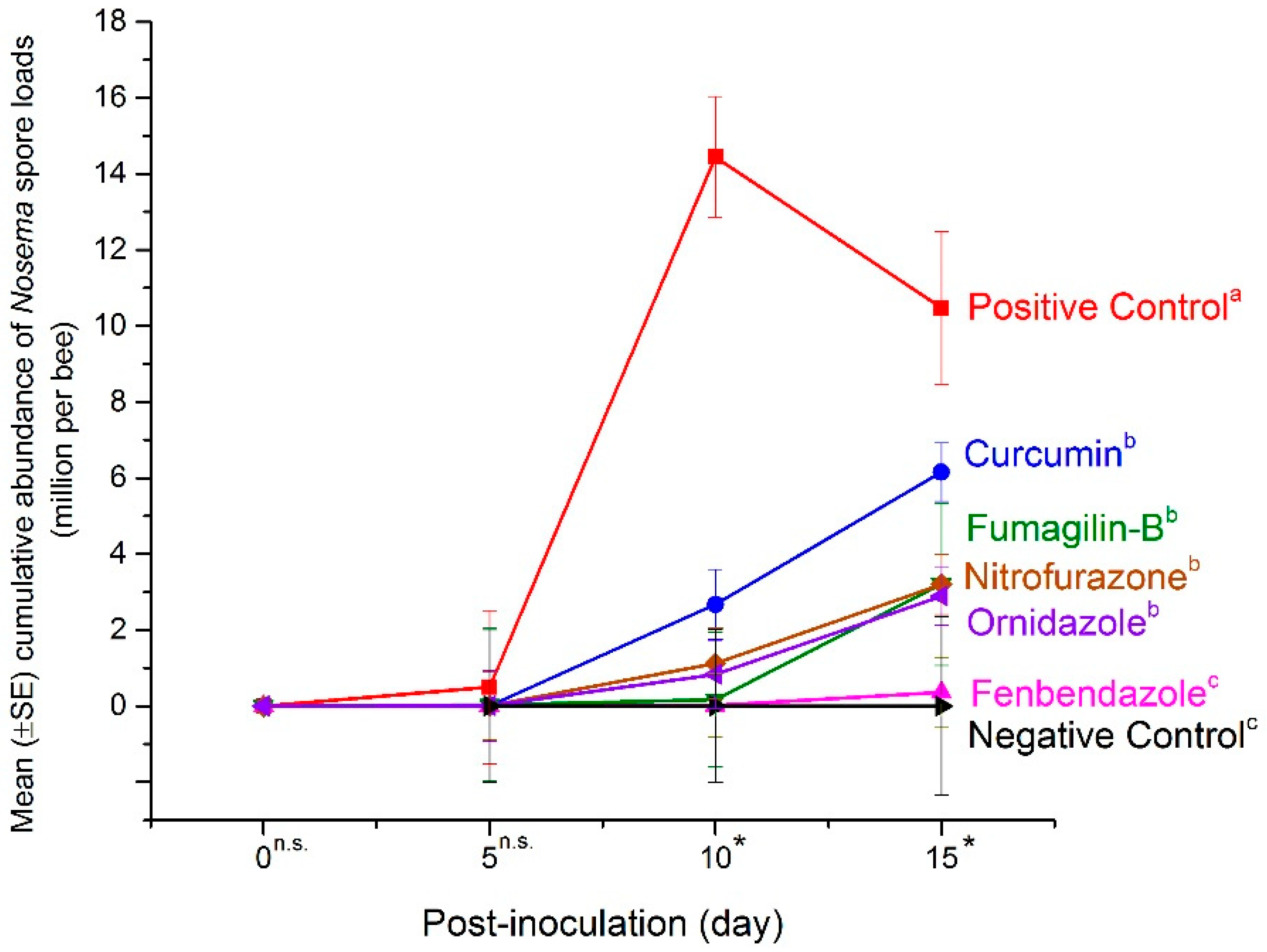
2.1. Mean Abundance of Nosema Spores in the Mid-Experiment Sample
2.2. Mean Abundance of Nosema Spores in the End-Experiment Samples
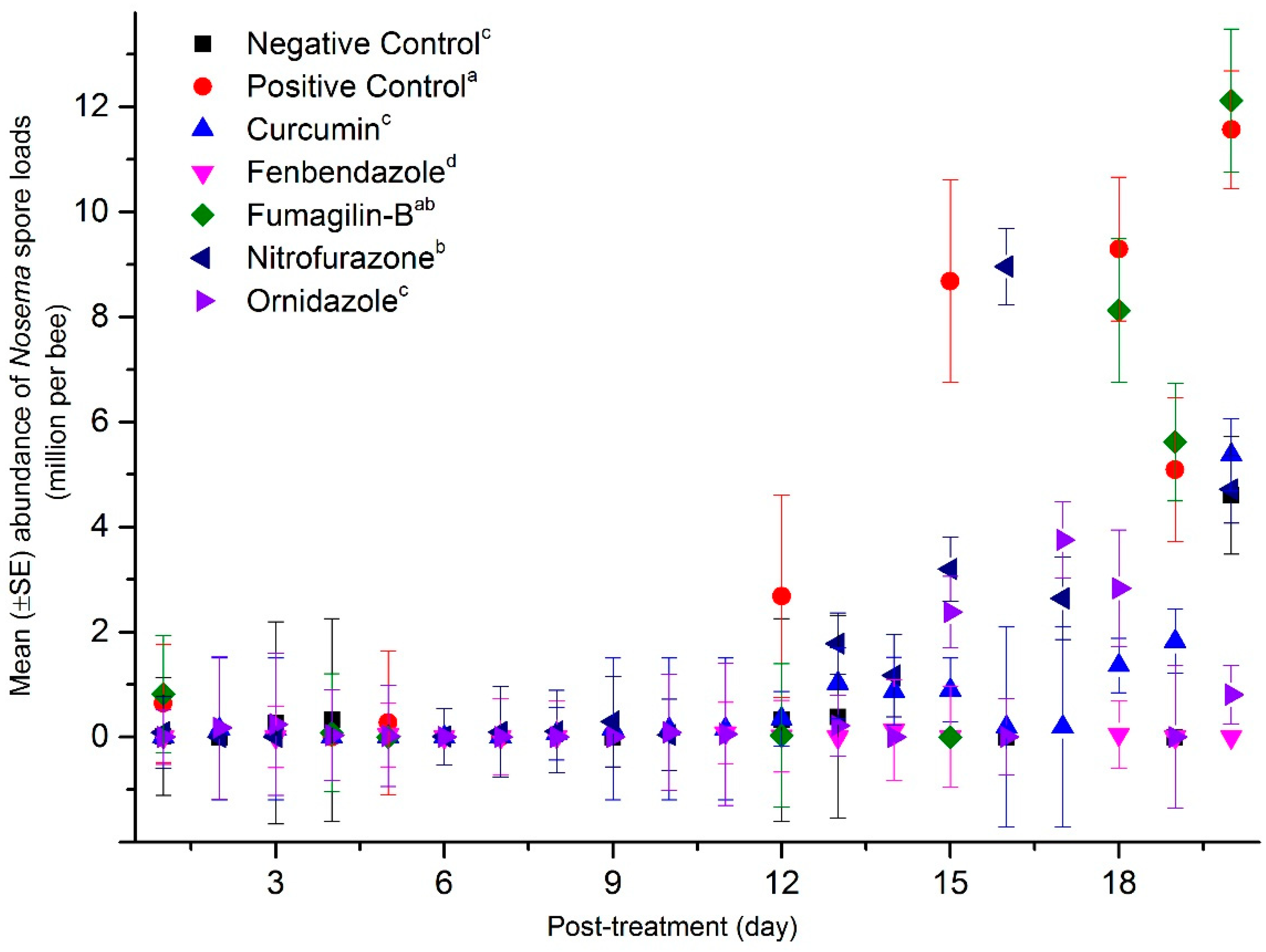
2.3. Mean Abundance of Nosema Spores in the Dead Bee Samples
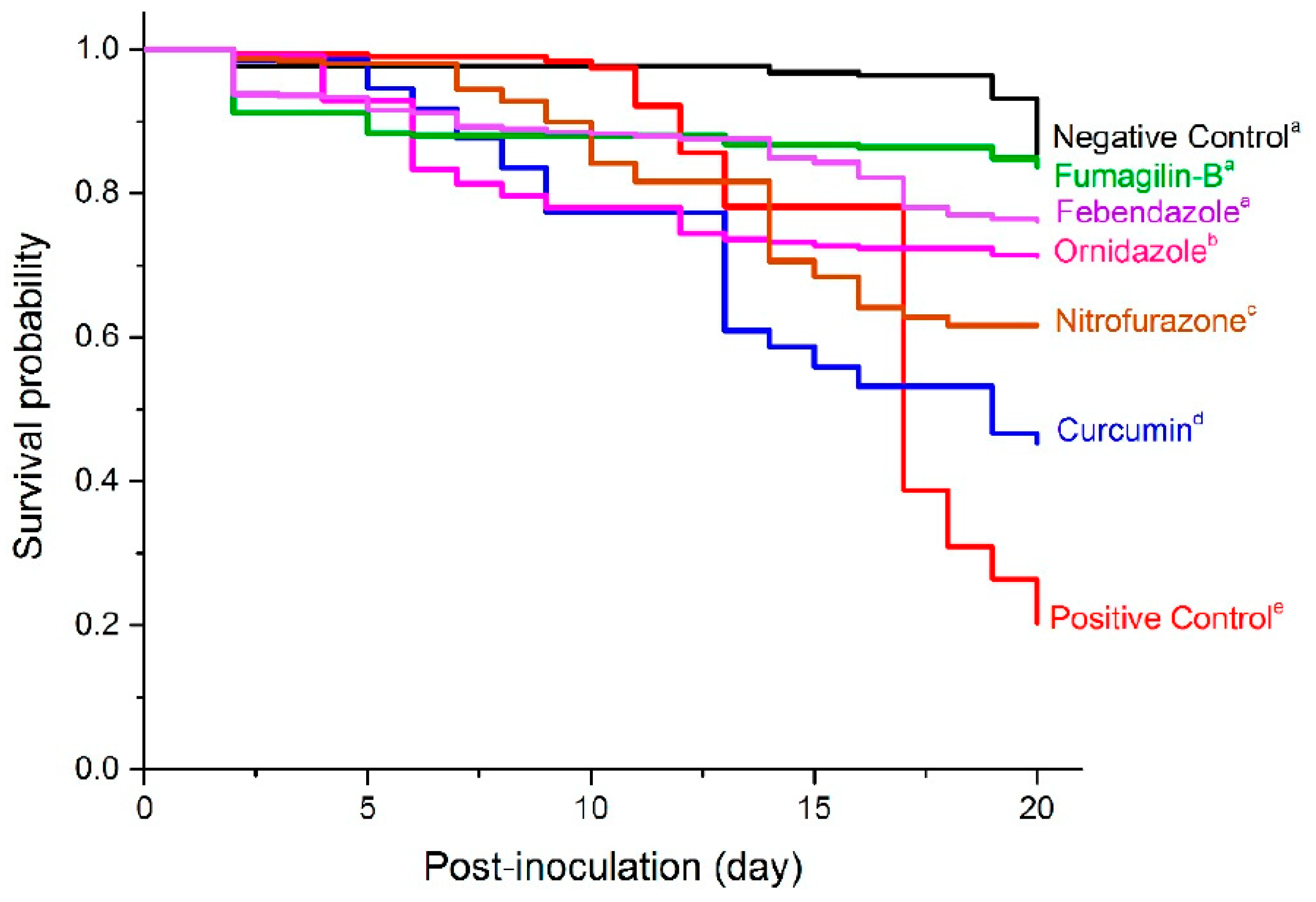
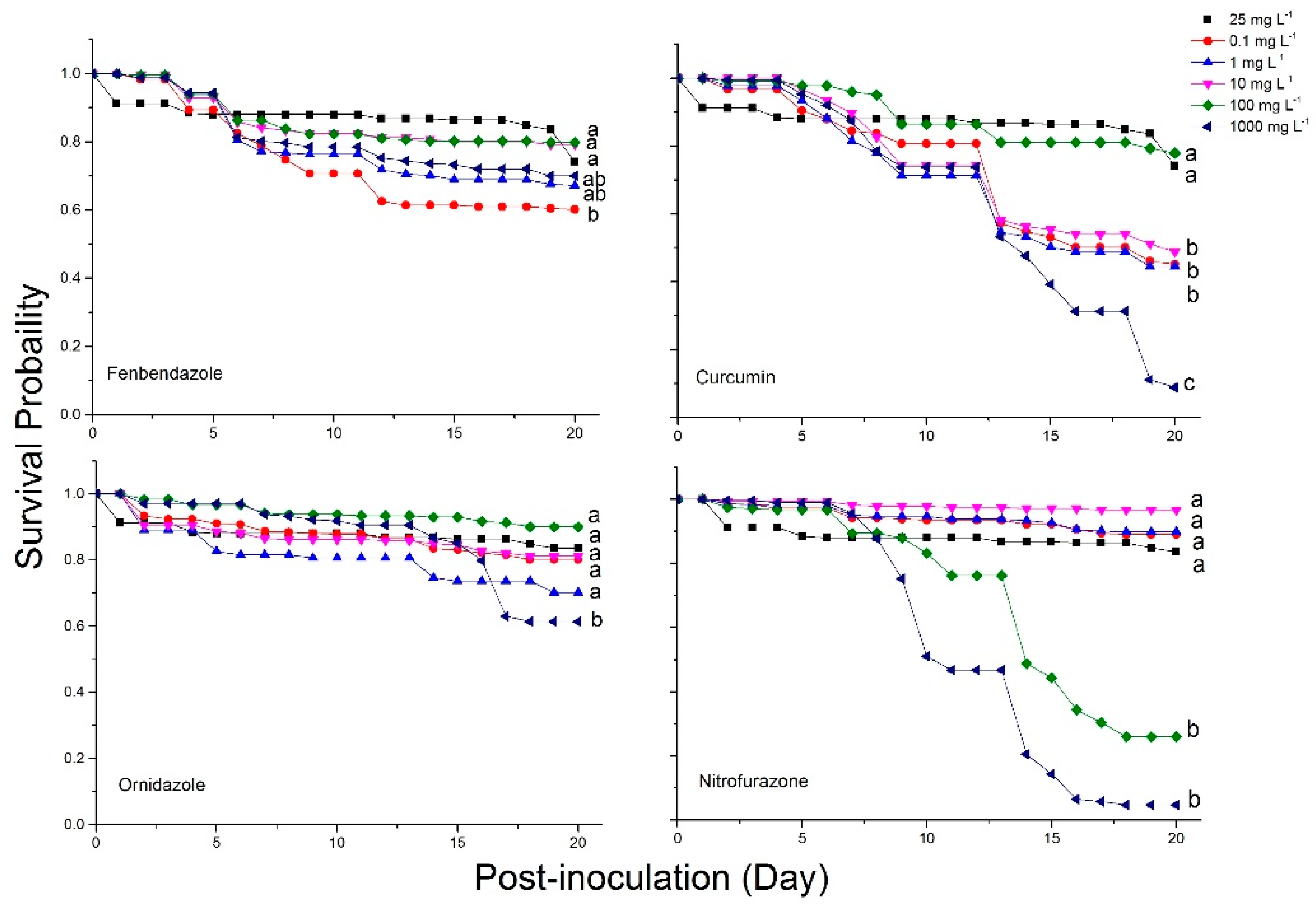
2.4. Bee Mortality and Survival Rates
3. Discussion
4. Materials and Methods
4.1. Isolation of Nosema Spores to Be Used for Inoculation
4.2. Newly Emerged Bee Collection and Caging
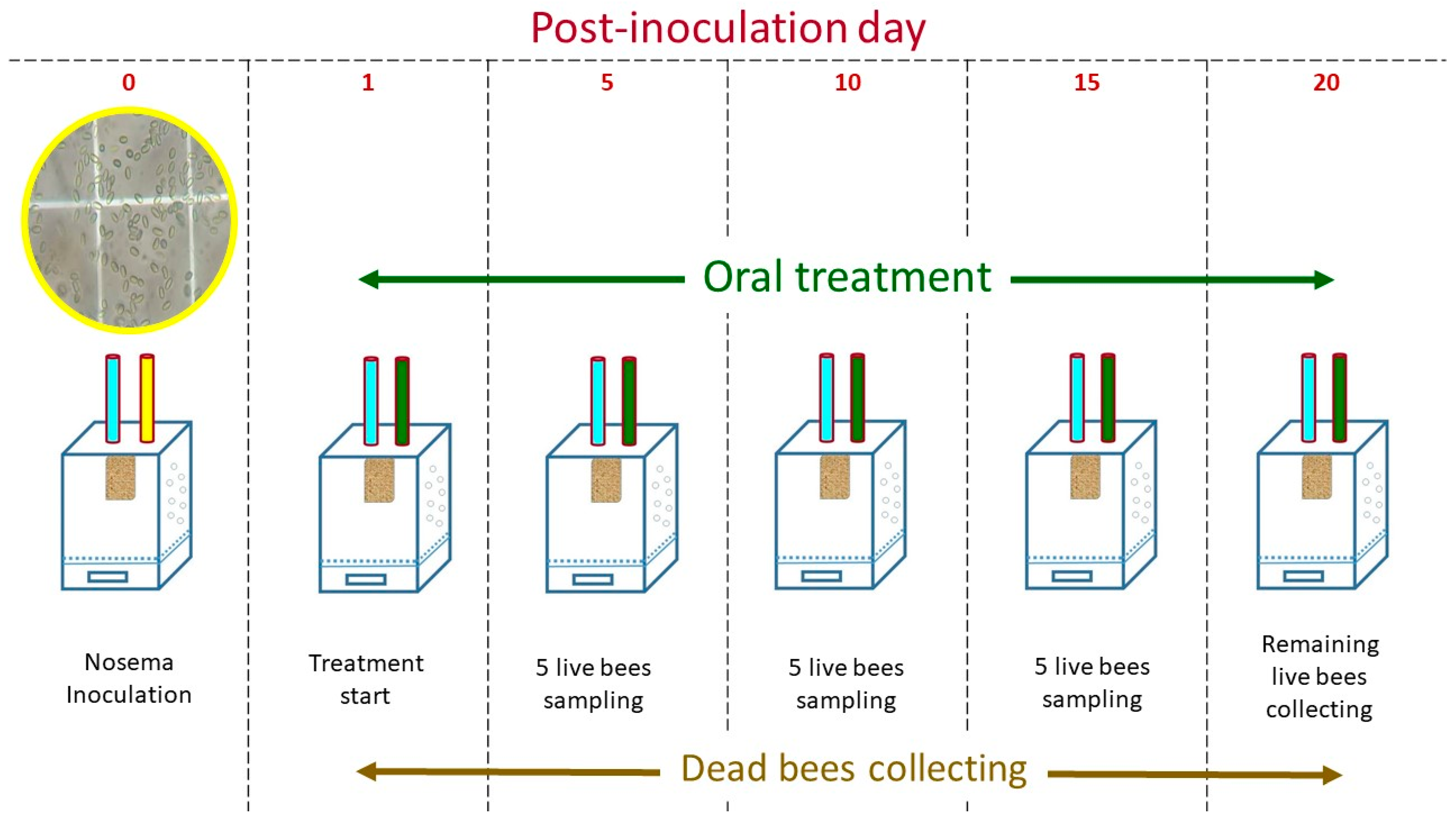
4.3. Experimental Procedures
4.4. Nosema Species Assessment
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chemurot, M.; De Smet, L.; Brunain, M.; De Rycke, R.; de Graaf, D.C. Nosema neumanni n. sp. (Microsporidia, Nosematidae), a new microsporidian parasite of honeybees, Apis mellifera in Uganda. Eur. J. Protistol. 2017, 61, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, Y.S.; Huang, W.F.; Solter, L.F.; Malysh, J.M.; Becnel, J.J.; Vossbrinck, C.R. A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J. Invertebr. Pathol. 2020, 169, 107279. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L. Honey Bee Pathology; Academic Press: London, UK, 1981. [Google Scholar]
- White, G.F. Nosema-Disease; US Department of Agriculture: Washington, DC, USA, 1919.
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee. Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E. Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Evans, J.D.; Zhou, L.; Boncristiani, H.; Kimura, K.; Xiao, T.; Litkowski, A.M.; Pettis, J.S. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J. Invertebr. Pathol. 2009, 101, 204–209. [Google Scholar] [CrossRef]
- Ozkırım, A.; Schiesser, A.; Keskin, N. Dynamics of Nosema apis and Nosema ceranae co-infection seasonally in honey bee (Apis mellifera L.) colonies. J. Apic. Sci. 2019, 63, 41–48. [Google Scholar] [CrossRef]
- Punko, R.N.; Currie, R.W.; Nasr, M.E.; Hoover, S.E. Epidemiology of Nosema spp. and the effect of indoor and outdoor wintering on honey bee colony population and survival in the Canadian Prairies. PLoS ONE 2021, 16, e0258801. [Google Scholar] [CrossRef]
- Emsen, B.; Guzman-Novoa, E.; Hamiduzzaman, M.M.; Eccles, L.; Lacey, B.; Ruiz-Perez, R.A.; Nasr, M. Higher prevalence and levels of Nosema ceranae than Nosema apis infections in Canadian honey bee colonies. Parasitol. Res. 2016, 115, 175–181. [Google Scholar] [CrossRef]
- Roberts, K.E.; Evison, S.E.; Baer, B.; Hughes, W.O. The cost of promiscuity: Sexual transmission of Nosema microsporidian parasites in polyandrous honey bees. Sci. Rep. 2015, 30, 10982. [Google Scholar] [CrossRef]
- Eiri, D.M.; Suwannapong, G.; Endler, M.; Nieh, J.C. Nosema ceranae can infect honey bee larvae and reduces subsequent adult longevity. PLoS ONE 2015, 10, e0126330. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Shutler, D.; Little, C.M.; Burgher-MacLellan, K.L.; Rogers, R.E.L. The microsporidian Nosema ceranae, the antibiotic Fumagilin-B®, and western honey bee (Apis mellifera) colony strength. Apidologie 2011, 42, 15–22. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Cook, S.C. Chronic Nosema ceranae infection inflicts comprehensive and persistent immunosuppression and accelerated lipid loss in host Apis mellifera honey bees. Int. J. Parasitol. 2018, 48, 433–444. [Google Scholar] [CrossRef]
