Influenza A Virus Exacerbates Group A Streptococcus Infection and Thwarts Anti-Bacterial Inflammatory Responses in Murine Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathogens
2.2. Animals
2.3. In Vivo Infection Models and Clinical Scoring
2.4. Eicosanoid Extraction and Analysis
2.5. Isolation of RNA and DNA from Lung Samples
2.6. Lung Pathogen Genetic Material and Gene Expression
2.7. Bone Marrow Derived Macrophage Infection Model
2.8. Single Cell Analysis by Flow Cytometry
2.9. Macrophage Gene Expression
2.10. Cytokine Analysis
2.11. Statistical Analysis
3. Results
3.1. Infection with Influenza A Virus H1N1 Caused Mild Symptoms and Reduced CCL2 in the Periphery
3.2. Group A Streptococcal Infection Was Aggravated Following Influenza A Virus Infection
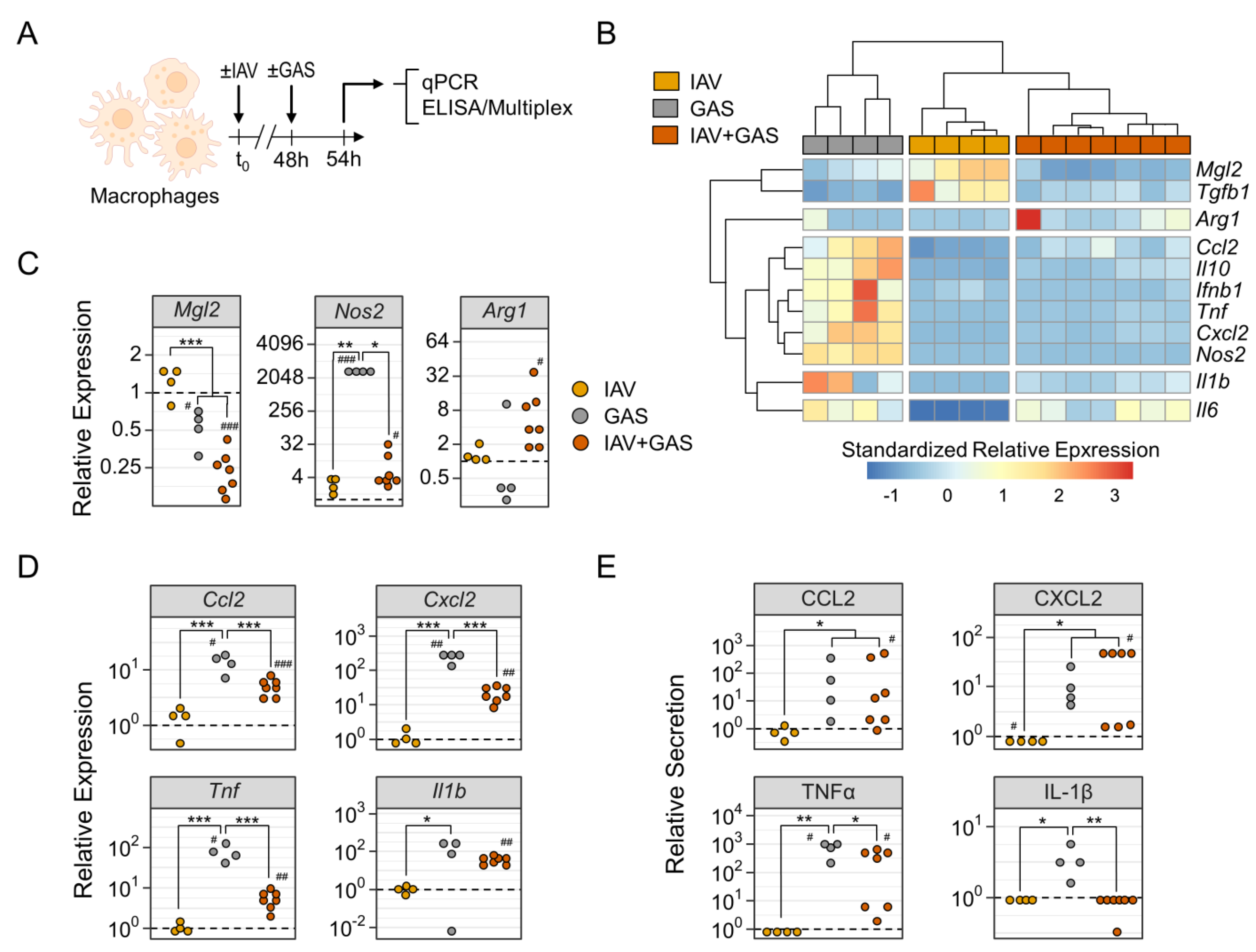
3.3. Preceding Influenza A Virus Infection Impacted on the Group A Streptococcus Induced Diversification of Macrophage Surface Expression Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siemens, N.; Oehmcke-Hecht, S.; Mettenleiter, T.C.; Kreikemeyer, B.; Valentin-Weigand, P.; Hammerschmidt, S. Port d’Entrée for Respiratory Infections—Does the Influenza A Virus Pave the Way for Bacteria? Front. Microbiol. 2017, 8, 2602. [Google Scholar] [CrossRef] [PubMed]
- Tjon-Kon-Fat, R.; Meerhoff, T.; Nikisins, S.; Pires, J.; Pereyaslov, D.; Gross, D.; Brown, C.; Drishti, A.; Hasibra, I.; Kota, M.; et al. The Potential Risks and Impact of the Start of the 2015–2016 Influenza Season in the WHO European Region: A Rapid Risk Assessment. Influenza Other Respi. Viruses 2016, 10, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHardy, A.C.; Adams, B. The Role of Genomics in Tracking the Evolution of Influenza A Virus. PLoS Pathog. 2009, 5, e1000566. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and Pandemic Potential of Swine-Origin H1N1 Influenza Virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The Mother of All Pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Brundage, J.F.; Shanks, G.D. Deaths from Bacterial Pneumonia during 1918–19 Influenza Pandemic. Emerg. Infect. Dis. 2008, 14, 1193–1199. [Google Scholar] [CrossRef]
- Tasher, D.; Stein, M.; Simões, E.A.F.; Shohat, T.; Bromberg, M.; Somekh, E. Invasive Bacterial Infections in Relation to Influenza Outbreaks, 2006-2010. Clin. Infect. Dis. 2011, 53, 1199–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, S.; Malito, E.; Rouse, S.L.; Abate, F.; Bensi, G.; Chiarot, E.; Micoli, F.; Mancini, F.; Gomes Moriel, D.; Grandi, G.; et al. Structure, Dynamics and Immunogenicity of a Catalytically Inactive CXC Chemokine-Degrading Protease SpyCEP from Streptococcus Pyogenes. Comput. Struct. Biotechnol. J. 2020, 18, 650–660. [Google Scholar] [CrossRef]
