Correlation between Clinical and Immunological Variables and Humoral Response to SARS-CoV-2 Vaccination in Adult Patients with Antibody Deficiency Disorders
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. ADD Patients and Healthy Controls
2.3. Method
2.4. Humoral Response to COVID-19 mRNA-Based Vaccine
2.5. Statistical Analysis
3. Results
3.1. SID and PID Patients’ Characteristics
3.2. Adverse Reactions to Vaccination
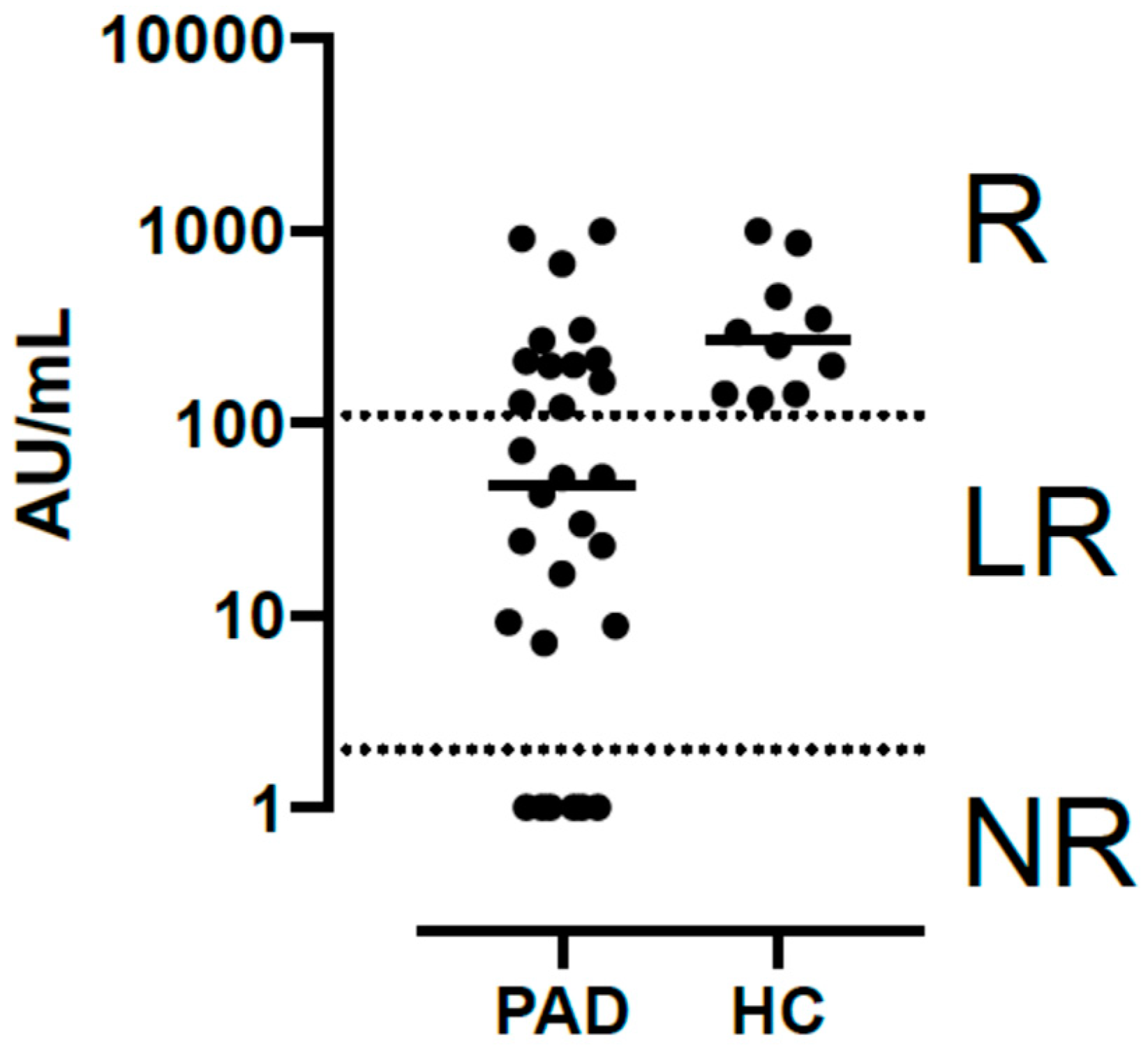
3.3. Two-Dose Vaccine-Induced IgG Immune Response Categorization and Related Variables
3.4. Third-Dose Vaccine-Induced IgG Immune Response against SARS-CoV-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Weekly Epidemiological Update on COVID-19—31 August 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---31-august-2022 (accessed on 15 September 2022).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yuan, Y.; Zhou, Y.; Deng, Z.; Zhao, J.; Feng, F.; Zou, H.; Sun, C. Safety of SARS-CoV-2 vaccines: A systematic review and meta-analysis of randomized controlled trials. Infect. Dis. Poverty 2021, 10, 94. [Google Scholar] [CrossRef]
- Shields, A.M.; Burns, S.O.; Savic, S.; Richter, A.G.; UK PIN COVID-19 Consortium. COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience. J. Allergy Clin. Immunol. 2021, 147, 870–875.e1. [Google Scholar] [CrossRef] [PubMed]
- Meyts, I.; Bucciol, G.; Quinti, I.; Neven, B.; Fischer, A.; Seoane, E.; Lopez-Granados, E.; Gianelli, C.; Robles-Marhuenda, A.; Jeandel, P.Y.; et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J. Allergy Clin. Immunol. 2021, 147, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Milito, C.; Lougaris, V.; Giardino, G.; Punziano, A.; Vultaggio, A.; Carrabba, M.; Cinetto, F.; Scarpa, R.; Delle Piane, R.M.; Baselli, L.; et al. Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity. J. Allergy Clin. Immunol. Pract. 2021, 9, 2904–2906.e2. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.L.; Chen, I.T.; Lai, M.Z. Infection-induced inflammation from specific inborn errors of immunity to COVID-19. FEBS J. 2021, 288, 5021–5041. [Google Scholar] [CrossRef] [PubMed]
- Goudouris, E.S.; Pinto-Mariz, F.; Mendonça, L.O.; Aranda, C.S.; Guimarães, R.R.; Kokron, C.; Barros, M.T.; Anísio, F.; Alonso, M.L.O.; Marcelino, F.; et al. Outcome of SARS-CoV-2 Infection in 121 Patients with Inborn Errors of Immunity: A Cross-Sectional Study. J. Clin. Immunol. 2021, 41, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168. [Google Scholar] [CrossRef]
- Luxi, N.; Giovanazzi, A.; Capuano, A.; Crisafulli, S.; Cutroneo, P.M.; Fantini, M.P.; Ferrajolo, C.; Moretti, U.; Poluzzi, E.; Raschi, E.; et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021, 44, 1247–1269. [Google Scholar] [CrossRef]