- Antunez, K.; Martin-Hernandez, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, R.; Currie, R.W. The influence of Nosema (Microspora: Nosematidae) infection on honey bee (Hymenoptera: Apidae) defense against Varroa destructor (Mesostigmata: Varroidae). J. Econ. Entomol. 2015, 132, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef]
- Katznelson, H.; Jamieson, C.A. Control of Nosema disease of honeybees with fumagillin. Science 1952, 115, 70–71. [Google Scholar] [CrossRef]
- Bailey, L. Effect of fumagillin upon Nosema apis (Zander). Nature 1953, 171, 212–213. [Google Scholar] [CrossRef]
- van den Heever, J.P.; Thompson, T.S.; Otto, S.J.G.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Evaluation of Fumagilini-B® and other potential alternative chemotherapies against Nosema ceranae-infected honeybees (Apis mellifera) in cage trial assay. Apidologie 2016, 47, 617–630. [Google Scholar] [CrossRef]
- van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Pernal, S.F. Stability of dicyclohexylamine and fumagillin in honey. Food Chem. 2015, 179, 152–158. [Google Scholar] [CrossRef] [PubMed]
- van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escape fumagillin control in honey bees. PLoS ONE 2013, 9, e1003185. [Google Scholar] [CrossRef]
- Glavinic, U.; Stevanovic, J.; Ristanic, M.; Rajkovic, M.; Davitkov, D.; Lakic, N.; Stanimirovic, Z. Potential of fumagillin and Agaricus blazei mushroom extract to reduce Nosema ceranae in honey bees. Insects 2021, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.B.; Paul, A.Y.; Zainal Abidin, B.A.H.; Sajap, A.S.; Hussan, A.K. Preliminary evidence on Nosema spp. infection diamondback moth larvae on Fumidil-B. Pak. J. Biol. Sci. 2001, 4, 329–330. [Google Scholar]
- Killough, J.H.; Magill, G.B.; Smith, R.C. The treatment of amebiasis with suramin. Science 1952, 115, 71–72. [Google Scholar] [CrossRef]
- Kulic, M.; Aleksic, N.; Stanimirovic, Z.; Ristic, S.; Medenica, S. Examination of genotoxic effects of fumagillin in vivo. Genetika 2009, 41, 329–338. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Aleksic, N.; Kulic, M.; Maletic, M. Fumagillin induced chromosome aberrations in mouse bone-marrow cells. Arch. Biol. Sci. 2010, 62, 47–55. [Google Scholar] [CrossRef]
- Gisder, S.; Genersch, E. Identification of candidate agents active against N. ceranae infection in honey bees: Establishment of a medium throughput screening assay based on N. ceranae infected culture cells. PLoS ONE 2015, 10, e0117200. [Google Scholar] [CrossRef]