- Chaussee, M.S.; Sandbulte, H.R.; Schuneman, M.J.; DePaula, F.P.; Addengast, L.A.; Schlenker, E.H.; Huber, V.C. Inactivated and Live, Attenuated Influenza Vaccines Protect Mice against Influenza:Streptococcus Pyogenes Super-Infections. Vaccine 2011, 29, 3773–3781. [Google Scholar] [CrossRef]
- Teymournejad, O.; Montgomery, C.P. Evasion of Immunological Memory by S. Aureus Infection: Implications for Vaccine Design. Front. Immunol. 2021, 12, 633672. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Kawabata, S.; Nakagawa, I.; Okuno, Y.; Goto, T.; Sano, K.; Hamada, S. Influenza A Virus-Infected Hosts Boost an Invasive Type of Streptococcus Pyogenes Infection in Mice. J. Virol. 2003, 77, 4104–4112. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, S.; Kawabata, S.; Terao, Y.; Fujitaka, H.; Okuno, Y.; Hamada, S. The Streptococcus Pyogenes Capsule Is Required for Adhesion of Bacteria to Virus-Infected Alveolar Epithelial Cells and Lethal Bacterial-Viral Superinfection. Infect. Immun. 2004, 72, 6068–6075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Sluijs, K.F.; Nijhuis, M.; Levels, J.H.M.; Florquin, S.; Mellor, A.L.; Jansen, H.M.; van der Poll, T.; Lutter, R. Influenza-Induced Expression of Indoleamine 2,3-Dioxygenase Enhances Interleukin-10 Production and Bacterial Outgrowth during Secondary Pneumococcal Pneumonia. J. Infect. Dis. 2006, 193, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Li, S.; Southern, P.J.; Cleary, P.P. Streptococcal Modulation of Cellular Invasion via TGF-Β1 Signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 2380–2385. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.L.; Suso, K.; Allison, S.; Simon, A.; Schlenker, E.; Huber, V.C.; Chaussee, M.S. Binding Host Proteins to the M Protein Contributes to the Mortality Associated with Influenza–Streptococcus Pyogenes Superinfections. Microbiology 2017, 163, 1445–1456. [Google Scholar] [CrossRef]
- Korteweg, C.; Gu, J. Pathology, Molecular Biology, and Pathogenesis of Avian Influenza A (H5N1) Infection in Humans. Am. J. Pathol. 2008, 172, 1155–1170. [Google Scholar] [CrossRef] [Green Version]
- Plotkowski, M.C.; Bajolet-Laudinat, O.; Puchelle, E. Cellular and Molecular Mechanisms of Bacterial Adhesion to Respiratory Mucosa. Eur. Respir. J. 1993, 6, 903–916. [Google Scholar]
- Sun, K.; Metzger, D.W. Inhibition of Pulmonary Antibacterial Defense by Interferon-γ during Recovery from Influenza Infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef]
- Navarini, A.A.; Recher, M.; Lang, K.S.; Georgiev, P.; Meury, S.; Bergthaler, A.; Flatz, L.; Bille, J.; Landmann, R.; Odermatt, B.; et al. Increased Susceptibility to Bacterial Superinfection as a Consequence of Innate Antiviral Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 15535–15539. [Google Scholar] [CrossRef] [Green Version]