- Ainsua-Enrich, E.; Pedreño, N.; Bracke, C.; Avila-Nieto, C.; Rodriguez de la Concepción, M.L.; Pradenas, E.; Trinite, B.; Marfil, S.; Miranda, C.; Gonzalez, S.; et al. Kinetics of immune responses elicited after three mRNA COVID-19 vaccine doses in predominantly antibody-deficient individuals. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Seidel, M.G.; Kindle, G.; Gathmann, B.; Quinti, I.; Buckland, M.; van Montfrans, J.; Scheible, R.; Rusch, S.; Gasteiger, L.M.; Grimbacher, B.; et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J. Allergy Clin. Immunol. Pract. 2019, 7, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Shields, A.M.; Faustini, S.E.; Hill, H.J.; Al-Taei, S.; Tanner, C.; Ashford, F.; Workman, S.; Moreira, F.; Verma, N.; Wagg, H.; et al. SARS-CoV-2 Vaccine Responses in Individuals with Antibody Deficiency: Findings from the COV-AD Study. J Clin Immunol 2022, 42, 923–934. [Google Scholar] [CrossRef]
- Shields, A.M.; Faustini, S.E.; Hill, H.J.; Al-Taei, S.; Tanner, C.; Ashford, F.; Workman, S.; Moreira, F.; Verma, N.; Wagg, H.; et al. Increased Seroprevalence and Improved Antibody Responses following Third Primary SARS-CoV-2 Immunisation: An Update from the COV-AD Study. Front. Immunol. 2022, 13, 912571. [Google Scholar] [CrossRef]
- Delmonte, O.M.; Bergerson, J.R.E.; Burbelo, P.D.; Durkee-Shock, J.R.; Dobbs, K.; Bosticardo, M.; Keller, M.D.; McDermott, D.H.; Rao, V.K.; Dimitrova, D.; et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J. Allergy Clin. Immunol. 2021, 148, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Amodio, D.; Ruggiero, A.; Sgrulletti, M.; Pighi, C.; Cotugno, N.; Medri, C.; Morrocchi, E.; Colagrossi, L.; Russo, C.; Zaffina, S.; et al. Humoral and Cellular Response following Vaccination With the BNT162b2 mRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front. Immunol. 2021, 12, 727850. [Google Scholar] [CrossRef]
- Van Leeuwen, L.P.M.; GeurtsvanKessel, C.H.; Ellerbroek, P.M.; de Bree, G.J.; Potjewijd, J.; Rutgers, A.; Jolink, H.; van de Veerdonk, F.; van Gorp, E.C.M.; de Wilt, F.; et al. Immunogenicity of the mRNA-1273 COVID-19 vaccine in adult patients with inborn errors of immunity. J. Allergy Clin. Immunol. 2022, 149, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.M.; Mrak, D.; Perkmann, T.; Haslacher, H.; Aletaha, D. SARS-CoV-2 vaccination in rituximab-treated patients: Evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021, 80, 1355–1356. [Google Scholar] [CrossRef]
- Cazzato, G.; Romita, P.; Foti, C.; Lobreglio, D.; Trilli, I.; Colagrande, A.; Ingravallo, G.; Resta, L. Development of Flat Warts on the Cheeks after BioNTech-Pfizer BNT162b2 Vaccine: Is There a Correlation? Vaccines 2022, 10, 532. [Google Scholar] [CrossRef]