- Botias, C.; Martin-Hernandez, R.; Dias, J.; Garcia-Palencia, P.; Matabuena, M.; Juarranz, A.; Barrios, L.; Meana, A.; Nanetti, A.; Higes, M. The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environ. Microbiol. 2012, 14, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Carbonell, V.; Sepulveda, B.; Delporte, C.; Valdovinos, C.E.; Martin-Hernandez, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.P.; Fernandez, N.J.; Garrido, P.M.; Gende, L.B.; Medici, S.K.; Eguaras, M.J. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 2011, 42, 700–707. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of Nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef]
- Costa, C.; Lodesani, M.; Maistrello, L. Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 2010, 41, 141–150. [Google Scholar] [CrossRef]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honey bees (Apis mellifera). Biochemistry 2014, 79, 1192–1201. [Google Scholar] [PubMed]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Curcumin stimulates biochemical mechanisms of Apis mellifera resistance and extends the apian lifespan. J. Apicult. Sci. 2015, 59, 129–141. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.K.; Lee, J.K. Evaluation of antimicrosporidian activity of plant extracts on Nosema ceranae. J. Apicult. Sci. 2016, 60, 167–178. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, J.H.; Mina, J.; Rangachari, B.; Park, J.K. Anti-nosemosis activity of Aster scaber and Artemisia dubia aqueous extracts. J. Apicult. Sci. 2018, 62, 27. [Google Scholar] [CrossRef]
- Pohorecka, K. Laboratory studies on the effect of standardized Artemisia absinthium L. extract on Nosema apis infection in the worker Apis mellifera. J. Apicul. Sci. 2004, 48, 131–136. [Google Scholar]
- Ptaszynska, A.A.; Zaluski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. roots: A new hope against honeybee death caused by nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Xu, Y.; Gong, H.; Wu, Y.; Chen, Y.; Hu, F.; Zheng, H. Protective potential of Chinese herbal extracts against microsporidian Nosema ceranae, an emergent pathogen of western honey bees, Apis mellifera L. J. Asia-Pac. Entomol. 2021, 24, 502–512. [Google Scholar] [CrossRef]
- El Khoury, S.; Rousseau, A.; Lecoeur, A.; Cheaib, B.; Bouslama, S.; Mercier, P.L.; Demey, V.; Castex, M.; Giovenazzo, P.; Derome, N. Deleterious interaction between honey bees (Apis mellifera) and its microsporidian intracellular parasite Nosema ceranae was mitigated by administrating either endogenous or allochthonous gut microbiota strains. Front. Ecol. Evol. 2018, 6, 58. [Google Scholar] [CrossRef]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honey bees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef] [PubMed]
- Klassen, S.S.; VanBlyderveen, W.; Eccles, L.; Kelly, P.G.; Borges, D.; Goodwin, P.H.; Petukhova, T.; Wang, Q.; Guzman-Novoa, E. Nosema ceranae infections in honey bees (Apis mellifera) treated with pre/probiotics and impacts on colonies in the field. Vet. Sci. 2021, 8, 107. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Marathe, S.A.; Kumar, R.; Ajitkumar, P.; Nagaraja, V.; Chakravortty, D. Curcumin reduces the antimicrobial activity of ciprofloxacin against Salmonella Typhimurium and Salmonella Typhi. J. Antimicrob. Chem. 2013, 68, 139–152. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, P.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Inter. 2014, 2014, 186864. [Google Scholar]
