β-Carboline Alkaloids from Peganum harmala Inhibit Fusarium oxysporum from Codonopsis radix through Damaging the Cell Membrane and Inducing ROS Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of F. oxysporum
2.2. Chemicals
2.3. Inhibition of Total Alkaloids on Mycelial Growth
2.4. Inhibition of Five βCs on Mycelial Growth and IC50 CALCULATION
2.5. Determination of Minimal Inhibitory Concentration (MIC)
2.6. Scanning Electron Microscopy (SEM)
2.7. Transmission Electron Microscopy (TEM)
2.8. Evaluation of Release of Cell Components
2.9. Measurement of Electrical Conductivity
2.10. ROS Assay
2.11. Annexin V-FITC/PI Double Staining Assay
2.12. Transcriptomic Analysis
2.13. Statistical Analysis
3. Results
3.1. Inhibition of Total Alkaloid Extracts from P. harmala on Mycelial Growth
3.2. Inhibition of Five Target βCs on Mycelial Growth
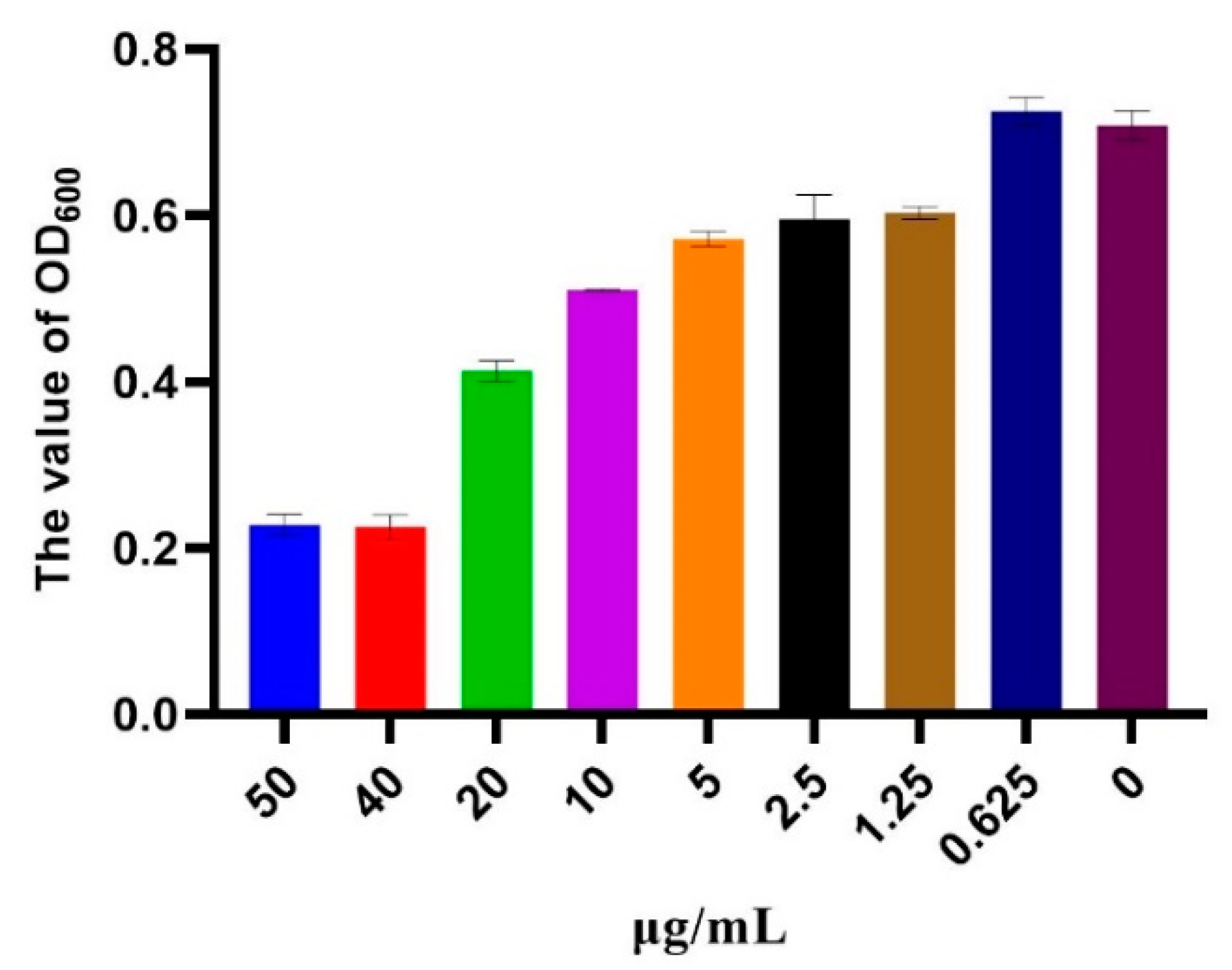
3.3. MIC
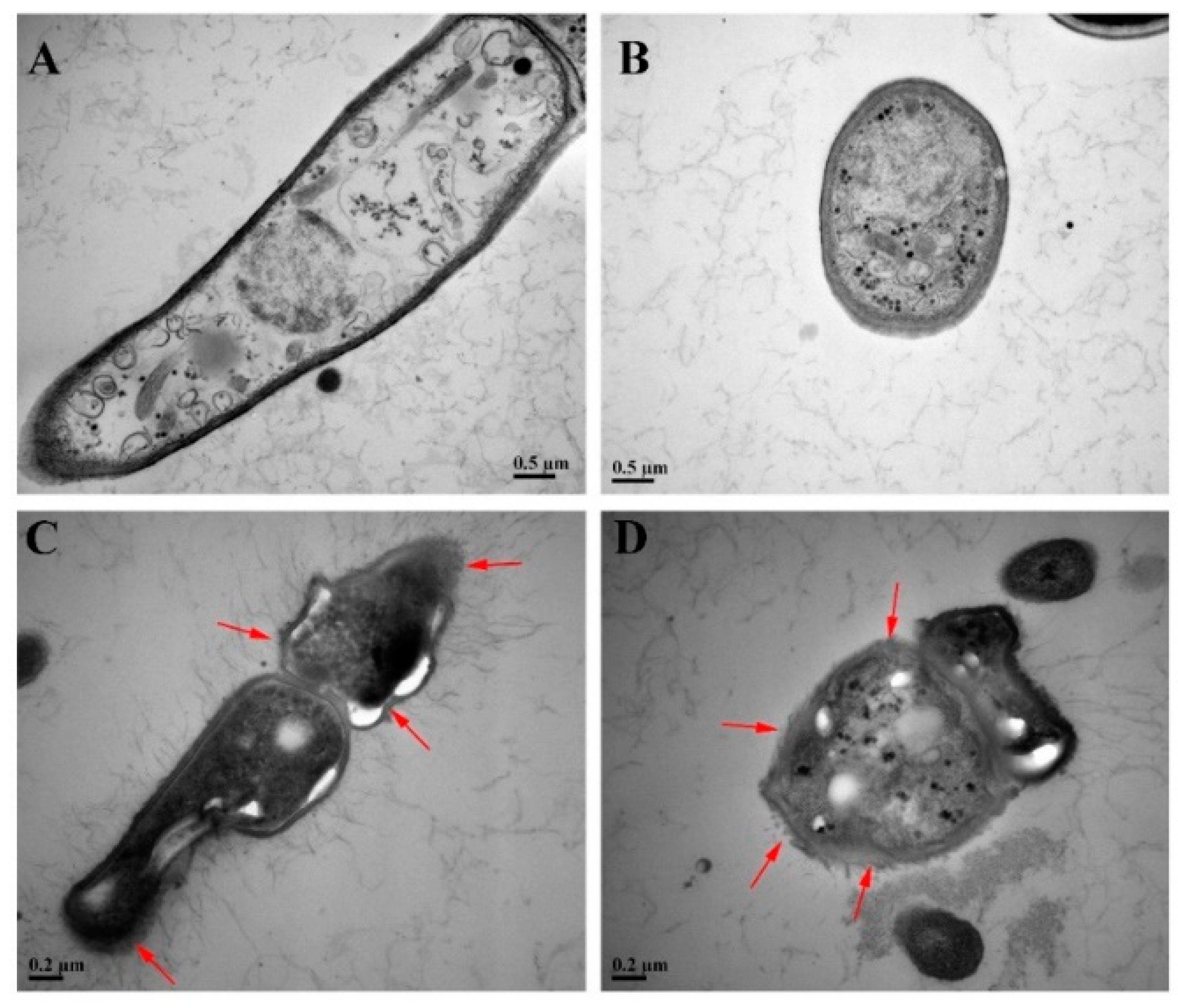
3.4. SEM
3.5. TEM
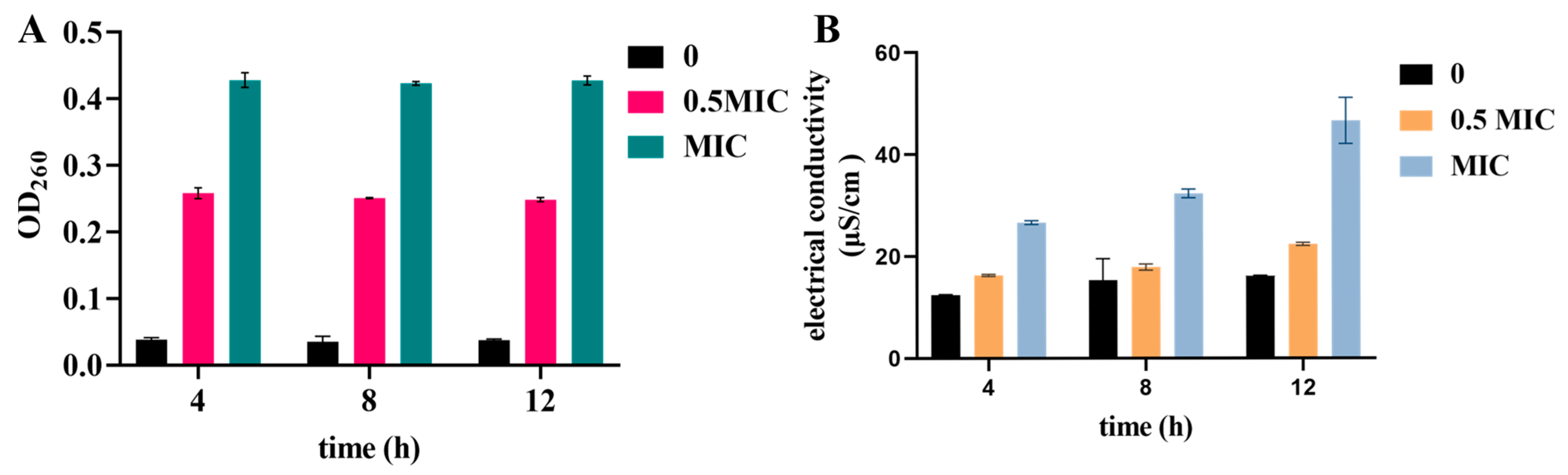
3.6. Detection of Release of Cell Components and Electrical Conductivity
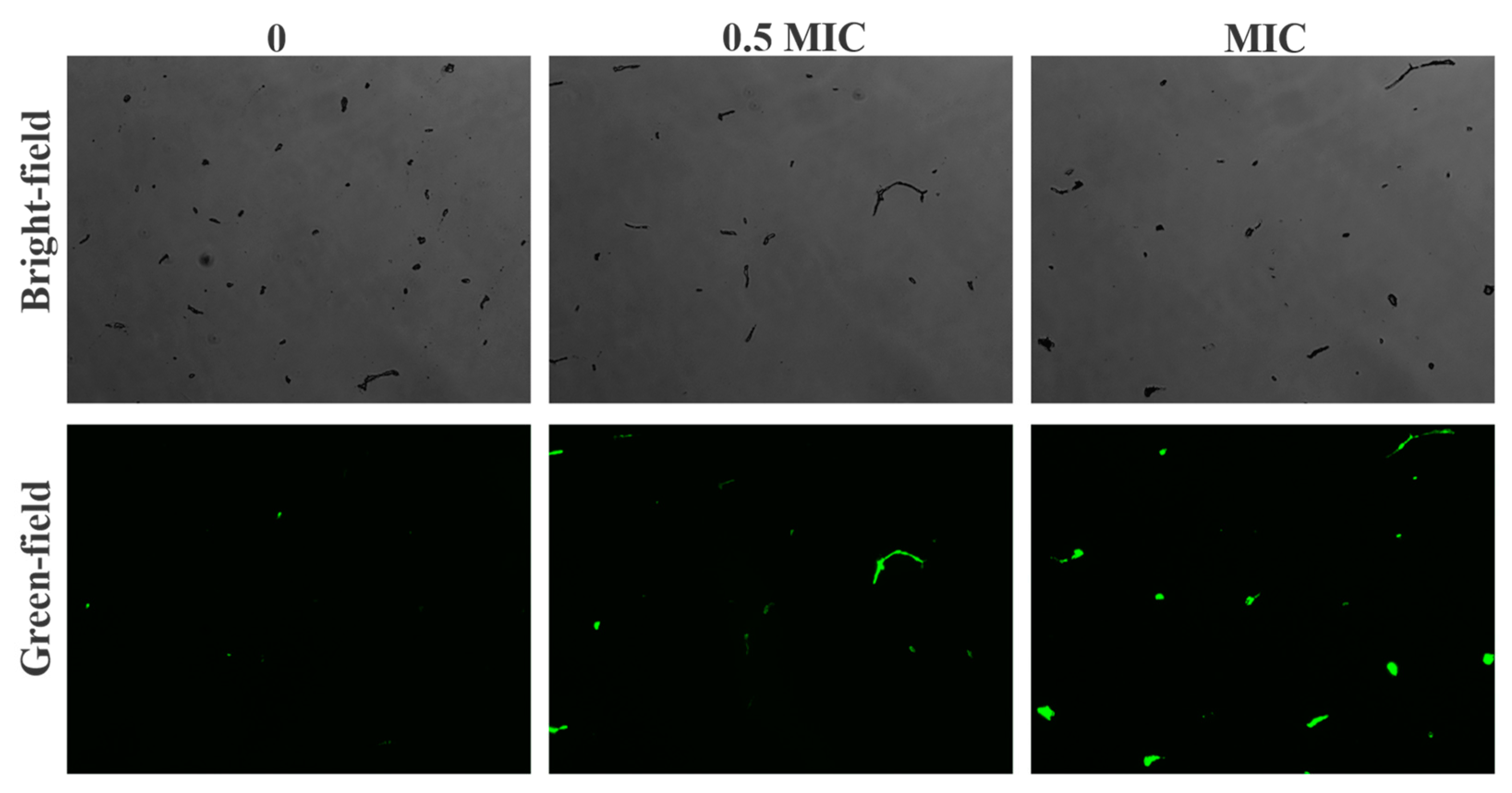
3.7. Harmane Induced Accumulation of ROS
3.8. Cell Death Analysis
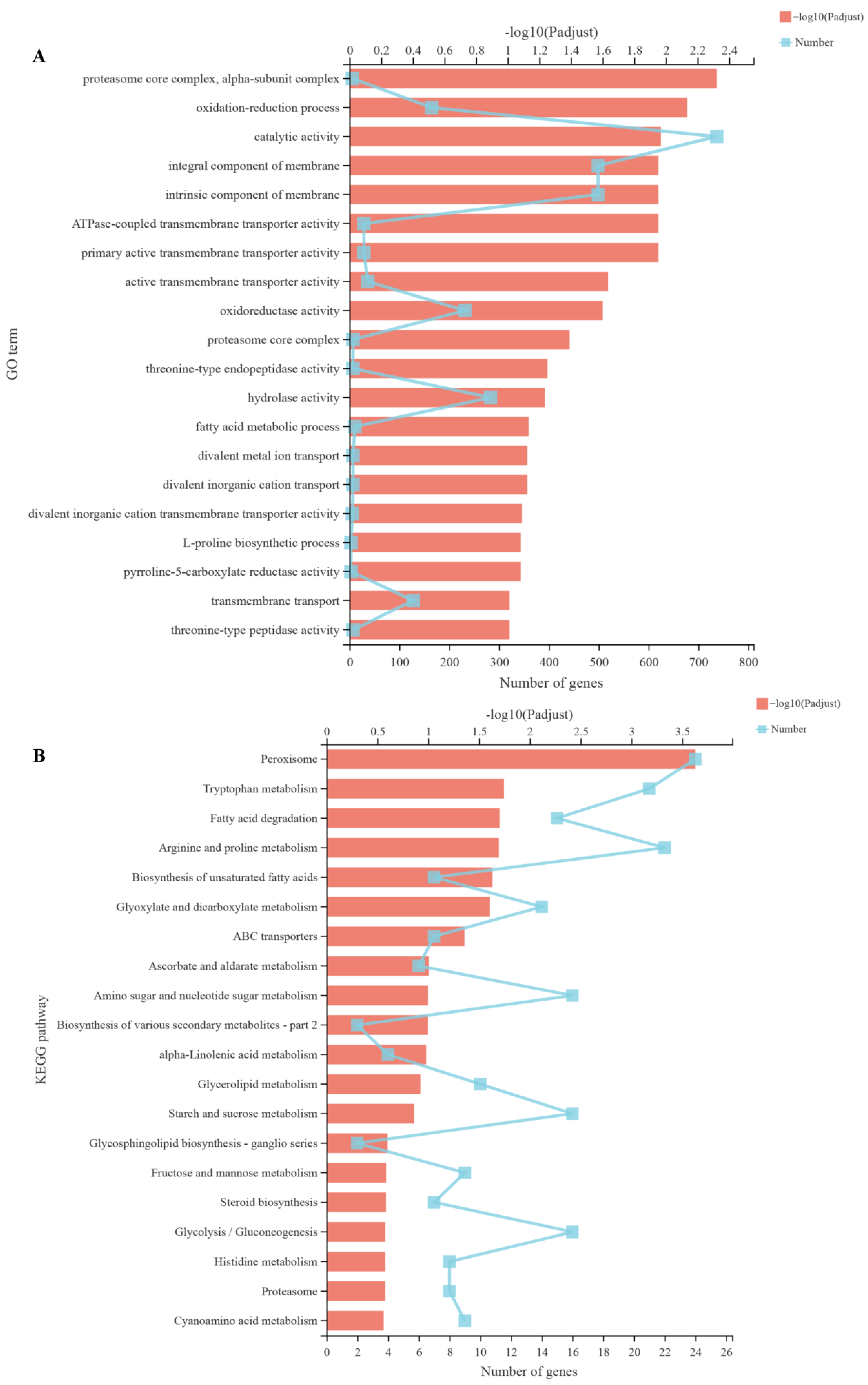
3.9. Effect of Harmane on the Transcriptome of F. oxysporum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, X.F.; Wang, S.; Kang, C.Z.; Lyu, C.G.; Guo, L.P.; Huang, L.Q. Chinese medicinal materials industry during the 14th Five-Year Plan period: Trends and development suggestions. China J. Chin. Mater. Med. 2022, 47, 1144–1152. [Google Scholar] [CrossRef]