- Metzger, D.W.; Sun, K. Immune Dysfunction and Bacterial Coinfections Following Influenza. J. Immunol. 2013, 191, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Shahangian, A.; Chow, E.K.; Tian, X.; Kang, J.R.; Ghaffari, A.; Liu, S.Y.; Belperio, J.A.; Cheng, G.; Deng, J.C. Type I IFNs Mediate Development of Postinfluenza Bacterial Pneumonia in Mice. J. Clin. Investig. 2009, 119, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.; Goulding, J.; Patel, S.; Snelgrove, R.; Low, L.; Bebien, M.; Lawrence, T.; Van Rijt, L.S.; Lambrecht, B.N.; Sirard, J.C.; et al. Sustained Desensitization to Bacterial Toll-like Receptor Ligands after Resolution of Respiratory Influenza Infection. J. Exp. Med. 2008, 205, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Davis, K.M.; Weiser, J.N. Synergistic Stimulation of Type I Interferons during Influenza Virus Coinfection Promotes Streptococcus Pneumoniae Colonization in Mice. J. Clin. Investig. 2011, 121, 3657–3665. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.W. Pathogenesis of Group A Streptococcal Infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Musher, D. Trends in Bacteremic Infection Due to Streptococcus Pyogenes (Group A Streptococcus), 1986-1995. Emerg. Infect. Dis. 1996, 2, 54–56. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.L.; Huber, V.C.; Chaussee, M.S. The Association between Invasive Group A Streptococcal Diseases and Viral Respiratory Tract Infections. Front. Microbiol. 2016, 7, 342. [Google Scholar] [CrossRef] [Green Version]
- Jean, C.; Louie, J.K.; Glaser, C.A.; Harriman, K.; Hacker, J.K.; Aranki, F.; Bancroft, E.; Farley, S.; Ginsberg, M.; Hernandez, L.B.; et al. Invasive Group a Streptococcal Infection Concurrent with 2009 H1n1 Influenza. Clin. Infect. Dis. 2010, 50, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Zakikhany, K.; Degail, M.A.; Lamagni, T.; Waight, P.; Guy, R.; Zhao, H.; Efstratiou, A.; Pebody, R.; George, R.; Ramsay, M. Increase in Invasive Streptococcus Pyogenes and Streptococcus Pneumoniae Infections in England, December 2010 to January 2011. Eurosurveillance 2011, 16, 1–4. [Google Scholar] [CrossRef]
- Okamoto, S.; Nagase, S. Pathogenic Mechanisms of Invasive Group A Streptococcus Infections by Influenza Virus–Group A Streptococcus Superinfection. Microbiol. Immunol. 2018, 62, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Goldmann, O.; Rohde, M.; Chhatwal, G.S.; Medina, E. Role of Macrophages in Host Resistance to Group A Streptococci. Infect. Immun. 2004, 72, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, H.E.; Thomas, P.G.; McCullers, J.A. Depletion of Alveolar Macrophages during Influenza Infection Facilitates Bacterial Superinfections. J. Immunol. 2013, 191, 1250–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, D.; Methling, K.; Rothe, M.; Lalk, M. Eicosanoid Profile of Influenza A Virus Infected Pigs. Metabolites 2019, 9, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Stabenow, J.M.; Parvathareddy, J.; Wodowski, A.J.; Fabrizio, T.P.; Bina, X.R.; Zalduondo, L.; Bina, J.E. Visualization of Murine Intranasal Dosing Efficiency Using Luminescent Francisella Tularensis: Effect of Instillation Volume and Form of Anesthesia. PLoS ONE 2012, 7, e31359. [Google Scholar] [CrossRef] [Green Version]