- Jokipii, L.; Jokipii, A.M. Treatment of giardiasis: Comparative evaluation of ornidazole and tinidazole as a single oral dose. Gastroenterology 1982, 83, 399–404. [Google Scholar] [CrossRef]
- Rossignol, J.F.; Maisonneuve, H.; Cho, Y.W. Nitroimidazoles in the treatment of trichomoniasis, giardiasis, and amebiasis. Int. J. Clin. Pharmacol. Ther. Toxicol. 1984, 22, 63–72. [Google Scholar]
- Singh, P.; Mittal, R.; Sharma, G.C.; Singh, S.; Singh, A. Ornidazole: Comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2003, 30, 123–184. [Google Scholar] [PubMed]
- Thulkar, J.; Kriplani, A.; Agarwal, N. A comparative study of oral single dose of metronidazole, tinidazole, secnidazole and ornidazole in bacterial vaginosis. Indian J. Pharmacol. 2012, 44, 243. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Nataraju, B.; Bashir, I. Efficacy of different benzimidazole derivatives on microsporidiosis of lamerin breed of the silkworm, Bombyx mori L. J. Entomol. Nematol. 2012, 4, 12–14. [Google Scholar]
- Xin, Q.; Yuan, M.; Li, H.; Song, X.; Lu, J.; Jing, T. In vitro and in vivo effects of 3-bromopyruvate against Echinococcus metacestodes. Vet. Res. 2019, 50, 96. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Weiss, L.M. Therapeutic targets for the treatment of microsporidiosis in humans. Expert Opin. Ther. Targets 2018, 22, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Suter, C.; Muler-Doblies, U.U.; Deplazes, P.; Hatt, J.M. Prevention and treatment of Encephalitozoon cuniculi infection in rabbits with fenbendazole. Vet. Rec. 2001, 148, 478–480. [Google Scholar] [CrossRef]
- La’Toya, V.L.; Bradley, C.W.; Wyre, N.R. Encephalitozoon cuniculi in pet rabbits: Diagnosis and optimal management. Vet. Med. Res. Rep. 2014, 5, 169. [Google Scholar]
- Baeder, C.; Bahr, H.; Christ, O.; Duwel, D.; Kellner, H.M.; Kirsch, R.; Loewe, H.; Schultes, E.; Schutz, E.; Westen, H. Fenbendazole: A new, highly effective anthelmintic. Experientia 1974, 30, 753–754. [Google Scholar] [CrossRef]
- Duwel, D. Fenbendazole. II. Biological properties and activity. Pestic. Sci. 1977, 8, 550–555. [Google Scholar] [CrossRef]
- Short, C.R.; Flory, W.; Hsieh, L.C.; Barker, S.A. The oxidative metabolism of fenbendazole: A comparative study. J. Vet. Pharmacol. Ther. 1988, 11, 50–55. [Google Scholar] [CrossRef]
- Yazwinski, T.A.; Tucker, C.; Stelzleni, A.; Johnson, Z.; Robins, J.; Downum, K.; Fincher, M.; Matlock, J.; Chapman, H.D. Subclinical effects and fenbendazole treatment of turkey ascaridiasis under simulated field conditions. Avian Dis. 2002, 46, 886–892. [Google Scholar] [CrossRef]
- Mackie, K.G.; Menzies, P.I.; Bateman, K.G.; Gordon, J.L. Efficacy of fenbendazole and ivermectin in treating gastrointestinal nematode infections in an Ontario cow-calf herd. Can. Vet. J. 2019, 60, 1213. [Google Scholar] [PubMed]
- Vesselova, S.; Karanikolova, M.; Nazarov, V.; Ivanova, S.; Petkov, S.; Kanora, A.; Depondt, W.; Claerhout, L. Maintenance of fenbendazole concentrations post oral administration and clinical effect of Pigfen® in pigs naturally infected with Ascaris Suum. In Proceedings of the International Pig Veterinary Society Congress, Rio de Janeiro, Brazil, 3–6 November 2020; p. 665. [Google Scholar]