- Zhao, X.; Yue, L.; Uwaremwe, C.; Liu, Y.; Tian, Y.; Zhao, H.J.; Zhou, Q.; Zhang, Y.B.; Wang, R.Y. First report of root rot caused by the Fusarium oxysporum species complex on Codonopsis pilosula in China. Plant Dis. 2021, 105, 3742. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Lei, M.Y.; Pu, S.C.; Xiao, Z.; Cao, H.Q.; Yang, C.Q. Fungal disease survey and pathogen identification on Codonopsis tangshen in Chongqing. J. Chin. Med. Mater. 2015, 38, 1119–1122. [Google Scholar]
- Punja, Z.K.; Wan, A.; Goswami, R.S. Root rot and distortion of ginseng seedling roots caused by Fusarium oxysporum. Can. J. Plant Pathol. 2008, 30, 565–574. [Google Scholar] [CrossRef]
- Di Primo, P.; Cappelli, C. Preliminary characterization of Fusarium oxysporum f. sp. gladioli causing Fusarium Corm rot of Saffron in Italy. Plant Dis. 2000, 84, 806. [Google Scholar] [CrossRef]
- Gao, W.W.; Zhang, X.M.; Tian, G.L.; Jiao, X.L. Plant diseases of traditional Chinese medicines:20 years of progress in research on understanding and management. Plant Prot. 2016, 42, 15–23. [Google Scholar]
- Gao, F.; Ren, X.X.; Wang, M.L.; Qin, X.M. Research progress in root rot diseases of Chinese herbal medicine and control strategy by antagonistic microorganisms. China J. Chin. Mater. Med. 2015, 40, 4122–4126. [Google Scholar]
- Raza, W.; Ling, N.; Zhang, R.F.; Huang, Q.W.; Xu, Y.C.; Shen, Q.R. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 2017, 37, 202–212. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.R.; Leandro, L.F.S.; Munkvold, G.P. Effects of temperature and pH on Fusarium oxysporum and soybean seedling disease. Plant Dis. 2019, 103, 3234–3243. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staver, C.; Pemsl, D.E.; Scheerer, L.; Perez Vicente, L.; Dita, M. Ex ante assessment of returns on research investments to address the impact of Fusarium Wilt tropical race 4 on global banana production. Front. Plant Sci. 2020, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Portal Gonzalez, N.; Soler, A.; Ribadeneira, C.; Solano, J.; Portieles, R.; Herrera Isla, L.; Companioni, B.; Borras-Hidalgo, O.; Santos Bermudez, R. Phytotoxic metabolites produce by Fusarium oxysporum f. sp. cubense race 2. Front. Microbiol. 2021, 12, 629395. [Google Scholar] [CrossRef]
- Wei, J.; Wu, B. Chemistry and bioactivities of secondary metabolites from the genus Fusarium. Fitoterapia 2020, 146, 104638. [Google Scholar] [CrossRef]
- Porter, J.K.; Bacon, C.W.; Wray, E.M.; Hagler, W.M., Jr. Fusaric acid in Fusarium moniliforme cultures, corn, and feeds toxic to livestock and the neurochemical effects in the brain and pineal gland of rats. Nat. Toxins 1995, 3, 91–100. [Google Scholar] [CrossRef]
- Ghazi, T.; Nagiah, S.; Dhani, S.; Chuturgoon, A.A. Fusaric acid-induced epigenetic modulation of hepatic H3K9me3 triggers apoptosis in vitro and in vivo. Epigenomics 2020, 12, 955–972. [Google Scholar] [CrossRef]
- Onwona-Kwakye, M.; Hogarh, J.N.; Van den Brink, P.J. Environmental risk assessment of pesticides currently applied in Ghana. Chemosphere 2020, 254, 126845. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, R.K.; Singh, N. Leaching behaviour of azoxystrobin and metabolites in soil columns. Pest Manag. Sci. 2009, 65, 1009–1014. [Google Scholar] [CrossRef]
- Kai, K.; Wang, R.; Bi, W.; Ma, Z.; Shi, W.; Ye, Y.; Zhang, D. Chlorogenic acid induces ROS-dependent apoptosis in Fusarium fujikuroi and decreases the postharvest rot of cherry tomato. World J. Microbiol. Biotechnol. 2021, 37, 93. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- De Oliveira Pereira, F.; Mendes, J.M.; de Oliveira Lima, E. Investigation on mechanism of antifungal activity of eugenol against Trichophyton rubrum. Med. Mycol. 2013, 51, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, C.Y.; Kai, K.; Wang, X.F.; Shi, W.; Zhang, D.F.; Liu, Y.S. Curcumin inhibits gray mold development in kiwifruit by targeting mitogen-activated protein kinase (MAPK) cascades in Botrytis cinerea. Postharvest Biol. Technol. 2019, 151, 152–159. [Google Scholar] [CrossRef]
- Li, S.; Cheng, X.; Wang, C. A review on traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the genus Peganum. J. Ethnopharmacol. 2017, 203, 127–162. [Google Scholar] [CrossRef]
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol. 2013, 55, 202–228. [Google Scholar] [CrossRef]
- Cheng, X.M.; Zhao, T.; Yang, T.; Wang, C.H.; Bligh, S.W.; Wang, Z.T. HPLC fingerprints combined with principal component analysis, hierarchical cluster analysis and linear discriminant analysis for the classification and differentiation of Peganum sp. indigenous to China. Phytochem. Anal. 2010, 21, 279–289. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, S.; Wang, C. Antibacterial, antifungal, antiviral, and antiparasitic activities of Peganum harmala and its ingredients: A review. Molecules 2022, 27, 4161. [Google Scholar] [CrossRef]
- Hajji, A.; Bnejdi, F.; Saadoun, M.; Ben Salem, I.; Nehdi, I.; Sbihi, H.; Alharthi, F.A.; El Bok, S.; Boughalleb-M’Hamdi, N. High reserve in delta-Tocopherol of Peganum harmala seeds oil and antifungal activity of oil against ten plant pathogenic fungi. Molecules 2020, 25, 4569. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wei, J.Y.; Wei, Y.Y.; Han, P.P.; Dai, K.; Zou, X.R.; Jiang, S.; Xu, F.; Wang, H.F.; Sun, J.C.; et al. Tea tree oil controls brown rot in peaches by damaging the cell membrane of Monilinia fructicola. Postharvest Biol. Technol. 2021, 175, 11474. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Zhao, W.; Xie, Y. (E)-2-Hexenal, as a potential natural antifungal compound, inhibits Aspergillus Flavus spore germination by disrupting mitochondrial energy metabolism. J. Agric. Food Chem. 2019, 67, 1138–1145. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, X.; Liu, W.; Chou, G.; Wang, Z.; Wang, C. Potent AChE and BChE inhibitors isolated from seeds of Peganum harmala Linn by a bioassay-guided fractionation. J. Ethnopharmacol. 2015, 168, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xu, Z.; Deng, R.; Huang, L.; Zheng, R.; Kong, Q. Peppermint essential oil suppresses Geotrichum citri-aurantii growth by destructing the cell structure, internal homeostasis, and cell cycle. J. Agric. Food Chem. 2021, 69, 7786–7797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, F.; Zeng, M.; Zhang, J.; Liu, Y.; Xin, C.; Mao, Y.; Song, Z. Antifungal activity of sodium new houttuyfonate against Aspergillus fumigatus in vitro and in vivo. Front. Microbiol. 2022, 13, 856272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qiang, R.; Zhou, Z.; Pan, Y.; Yu, S.; Yuan, W.; Cheng, J.; Wang, J.; Zhao, D.; Zhu, J.; et al. Biocontrol and action mechanism of Bacillus subtilis lipopeptides’ fengycins Against Alternaria solani in potato as assessed by a transcriptome analysis. Front. Microbiol. 2022, 13, 861113. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Synergistic inhibition effect of citral and eugenol against Aspergillus niger and their application in bread preservation. Food Chem. 2020, 310, 125974. [Google Scholar] [CrossRef]