- Greenhawt, M.; Abrams, E.M.; Shaker, M.; Chu, D.K.; Khan, D.; Akin, C.; Alqurashi, W.; Arkwright, P.; Baldwin, J.L.; Ben-Shoshan, M.; et al. The Risk of Allergic Reaction to SARS-CoV-2 Vaccines and Recommended Evaluation and Management: A Systematic Review, Meta-Analysis, GRADE Assessment, and International Consensus Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 3546–3567. [Google Scholar] [CrossRef]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022, 399, 36–49. [Google Scholar] [CrossRef]
- Antolí, A.; Rocamora-Blanch, G.; Framil, M.; Mas-Bosch, V.; Navarro, S.; Bermudez, C.; Martinez-Yelamos, S.; Dopico, E.; Calatayud, L.; Garcia-Muñoz, N.; et al. Evaluation of Humoral and Cellular Immune Responses to the SARS-CoV-2 Vaccine in Patients with Common Variable Immunodeficiency Phenotype and Patient Receiving B-Cell Depletion Therapy. Front. Immunol. 2022, 13, 895209. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef]
- Blanco, E.; Pérez-Andrés, M.; Arriba-Méndez, S.; Contreras-Sanfeliciano, T.; Criado, I.; Pelak, O.; Serra-Caetano, A.; Romero, A.; Puig, N.; Remesal, A.; et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J. Allergy Clin. Immunol. 2018, 141, 2208–2219.e16. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J.; Burrows, P.D.; Wang, J.Y. B Cell Development and Maturation. Adv. Exp. Med. Biol. 2020, 1254, 1–22. [Google Scholar]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Hagin, D.; Freund, T.; Navon, M.; Halperin, T.; Adir, D.; Marom, R.; Levi, I.; Benor, S.; Alcalay, Y.; Freund, N.T. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J. Allergy Clin. Immunol. 2021, 148, 739–749. [Google Scholar] [CrossRef]
- Abramowicz, M.; Zucotti, G.; Pflomm, M. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA 2022, 327, 384–385. [Google Scholar]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. 2021, 15, 102329. [Google Scholar] [CrossRef] [PubMed]
| Variable | n/30 (%) |
|---|---|
| Age in years—mean (range) | 55.6 (29–85) |
| Gender | Female 15 (50%) |
| Male 15 (50%) | |
| Underlying or related diseases | Lymphoma 2 (6.7%) |
| Leukemia 1 (3.3%) | |
| Solid cancer 7 (23.2%) | |
| Basocellular carcinoma 2 (6.7%) | |
| Breast carcinoma 2 (6.7%) | |
| Lung adenocarcinoma 1 (3.3%) | |
| Seminoma 1 (3.3%) | |
| Thymoma 1 (3.3%) | |
| Chronic liver disease 1 (3.3%) | |
| Chronic kidney disease 1 (3.3%) | |
| Nephrotic syndrome 1 (3.3%) | |
| Autoimmune diseases (33.3%) | |
| Thrombotic thrombocytopenic purpura 2 (6.7%) | |
| Celiac disease 1 (3.3%) | |
| Collagenous colitis 1 (3.3%) | |
| Crohn’s disease 1 (3.3%) | |
| Ulcerative proctitis 1 (3.3%) | |
| Autoimmune anemia 1 (3.3%) | |
| Lupus-like syndrome 1 (3.3%) | |
| Membranoproliferative glomerulonephritis type 1 1 (3.3%) | |
| PR3-ANCA-associated vasculitis 1 (3.3%) | |
| GLILD 3 (10%) | |
| Asthma 6 (20%) | |
| Drug or food allergies 9 (30%) | |
| Immunosuppressive agents used in the last 3 months | Azathioprine 2 (6.7%) |
| Mycophenolate 1 (3.3%) | |