- Park, K.; Bang, H.W.; Park, J.; Kwak, I.S. Ecotoxicological multilevel-evaluation of the effects of fenbendazole exposure to Chironomus riparius larvae. Chemosphere 2009, 77, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Schmahl, G.; Benini, J. Treatment of fish parasites. 11. Effects of different benzimidadole derivates (albendazole, mebendazole, fenbendazole) on Gluglea anomalia, Moniez, 1887 (Microsporidia): Ultrastructural aspects and efficacy studies. Parasitol. Res. 1998, 84, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.L.; Stiebel, C.L.; Grizzle, J.M. Acute toxicity of nitrofurazone to channel catfish Ictalurus punctatus, and goldfish, Carassius auratus. Bull. Environ. Contam. Toxicol. 1987, 38, 42–46. [Google Scholar] [CrossRef]
- Kotsiou, A.; Tesseromatis, C. Hypericum perforatum for experimental skin burns treatment in rats in comparison to nitrofurazone. J. Med. Plants 2020, 8, 227–231. [Google Scholar]
- Ona, K.R.; Courcelle, C.T.; Courcelle, J. Nucleotide excision repair is a predominant mechanism for processing nitrofurazone-induced DNA damage in Escherichia coli. J. Bacteriol. 2009, 191, 4959–4965. [Google Scholar] [CrossRef]
- McGowan, J.; De la Mora, A.; Goodwin, P.H.; Habash, M.; Hamiduzzaman, M.M.; Kelly, P.G.; Guzman-Novoa, E. Viability and infectivity of fresh and cryopreserved Nosema ceranae spores. J. Microbiol. Methods 2016, 131, 16–22. [Google Scholar] [CrossRef]
- Valizadeh, P.; Guzman-Novoa, E.; Goodwin, P.H. Effect of immune inducers on Nosema ceranae multiplication and their impact on honey bee (Apis mellifera L.) survivorship and behaviors. Insects 2020, 11, 572. [Google Scholar] [CrossRef]
- Milbrath, M.O.; van Tran, T.; Huang, W.F.; Solter, L.F.; Tarpy, D.R.; Lawrence, F.; Huang, Z.Y. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera). J. Invertebr. Pathol. 2015, 125, 9–15. [Google Scholar] [CrossRef]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Tesovnik, T.; Stevanovic, J.; Zorc, M.; Cizelj, I.; Stanimirovic, Z.; Narat, M. Response of adult honey bees treated in larval stage with prochloraz to infection with Nosema ceranae. Peer J. 2019, 7, e6325. [Google Scholar] [CrossRef] [PubMed]
- Braglia, C.; Alberoni, D.; Porrini, M.P.; Garrido, P.M.; Baffoni, L.; Di Gioia, D. Screening of dietary ingredients against the honey bee parasite Nosema ceranae. Pathogens 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.D.; Kemmelmeier, C.; Arroteia, C.C.; da Costa, C.L.; Mallmann, C.A.; Janeiro, V.; Ferreira, F.M.D.; Mossini, S.A.G.; Silva, E.L.; Machinski, M., Jr. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chem. 2013, 136, 789–793. [Google Scholar] [CrossRef]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial activity of curcumin against Periodontopathic bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef]
- Andromeda, S.E.; Berbudi, A. The role of curcumin as an antimalarial agent. Sys. Rev. Pharm. 2020, 11, 18–25. [Google Scholar]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Evens, F.; Niemegeers, K.; Packchanian, A. Nitrofurazone therapy of Trypanosoma gambiense sleeping sickness in man. Am. J. Trop. Med. Hyg. 1957, 6, 665–678. [Google Scholar] [CrossRef]