- Sun, C.Q.; Peng, J.; Yang, L.B.; Jiao, Z.L.; Zhou, L.X.; Tao, R.Y.; Zhu, L.J.; Tian, Z.Q.; Huang, M.J.; Guo, G. A cecropin-4 derived peptide c18 inhibits Candida albicans by disturbing mitochondrial function. Front. Microbiol. 2022, 13, 872322. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and prospects of botanical biopesticides in Europe and Mediterranean countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, M.; Luo, H. Identification and antimicrobial activity of two alkaloids from traditional Chinese medicinal plant Tinospora capillipes. Ind. Crops Prod. 2012, 37, 298–302. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, J.; Hui, N.; Zhou, T.; Wang, L.; Ma, Y.; Zhang, X. Identification of root rot pathogen of Glycyrrhiza and indoor toxicity text. Acta Agric. Boreali-Occident. Sin. 2013, 22, 98–102. [Google Scholar]
- Mbasa, W.V.; Nene, W.A.; Kapinga, F.A.; Lilai, S.A.; Tibuhwa, D.D. Characterization and chemical management of cashew fusarium wilt disease caused by Fusarium oxysporum in Tanzania. Crop. Prot. 2021, 139, 105379. [Google Scholar] [CrossRef]
- Sekhon, A.S.; Padhye, A.A.; Garg, A.K.; Ahmad, H.; Moledina, N. In vitro sensitivity of medically significant Fusarium species to various antimycotics. Chemotherapy 1994, 40, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, Z.; Li, M.; Xing, M.; Xian, T.; Tu, K. Carvacrol and eugenol effectively inhibit Rhizopus stolonifer and control postharvest soft rot decay in peaches. J. Appl. Microbiol. 