Culture-Independent Genotyping Improves Surveillance of Neisseria gonorrhoeae, Especially in Oropharyngeal Samples, the Netherlands, 2017 to 2018
Abstract
1. Introduction
2. Results
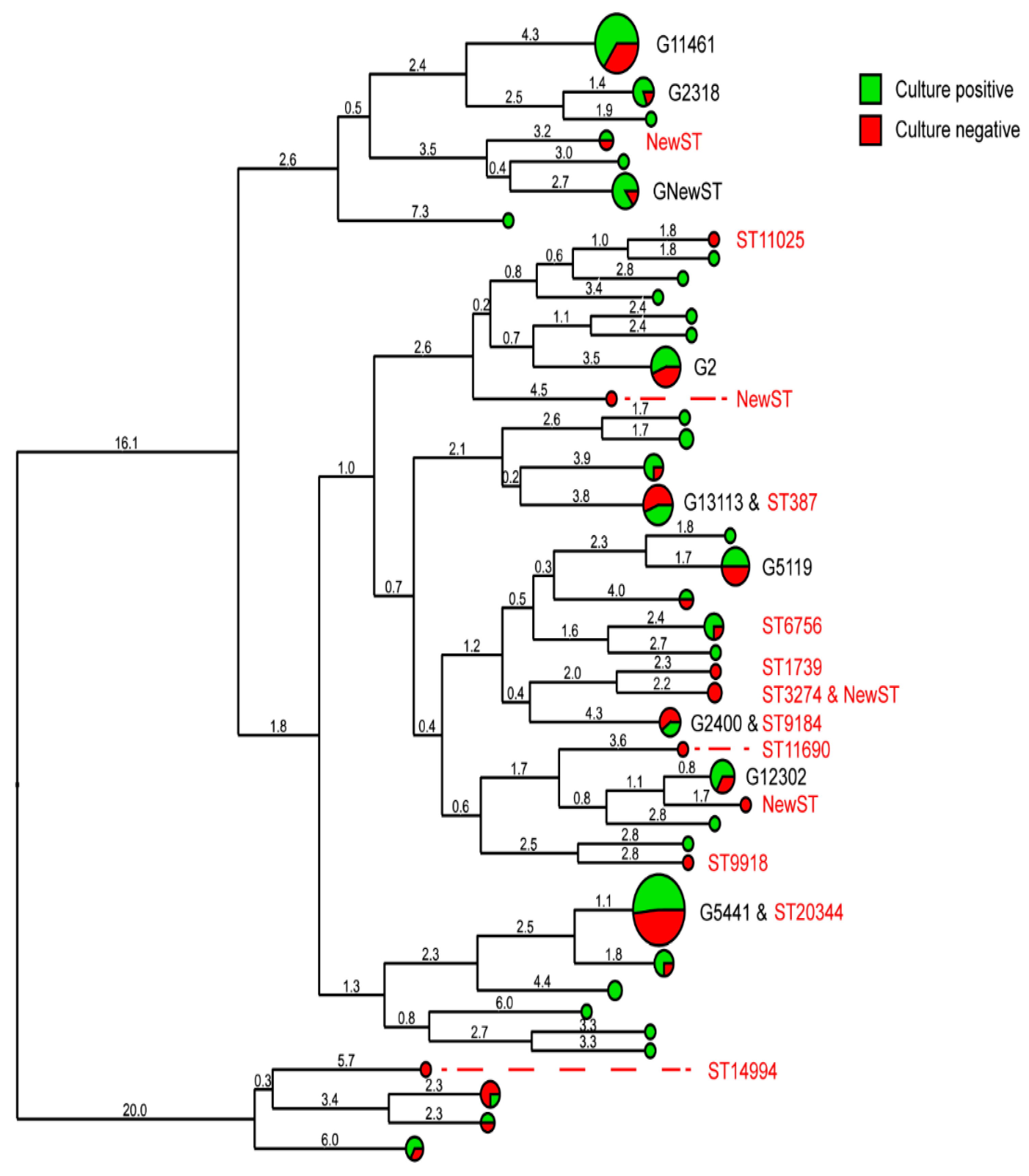
2.1. Culture-Independent Typing of Clinical Samples
2.2. Comparison of Surveillance Methods
2.3. Sequence Types and Genogroups
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. Culture-Independent Genotyping
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Global Health Sector Strategy on Sexually Transmitted INFECTIONS, 2016–2021; World Health Organisation: Geneva, Switerland, 2016.