- Nagel, M.L.; Summerfelt, R.C. Nitrofurazone for control of the microsporidan parasite Pleistophora ovariae in golden shiners. Progress. Fish-Cult. 1977, 39, 18–23. [Google Scholar] [CrossRef]
- Bruggemann, J.; Bronsch, K.; Heigner, H.; Knapstein, H. Feed additives: Nitrofurazone determination in poultry feed by phenylhydrazine or alkali reaction. J. Agr. Food Chern. 1962, 10, 108. [Google Scholar] [CrossRef]
- Moffett, J.O.; Lackett, J.J.; Hitchcock, J.D. Compounds tested for control of Nosema in honey bees. J. Eco. Entomol. 1969, 62, 886–889. [Google Scholar] [CrossRef]
- Bustos, M.D.; Salazar, N.; Espino, F.E.; Montalban, C.; Sabordo, N.; Laurente, M. Ornidazole in the treatment of giardiasis in an institution for the mentally retarded. Philipp. J. Microbiol. Infect. Dis. 1991, 20, 13–16. [Google Scholar]
- Bulut, B.U.; Gulnar, S.B.; Aysev, D. Alternative treatment protocols in giardiasis: A pilot study. Scand. J. Infect. Dis. 1996, 28, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.C.; Bowman, D.D.; Heller, R.L. Efficacy of fenbendazole against giardiasis in dogs. Am. J. Vet. Res. 1994, 55, 988–990. [Google Scholar] [PubMed]
- O’Handley, R.M.; Buret, A.G.; McAllister, T.A.; Jelinski, M.; Olson, M.E. Giardiasis in dairy calves: Effects of fenbendazole treatment on intestinal structure and function. Inter. J. Parasitol. 2001, 31, 73–79. [Google Scholar] [CrossRef]
- Guerrini, A.; Carminati, A.; Stancampiano, L.; Roncada, P.; Frasnelli, M. Use of flubendazole and fenbendazole for treatment of lung severe infection by the gapeworm Cyathostoma bronchialis (Nematoda: Syngamidae) in Branta hutchinsii, Anser indicus and B. leucopsis exotic geese: An interesting case. Vet. Sci. 2021, 8, 147. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Joffe, L.S.; Simon, K.S.; Castelli, R.F.; Reis, F.C.; Bryan, A.M.; Borges, B.S.; Medeiros, L.C.; Bocca, A.L.; Del Poeta, M.; et al. Fenbendazole controls in vitro growth, virulence potential, and animal infection in the Cryptococcus model. Antimicrob. Agents Chemother. 2020, 64, e00286-20. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis 2003, 8, 413–450. [Google Scholar] [CrossRef]
- Martin, F.; Halvarsson, P.; Delhomme, N.; Hoglund, J.; Tyden, E. Exploring the β-tubulin gene family in a benzimidazole-resistant Parascaris univalens population. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 84–91. [Google Scholar] [CrossRef]
- Collins, J.B.; Jordan, B.; Baldwin, L.; Hebron, C.; Paras, K.; Vidyashankar, A.N.; Kaplan, R.M. Resistance to fenbendazole in Ascaridia dissimilis, an important nematode parasite of turkeys. Poult. Sci. 2019, 98, 5412–5415. [Google Scholar] [CrossRef] [PubMed]