2018, 124, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Khadraoui, N.; Essid, R.; Jallouli, S.; Damergi, B.; Ben Takfa, I.; Abid, G.; Jedidi, I.; Bachali, A.; Ayed, A.; Limam, F.; et al. Antibacterial and antibiofilm activity of Peganum harmala seed extract against multidrug-resistant Pseudomonas aeruginosa pathogenic isolates and molecular mechanism of action. Arch. Microbiol. 2022, 204, 133. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, G.M.; Cerioni, L.; Gonzalez, M.M.; Cabrerizo, F.M.; Volentini, S.I.; Rapisarda, V.A. UVA photoactivation of harmol enhances its antifungal activity against the phytopathogens Penicillium digitatum and Botrytis cinerea. Front. Microbiol. 2017, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dangol, S.; Wang, J.; Jwa, N.S. Focal accumulation of ros can block Pyricularia Oryzae effector BAS4-expression and prevent infection in rice. Int. J. Mol. Sci. 2020, 21, 6196. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Hwang, I.S.; Liu, Q.H.; Woo, E.R.; Lee, D.G. (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie 2012, 94, 1784–1793. [Google Scholar] [CrossRef]
- Hirayama, T.; Miyazaki, T.; Sumiyoshi, M.; Ashizawa, N.; Takazono, T.; Yamamoto, K.; Imamura, Y.; Izumikawa, K.; Yanagihara, K.; Kohno, S.; et al. ERG3-encoding sterol C5,6-Desaturase In Candida albicans is required for virulence in an enterically infected invasive Candidiasis mouse model. Pathogens 2020, 10, 23. [Google Scholar] [CrossRef]
- Liu, J.; Chai, X.; Guo, T.; Wu, J.; Yang, P.; Luo, Y.; Zhao, H.; Zhao, W.; Nkechi, O.; Dong, J.; et al. Disruption of the ergosterol biosynthetic pathway results in increased membrane permeability, causing overproduction and secretion of extracellular monascus pigments in submerged fermentation. J. Agric. Food Chem. 2019, 67, 13673–13683. [Google Scholar] [CrossRef]