| Corticosteroids 1 (3.3%) | |
| Rituximab + corticosteroids 1 (3.3%) | |
| Previous rituximab | 6 (20%) |
| ESID registry for Immunodeficiency [12] | Combined Immunodeficiency 1 (3.3%) |
| Common Variable Disorders 14 (46.7%) | |
| Secondary Immunodeficiency 3 (10%) | |
| Thymoma with Immunodeficiency 1 (3.3%) | |
| Unclassified Antibody Deficiency 11 (36.7%) | |
| Years since diagnose diagnosis—mean (range) | 7.87 (0–31) |
| Type of Immunoglobulin deficiency in PID/SID | IgG 6 (20%) |
| IgG + IgA 4 (13.3%) | |
| IgG + IgA + IgM 16 (53.3%) | |
| IgG + IgM 4 (13.3%) | |
| IgG subclass deficiency | IgG 1 4 (13.3%) |
| IgG 1 + 2 + 3 1 (3.3%) | |
| IgG 1 + 2 + 3 + 4 6 (20%) | |
| IgG 1 + 2 + 4 13 (43.3%) | |
| No subclass affected 6 (20%) | |
| Isohemagglutinin evaluation | Total evaluated 23 (76.7%) |
| High rates 8 (26.7%) | |
| Low rates 13 (43.3%) | |
| Not evaluable 1 2 (6.7%) | |
| Polysaccharide Typhim Vi® Antibody Response | Total evaluated 13/30 (43.3%) |
| Adequate 6 (20%) | |
| Non-adequate 7 (23.3%) | |
| Type of Immunoglobulin Replacement Therapy (IRT) | Subcutaneous 17 (56.6%) |
| Intravenous 13 (43.4%) | |
| Years in IRT | <1 year 5 (16.6%) |
| 1–5 years 13 (43.3%) | |
| 5–10 years 6 (20%) | |
| >10 years 6 (20%) | |
| Last available IgG trough levels—mean (range) | 797 mg/dL (233–1112) |
| Previous COVID-19 infection | 4 (13.3%) |
| WHO-SCORE in COVID-19 infection [13] | Score 1 1 (3.3%) |
| Score 2 1 (3.3%) | |
| Score 5 2 (6.7%) | |
| IgG SARS-CoV-2 (June/2020) | Positive 1 (3.3%) |
| Negative 28 (93.3%) |
| Adverse Events n/30 (%) | First Dose | Second Dose | Third Dose |
|---|---|---|---|
| Local Pain | 25 (83.3%) | 27 (90%) | 25 (83.3%) |
| Local Blush | 3 (10%) | 5 (16.7%) | 7 (23.3%) |
| Local Inflammation | 3 (10%) | 5 (16.7%) | 8 (26.7%) |
| Paresthesia | 0 (0%) | 1 (3.3%) | 2 (6.7%) |
| Headache | 9 (30%) | 8 (26.7%) | 9 (30%) |
| Shivers | 6(20%) | 10 (33.3%) | 12 (40%) |
| Arthromyalgia | 7 (23.3%) | 12 (40%) | 12 (40%) |
| Asthenia | 12 (40%) | 15 (50%) | 17 (56.7%) |
| Dizziness | 1 (3.3%) | 4 (13.3%) | 4 (13.3%) |
| Syncope | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea/Vomiting | 1 (3.3%) | 2 (6.6%) | 1 (3.3%) |
| Diarrhea | 2 (6.6%) | 1 (3.3%) | 3 (10%) |
| Fever | 4 (13.3%) | 10 (33.3%) | 12 (40%) |
| Local Adenopathy | 0 (0%) | 0 (0%) | 1 (3.3%) |
| Anaphylaxis | 0 (0%) | 0 (0%) | 0 (0%) |
| Other Adverse Events | 2 (6.6%) | 5 (16.7%) | 5 (16.7%) |
| Medical Assistance | 0 (0%) | 1 (3.3%) | 0 (0%) |
| Sick Leave | 1 (3.3%) | 1 (3.3%) | 0 (0%) |
| Days of Sick Leave | 2 | 3 | 0 |
| Variable | NR | LR | HR | p Value |
|---|---|---|---|---|
| Absolute B lymphocyte count (cel/µL) | 102 | 137 | 262 | >0.05 |
| Mean Memory B Cells (%) | 7.72 | 18.11 | 10.95 | >0.05 |
| Mean IgM memory B Cells (%) | 1.42 | 2.10 | 1.6 | >0.05 |
| Mean Transitional B Cells (%) | 12.7 | 9.15 | 8.25 | >0.05 |