- Sak, B.; Brdickova, K.; Holubova, N.; Kvetonova, D.; Hlaskova, L.; Kvac, M. Encephalitozoon cuniculi genotype III evinces a resistance to albendazole treatment in both immunodeficient and immunocompetent mice. Antimicrob. Agents Chemother. 2020, 64, e00058-20. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Shirato, M.; Toya, M.; Sato, M. Dual impact of a benzimidazole resistant β-tubulin on microtubule behavior in fission yeast. Cells 2021, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Winterrowd, C.A.; Pomroy, W.E.; Sangster, N.C.; Johnson, S.S.; Geary, T.G. Benzimidazole-resistant β-tubulin alleles in a population of parasitic nematodes (Cooperia oncophora) of cattle. Vet. Parasitol. 2003, 117, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, D.E.; Weiss, L.M.; Feng, X.; Williams, B.A.; Keeling, P.J.; Zhang, Q.; Tzipori, S. Analysis of the β-Tubulin Genes from Enterocytozoon bieneusi Isolates from a human and Rhesus Macaque. J. Euk. Microbiol. 2007, 54, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Franzen, C.; Salzberger, B. Analysis of the β-tubulin gene from Vittaforma corneae suggests benzimidazole resistance. Antimicrob. Agents Chemother. 2008, 52, 790–793. [Google Scholar] [CrossRef][Green Version]
- Kochansky, J.; Nasr, M. Laboratory studies on the photostability of fumagillin, the active ingredient of Fumidil-B. Apidologie 2004, 35, 301–310. [Google Scholar] [CrossRef]
- Mattila, H.R.; Harris, J.L.; Otis, G.W. Timing of production of winter bees in honey bee (Apis mellifera) colonies. Insectes Sociaux 2001, 48, 88–93. [Google Scholar] [CrossRef]
- Amdam, G.V.; Omholt, S.W. The regulatory anatomy of honeybee lifespan. J. Theoretical Biol. 2002, 216, 209–228. [Google Scholar] [CrossRef]
- Otis, G.W.; Wheeler, D.E.; Buck, N.; Mattila, H.R. Storage proteins in winter honey bees. Apiacata 2004, 38, 352–357. [Google Scholar]
- Aurori, C.M.; Buttstedt, A.; Dezmirean, D.S.; Marghitas, L.A.; Moritz, R.F.; Erler, S. What is the main driver of ageing in long-lived winter honeybees: Antioxidant enzymes, innate immunity, or vitellogenin? J. Gerontol. A Biom. Sci. Med. Sci. 2014, 69, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Kesnerova, L.; Emery, O.; Troilo, M.; Liberti, J.; Erkosar, B.; Engel, P. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 2020, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; Arathi, H.S. Dietary phytochemicals, honey bee longevity and pathogen tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef]
- Ingber, D.; Fujita, T.; Kishimoto, S.; Sudo, K.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Sin, N.; Meng, L.; Wang, M.Q.; Wen, J.J.; Bornmann, W.G.; Crews, C.M. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 1997, 94, 6099–6103. [Google Scholar] [CrossRef]
- Korzybski, T.; Kowszyk-Gindifer, Z.; Kurylowicz, W. Antibiotics isolated from the genus Bacillus (Bacillaceae). Antibiot. Orig. Nat. Prop. 1978, 3, 1529–1661. [Google Scholar]
- Villar, D.; Cray, C.; Zaias, J.; Altman, N.H. Biologic effects of fenbendazole in rats and mice: A review. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 8–15. [Google Scholar]
- Canadian Honey Council, Best Management Practices (BMPs) for the Honey Bee Health. Available online: http://www.honeycouncil.ca/images2/pdfs/BMP_manual_-_Les_Eccles_Pub_22920_-_FINAL_-_low-res_web_-_English.pdf (accessed on 25 September 2022).