- Suay-García, B.; Pérez-Gracia, M.T. Drug-resistant Neisseria gonorrhoeae: Latest developments. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1065–1071. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Response Plan to Control and Manage the Threat of Multidrug-Resistant Gonorrhoea in Europe. 2019. Available online: http://www.ecdc.europa.eu/en/publications/Publications/1206-ECDC-MDR-gonorrhoea-response-plan.pdf (accessed on 27 June 2022).
- Cole, M.J.; Quinten, C.; Jacobsson, S.; Day, M.; Amato-Gauci, A.J.; Woodford, N.; Spiteri, G.; Unemo, M.; Stary, A.; Haller, M.; et al. The European gonococcal antimicrobial surveillance programme (Euro-GASP) appropriately reflects the antimicrobial resistance situation for Neisseria gonorrhoeae in the European Union/European Economic Area. BMC Infect. Dis. 2019, 19, 1040. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Gonococcal Antimicrobial Susceptibility Surveillance in Europe 2013; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2013; ISBN 9789291932351. [Google Scholar]
- Martin, I.M.C.; Ison, C.A.; Aanensen, D.M.; Fenton, K.A.; Spratt, B.G. Rapid Sequence-Based Identification of Gonococcal Transmission Clusters in a Large Metropolitan Area. J. Infect. Dis. 2004, 189, 1497–1505. [Google Scholar] [CrossRef]
- Unemo, M.; Dillon, J.A.R. Review and international recommendation of methods for typing neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin. Microbiol. Rev. 2011, 24, 447–458. [Google Scholar] [CrossRef]
- Chisholm, S.A.; Unemo, M.; Quaye, N.; Johansson, E.; Cole, M.J.; Ison, C.A.; Van de Laar, M.J.W. Molecular epidemiological typing within the European Gonococcal Antimicrobial Resistance Surveillance Programme reveals predominance of a multidrugresistant clone. Eurosurveillance 2013, 18, 20358. [Google Scholar] [CrossRef] [PubMed]
- Papp, J.R.; Schachter, J.; Gaydos, C.A.; Pol, B. Van Der Recommendations for the Laboratory-Based Detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014, 63, 1–19. [Google Scholar]
- Staritsky, L.E.; Visser, M.; van Aar, F.; Op de Coul, E.L.M.; Heijne, J.C.M.; van Wees, D.A.; Kusters, J.M.A.; Alexiou, Z.W.; de Vries, A.; Götz, H.M.; et al. Sexually Transmitted Infections in the Netherlands in 2020. 2020. Available online: https://www.rivm.nl/bibliotheek/rapporten/2021-0052.pdf (accessed on 27 June 2022).
- Lewis, D.A. Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex. Transm. Infect. 2015, 91, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.; Rane, V.; Fairley, C.K.; Whiley, D.M.; Bradshaw, C.S.; Bissessor, M.; Chen, M.Y. Sampling technique is important for optimal isolation of pharyngeal gonorrhoea. Sex. Transm. Infect. 2013, 89, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, L.H.; Johnson, R.E.; Cheng, H.; Markowitz, L.E.; Papp, J.R.; Edward, I.W.H. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J. Clin. Microbiol. 2009, 47, 902–907. [Google Scholar] [CrossRef] [PubMed]
- van der Veer, B.M.J.W.; Wolffs, P.F.G.; Hoebe, C.J.P.A.; Dukers-Muijrers, N.H.T.M.; van Alphen, L.B. Culture-free genotyping of Neisseria gonorrhoeae revealed distinct strains at different anatomical sites in a quarter of patients, the Netherlands, 2012 to 2016. Eurosurveillance 2018, 23, 1800253. [Google Scholar] [CrossRef]