| Mean Switched B Cells (%) | 1.74 | 15.46 | 11.80 | 0.05 |
| Mean absolute CD8 Count (cells/µL) (range) | 561.6 (56–1068) | 688 (265–1360) | 350 (338–362) | >0.05 |
| Mean absolute CD4 Count (cells/µL) (range) | 578.2 (105–1022) | 670.75 (274–1133) | 1332 (1189–1476) | >0.05 |
| Non-Responders n (%) | Low Responders n (%) | Responders n (%) | p (Ẋ2) | |
|---|---|---|---|---|
| ESID registry classification | 0.005 | |||
| CID | 1/1 (100) | 0 | 0 | |
| CVID | 5/14 (35.7) | 7/14 (50) | 2/14 (14.3) | |
| SID | 0 | 0 | 3/3 (100) | |
| TI | 1/1 (100) | 0 | 0 | |
| UnPAD | 0 | 4/11 (36.4) | 7/11 (63.6) | |
| Type of Ig defciency | 0.02 | |||
| IgG | 0/6 | 1/6 (16.7) | 5/6 (83.3) | |
| IgG + IgM | 0/4 | 1/4 (25) | 3/4 (75) | |
| IgG + IgA | 0/4 | 2/4 (50) | 2/4 (50) | |
| IgG + IgA + IgM | 7/16 (43.8) | 7/16 (43.8) | 2/16 (12.5) | |
| GLILD | 0.004 | |||
| YES | 3/3 (100) | 0 | 0 | |
| NO | 12/27 (44.4) | 11/27 (40.7%) | 4/27 (14.8) | |
| Immunosuppressive drugs < 3 months | 0.05 | |||
| YES | 3/5 (60) | 0 | 2/5 (40) | |
| NO | 4/25 (16) | 11/25 (44) | 10/25 (40) | |
| Switched B cells (mean%, range) | 1.74 (0–9) | 15.46 (0–59) | 11.80 (5–19) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracke, C.; Miranda, C.; González, S.; Casas, I.; Cardona, P.J.; Benitez, R.M.; Sopena, N.; Reynaga, E.A.; Massanella, M.; Clotet, B.; et al. Correlation between Clinical and Immunological Variables and Humoral Response to SARS-CoV-2 Vaccination in Adult Patients with Antibody Deficiency Disorders. Pathogens 2022, 11, 1364. https://doi.org/10.3390/pathogens11111364
Bracke C, Miranda C, González S, Casas I, Cardona PJ, Benitez RM, Sopena N, Reynaga EA, Massanella M, Clotet B, et al. Correlation between Clinical and Immunological Variables and Humoral Response to SARS-CoV-2 Vaccination in Adult Patients with Antibody Deficiency Disorders. Pathogens. 2022; 11(11):1364. https://doi.org/10.3390/pathogens11111364
Chicago/Turabian StyleBracke, Carmen, Cristina Miranda, Sandra González, Irma Casas, Pere Joan Cardona, Rosa Maria Benitez, Nieves Sopena, Esteban Alberto Reynaga, Marta Massanella, Bonaventura Clotet, and et al. 2022. "Correlation between Clinical and Immunological Variables and Humoral Response to SARS-CoV-2 Vaccination in Adult Patients with Antibody Deficiency Disorders" Pathogens 11, no. 11: 1364. https://doi.org/10.3390/pathogens11111364
APA StyleBracke, C., Miranda, C., González, S., Casas, I., Cardona, P. J., Benitez, R. M., Sopena, N., Reynaga, E. A., Massanella, M., Clotet, B., Carrillo, J., Mateu, L., & Pedro-Botet, M. L. (2022). Correlation between Clinical and Immunological Variables and Humoral Response to SARS-CoV-2 Vaccination in Adult Patients with Antibody Deficiency Disorders. Pathogens, 11(11), 1364. https://doi.org/10.3390/pathogens11111364