- Government of Alberta, Honey Bee Pests and Diseases–Best Management Practices. Available online: https://open.alberta.ca/dataset/737f60e0-5426-463a-a95b-e0d8f0773c9e/resource/1dbd2659-247f-4603-a1c5-73fcc6e1e72c/download/af-honey-bee-pests-and-diseases-best-management-practices-2020-05.pdf (accessed on 25 September 2022).
- Gruszka, J.; Currie, R.W.; Dixon, D.; Tuckey, K.; van Westendorp, P. Beekeeping in Western Canada. Alberta Agriculture; Food and Rural Development: Edmonton, AB, Canada, 1998. [Google Scholar]
- Cantwell, G.E. Standard method for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.P.; Clafen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R.; et al. Standard methods for toxicology research in Apis mellifera. J. Apicult. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef]
- Gough, H.J.; McIndoe, E.C.; Lewis, B.G. The use of dimethoate as a reference compound in laboratory acute toxicity tests on honey bees (Apis mellifera L.) 1981–1992. J. Apicult. Res. 1994, 33, 119–125. [Google Scholar] [CrossRef]
- Copley, T.R.; Jabaji, S.H. Honeybee glands as possible infection reservoirs of Nosema ceranae and Nosema apis in naturally infected forager bees. J. Appl. Microbiol. 2012, 112, 15–24. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/STATVR 9.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; The Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Thompson, T.S.; van den Heever, J.P.; Pernal, S.F. Determination of dicyclohexylamine in beeswax by aqueous normal phase liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. Sci. 2018, 56, 262–269. [Google Scholar] [CrossRef] [PubMed]



| Treatment | 1000 mg L−1 | 100 mg L−1 | 10 mg L−1 | 1 mg L−1 | 0.1 mg L−1 | Fumagilin-B® |
|---|---|---|---|---|---|---|
| Curcumin | 13.75 ± 2.91 a | 16.44 ± 2.06 a | 5.55 ± 2.52 b | 13.73 ± 2.48 a | 12.02 ± 2.52 a | 5.57 ± 3.17 b |
| Fenbendazole | 0.25 ± 2.26 b | 0.42 ± 2.06 b | 0.4 ± 2.26 b | 0.43 ± 2.52 b | 0.29 ± 2.91 b | 5.57 ± 3.17 a |
| Nitrofurazone | 5.54 ± 5.05 bc | 7.38 ± 3.57 b | 9.28 ± 1.91 b | 11.94 ± 2.06 a | 9.98 ± 2.06 b | 5.57 ± 3.17 c |
| Ornidazole | 2.73 ± 2.52 b | 11.05 ± 2.1 a | 8.5 ± 2.06 b | 18.64 ± 2.52 a | 13.23 ± 2.1 a | 5.57 ± 3.17 b |
| Treatment | 1000 mg L−1 | 100 mg L−1 | 10 mg L−1 | 1 mg L−1 | 0.1 mg L−1 | Fumagilin-B® |
|---|---|---|---|---|---|---|
| Curcumin | 94.44 ± 9.35 a | 24.22 ± 9.55 b | 50.67 ± 9.5 ab | 53.69 ± 9.3 ab | 55.44 ± 9.45 ab | 24.43 ± 14.28 b |
| Fenbendazole | 31.22 ± 8.5 a | 21.23 ± 8.99 a | 20.92 ± 7.99 a | 34.79 ± 9.31 a | 44.06 ± 9.55 a | 24.43 ± 14.28 a |
| Nitrofurazone | 98.26 ± 9.25 a | 81.75 ± 9.62 a | 4.41 ± 8.77 c | 12.95 ± 8.9 bc | 17.4 ± 9.11 bc | 24.43 ± 14.28 b |
| Ornidazole | 56.35 ± 9.33 a | 12.41 ± 9.05 b | 22.16 ± 8.88 b | 23.95 ± 9.74 b | 25.68 ± 9.67 b | 24.43 ± 14.28 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahreini, R.; Nasr, M.; Docherty, C.; de Herdt, O.; Feindel, D.; Muirhead, S. In Vivo Inhibitory Assessment of Potential Antifungal Agents on Nosema ceranae Proliferation in Honey Bees. Pathogens 2022, 11, 1375. https://doi.org/10.3390/pathogens11111375
Bahreini R, Nasr M, Docherty C, de Herdt O, Feindel D, Muirhead S. In Vivo Inhibitory Assessment of Potential Antifungal Agents on Nosema ceranae Proliferation in Honey Bees. Pathogens. 2022; 11(11):1375. https://doi.org/10.3390/pathogens11111375
Chicago/Turabian StyleBahreini, Rassol, Medhat Nasr, Cassandra Docherty, Olivia de Herdt, David Feindel, and Samantha Muirhead. 2022. "In Vivo Inhibitory Assessment of Potential Antifungal Agents on Nosema ceranae Proliferation in Honey Bees" Pathogens 11, no. 11: 1375. https://doi.org/10.3390/pathogens11111375
APA StyleBahreini, R., Nasr, M., Docherty, C., de Herdt, O., Feindel, D., & Muirhead, S. (2022). In Vivo Inhibitory Assessment of Potential Antifungal Agents on Nosema ceranae Proliferation in Honey Bees. Pathogens, 11(11), 1375. https://doi.org/10.3390/pathogens11111375