- Korbie, D.J.; Mattick, J.S. Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nat. Protoc. 2008, 3, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Lynn, F.; Hobbs, M.M.; Zenilman, J.M.; Behets, F.M.T.F.; Van Damme, K.; Rasamindrakotroka, A.; Bash, M.C. Genetic typing of the porin protein of Neisseria gonorrhoeae from clinical noncultured samples for strain characterization and identification of mixed gonococcal infections. J. Clin. Microbiol. 2005, 43, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Trembizki, E.; Doyle, C.; Buckley, C.; Jennison, A.; Smith, H.; Bates, J.; Sloots, T.; Nissen, M.; Lahra, M.M.; Whiley, D. Estimating the prevalence of mixed-type gonococcal infections in Queensland, Australia. Sex. Health 2015, 12, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st Century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed]
- Slurink, I.; van Aar, F. Sexually Transmitted Infections in the Netherlands in 2018; National Insitute for Public Health and the Enviroment RIVM: Bilthoven, The Netherland, 2018. [Google Scholar]
- Bissessor, M.; Tabrizi, S.N.; Fairley, C.K.; Danielewski, J.; Whitton, B.; Bird, S.; Garland, S.; Chen, M.Y. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: Implications for gonococcal detection, transmission, and control. J. Clin. Microbiol. 2011, 49, 4304–4306. [Google Scholar] [CrossRef] [PubMed]
- Van Dommelen, L.; Wolffs, P.F.G.; Van Tiel, F.H.; Dukers, N.; Herngreen, S.B.; Bruggeman, C.A.; Hoebe, C.J.P.A. Influence of temperature, medium, and storage duration on Chlamydia trachomatis DNA detection by PCR. J. Clin. Microbiol. 2013, 51, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Bilek, N.; Martin, I.M.; Bell, G.; Kinghorn, G.R.; Ison, C.A.; Spratt, B.G. Concordance between Neisseria gonorrhoeae genotypes recovered from known sexual contacts. J. Clin. Microbiol. 2007, 45, 3564–3567. [Google Scholar] [CrossRef] [PubMed]

| Culture-Positive | Culture-Negative | Typing-Rate Culture-Independent | Typing-Rate Culture-Dependent * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample material | Typed | Failed | Total | Typed | Failed | Total | Culture positive | Culture negative | Total | Total | p-value ** |
| Urine (n = 102) | 60 (2 mixed) | 15 (4 mixed) | 75 | 19 | 8 (2 mixed) | 27 | 80.0% | 70.4% | 77.5% | 73.5% (75/102) | 0.63 |
| Vaginal swab (n = 22) | 8 | 1 (1 mixed) | 9 | 11 | 2 | 13 | 88.9% | 84.6% | 86.4% | 40.9% (9/22) | 0.002 |
| Anorectal swab (n = 56) | 23 | 7 (2 mixed) | 30 | 18 | 8 (1 mixed) | 26 | 76.7% | 69.2% | 73.2% | 53.6% (30/56) | 0.03 |
| Oropharyngeal swab (n = 16) | 1 | 0 | 1 | 12 | 3 (1 mixed) | 15 | 100.0% | 80.0% | 81.3% | 6.3% (1/16) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slaats, M.H.C.; van der Veer, B.M.J.W.; van Alphen, L.B.; Hoebe, C.J.P.A.; Dukers-Muijrers, N.H.T.M.; Wolffs, P.F.G. Culture-Independent Genotyping Improves Surveillance of Neisseria gonorrhoeae, Especially in Oropharyngeal Samples, the Netherlands, 2017 to 2018. Pathogens 2022, 11, 1344. https://doi.org/10.3390/pathogens11111344
Slaats MHC, van der Veer BMJW, van Alphen LB, Hoebe CJPA, Dukers-Muijrers NHTM, Wolffs PFG. Culture-Independent Genotyping Improves Surveillance of Neisseria gonorrhoeae, Especially in Oropharyngeal Samples, the Netherlands, 2017 to 2018. Pathogens. 2022; 11(11):1344. https://doi.org/10.3390/pathogens11111344
Chicago/Turabian StyleSlaats, Michiel H. C., Brian M. J. W. van der Veer, Lieke B. van Alphen, Christian J. P. A. Hoebe, Nicole H. T. M. Dukers-Muijrers, and Petra F. G. Wolffs. 2022. "Culture-Independent Genotyping Improves Surveillance of Neisseria gonorrhoeae, Especially in Oropharyngeal Samples, the Netherlands, 2017 to 2018" Pathogens 11, no. 11: 1344. https://doi.org/10.3390/pathogens11111344
APA StyleSlaats, M. H. C., van der Veer, B. M. J. W., van Alphen, L. B., Hoebe, C. J. P. A., Dukers-Muijrers, N. H. T. M., & Wolffs, P. F. G. (2022). Culture-Independent Genotyping Improves Surveillance of Neisseria gonorrhoeae, Especially in Oropharyngeal Samples, the Netherlands, 2017 to 2018. Pathogens, 11(11), 1344. https://doi.org/10.3390/pathogens11111344