- OuYang, Q.; Liu, Y.; Oketch, O.R.; Zhang, M.; Shao, X.; Tao, N. Citronellal exerts its antifungal activity by targeting ergosterol biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Famous, E.; Pan, S.; Peng, X.; Tian, J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021, 346, 128845. [Google Scholar] [CrossRef]


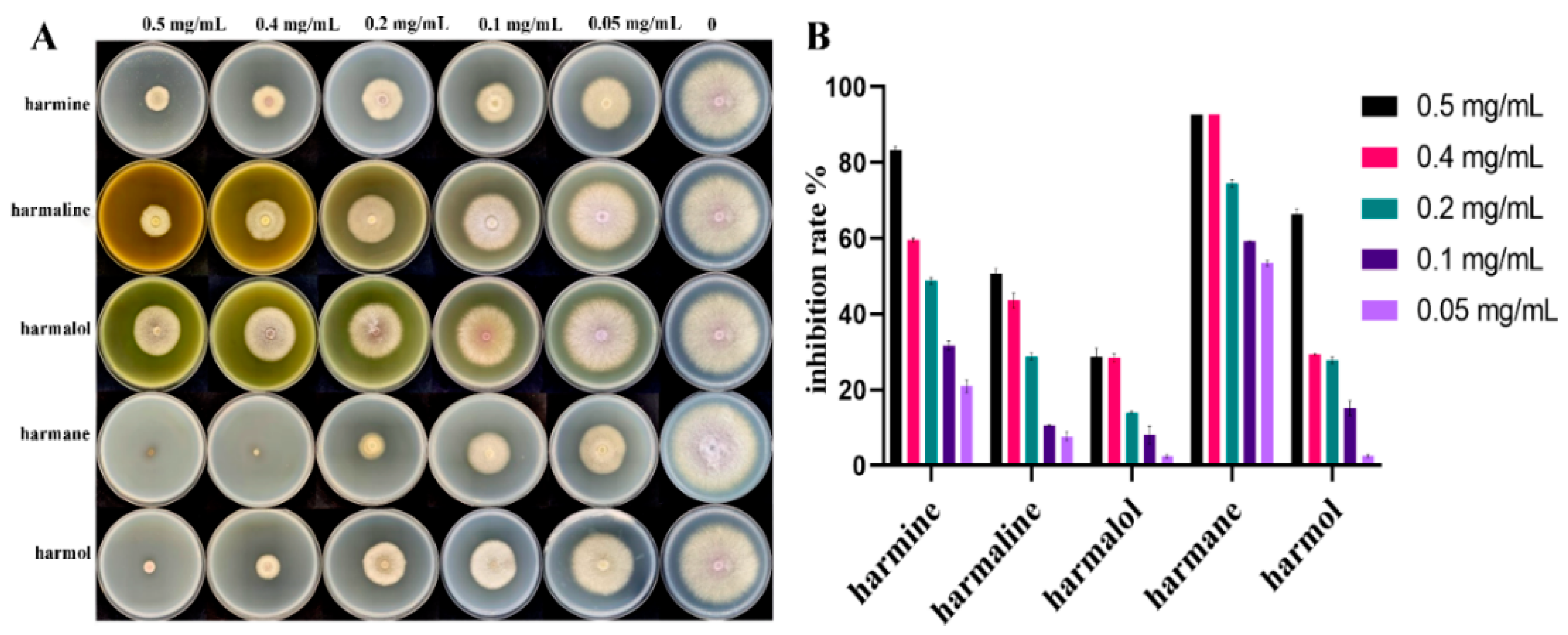


| βCs | Harmaine | Harmaline | Harmalol | Harmane | Harmol |
|---|---|---|---|---|---|
| IC50 (mg/mL) | 0.143 | 0.331 | 0.798 | 0.050 | 0.161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhao, S.; Wang, C. β-Carboline Alkaloids from Peganum harmala Inhibit Fusarium oxysporum from Codonopsis radix through Damaging the Cell Membrane and Inducing ROS Accumulation. Pathogens 2022, 11, 1341. https://doi.org/10.3390/pathogens11111341
Zhu Z, Zhao S, Wang C. β-Carboline Alkaloids from Peganum harmala Inhibit Fusarium oxysporum from Codonopsis radix through Damaging the Cell Membrane and Inducing ROS Accumulation. Pathogens. 2022; 11(11):1341. https://doi.org/10.3390/pathogens11111341
Chicago/Turabian StyleZhu, Zihao, Shujuan Zhao, and Changhong Wang. 2022. "β-Carboline Alkaloids from Peganum harmala Inhibit Fusarium oxysporum from Codonopsis radix through Damaging the Cell Membrane and Inducing ROS Accumulation" Pathogens 11, no. 11: 1341. https://doi.org/10.3390/pathogens11111341
APA StyleZhu, Z., Zhao, S., & Wang, C. (2022). β-Carboline Alkaloids from Peganum harmala Inhibit Fusarium oxysporum from Codonopsis radix through Damaging the Cell Membrane and Inducing ROS Accumulation. Pathogens, 11(11), 1341. https://doi.org/10.3390/pathogens11111341