- Volzke, J.; Schultz, D.; Kordt, M.; Müller, M.; Bergmann, W.; Methling, K.; Kreikemeyer, B.; Müller-Hilke, B. Inflammatory Joint Disease Is a Risk Factor for Streptococcal Sepsis and Septic Arthritis in Mice. Front. Immunol. 2020, 11, 579475. [Google Scholar] [CrossRef]
- Shrum, B.; Anantha, R.V.; Xu, S.X.; Donnelly, M.; Haeryfar, S.; McCormick, J.K.; Mele, T. A Robust Scoring System to Evaluate Sepsis Severity in an Animal Model. BMC Res. Notes 2014, 7, 233. [Google Scholar] [CrossRef] [Green Version]
- Amend, S.R.; Valkenburg, K.C.; Pienta, K.J. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J. Vis. Exp. 2016, 110, 53936. [Google Scholar] [CrossRef] [Green Version]
- Belkina, A.C.; Ciccolella, C.O.; Anno, R.; Halpert, R.; Spidlen, J.; Snyder-Cappione, J.E. Automated Optimized Parameters for T-Distributed Stochastic Neighbor Embedding Improve Visualization and Analysis of Large Datasets. Nat. Commun. 2019, 10, 5415. [Google Scholar] [CrossRef] [Green Version]
- Van Gassen, S.; Callebaut, B.; Van Helden, M.J.; Lambrecht, B.N.; Demeester, P.; Dhaene, T.; Saeys, Y. FlowSOM: Using Self-Organizing Maps for Visualization and Interpretation of Cytometry Data. Cytom. Part A 2015, 87, 636–645. [Google Scholar] [CrossRef]
- Gouwy, M.; Struyf, S.; Leutenez, L.; Pörtner, N.; Sozzani, S.; Van Damme, J. Chemokines and Other GPCR Ligands Synergize in Receptor-Mediated Migration of Monocyte-Derived Immature and Mature Dendritic Cells. Immunobiology 2014, 219, 218–229. [Google Scholar] [CrossRef]
- Gomes, R.N.; Teixeira-Cunha, M.G.A.; Figueiredo, R.T.; Almeida, P.E.; Alves, S.C.; Bozza, P.T.; Bozza, F.A.; Bozza, M.T.; Zimmerman, G.A.; Castro-Faria-Neto, H.C. Bacterial Clearance in Septic Mice Is Modulated by MCP-1/CCL2 and Nitric Oxide. Shock 2013, 39, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Taut, K.; Srivastava, M.; Länger, F.; Mack, M.; Briles, D.E.; Paton, J.C.; Maus, R.; Welte, T.; Gunn, M.D.; et al. Lung-Specific Overexpression of CC Chemokine Ligand (CCL) 2 Enhances the Host Defense to Streptococcus Pneumoniae Infection in Mice: Role of the CCL2-CCR2 Axis. J. Immunol. 2007, 178, 5828–5838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrama, J.A.; Nizet, V. Group A Streptococcus Encounters with Host Macrophages. Future Microbiol. 2018, 13, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Carreno, D.; Wanford, J.J.; Jasiunaite, Z.; Hames, R.G.; Chung, W.Y.; Dennison, A.R.; Straatman, K.; Martinez-Pomares, L.; Pareek, M.; Orihuela, C.J.; et al. Splenic Macrophages as the Source of Bacteraemia during Pneumococcal Pneumonia. EBioMedicine 2021, 72, 103601. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, G.; Fernandes, V.E.; Chung, W.Y.; Wanford, J.J.; Thomson, S.; Bayliss, C.D.; Straatman, K.; Crocker, P.R.; Dennison, A.; Martinez-Pomares, L.; et al. Intracellular Replication of Streptococcus Pneumoniae inside Splenic Macrophages Serves as a Reservoir for Septicaemia. Nat. Microbiol. 2018, 3, 600–610. [Google Scholar] [CrossRef]
- Horino, T.; Matsumoto, T.; Ishikawa, H.; Kimura, S.; Uramatsu, M.; Tanabe, M.; Tateda, K.; Miyazaki, S.; Aramaki, Y.; Iwakura, Y.; et al. Interleukin-1 Deficiency in Combination with Macrophage Depletion Increases Susceptibility to Pseudomonas Aeruginosa Bacteremia. Microbiol. Immunol. 2009, 53, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.H. Review of Septic Arthritis throughout the Antibiotic Era. Ann. Rheum. Dis. 1976, 35, 198–205. [Google Scholar] [CrossRef]
- Layne, S.P.; Beugelsdijk, T.J.; Patel, C.K.N.; Taubenberger, J.K.; Cox, N.J.; Gust, I.D.; Hay, A.J.; Tashiro, M.; Lavanchy, D. A Global Lab Against Influenza. Science 2001, 293, 1729. [Google Scholar] [CrossRef] [Green Version]
- Manicassamy, B.; Manicassamy, S.; Belicha-Villanueva, A.; Pisanelli, G.; Pulendran, B.; Garcia-Sastre, A. Analysis of in Vivo Dynamics of Influenza Virus Infection in Mice Using a GFP Reporter Virus. Proc. Natl. Acad. Sci. USA 2010, 107, 11531–11536. [Google Scholar] [CrossRef] [Green Version]
- Perrone, L.A.; Plowden, J.K.; García-Sastre, A.; Katz, J.M.; Tumpey, T.M. H5N1 and 1918 Pandemic Influenza Virus Infection Results in Early and Excessive Infiltration of Macrophages and Neutrophils in the Lungs of Mice. PLoS Pathog. 2008, 4, e1000115. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, A.; Pillai, P.S. Innate Immunity to Influenza Virus Infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tough, D.F.; Borrow, P.; Sprent, J. Induction of Bystander T Cell Proliferation by Viruses and Type I Interferon in Vivo. Science 1996, 272, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, S.; Pirhonen, J.; Miettinen, M.; Lehtonen, A.; Govenius-Vintola, C.; Sareneva, T.; Julkunen, I. Influenza A and Sendai Viruses Induce Differential Chemokine Gene Expression and Transcription Factor Activation in Human Macrophages. Virology 2000, 276, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, D.K.M.; Lau, L.L.H.; Leung, N.H.L.; Fang, V.J.; Chan, K.-H.; Chu, D.K.W.; Leung, G.M.; Peiris, J.S.M.; Uyeki, T.M.; Cowling, B.J. Viral Shedding and Transmission Potential of Asymptomatic and Pauci-Symptomatic Influenza Virus Infections in the Community. Clin. Infect. Dis. 2016, 64, 736–742. [Google Scholar] [CrossRef]
- Schwaiger, T.; Sehl, J.; Karte, C.; Schäfer, A.; Hühr, J.; Mettenleiter, T.C.; Schröder, C.; Köllner, B.; Ulrich, R.; Blohm, U. Experimental H1N1pdm09 Infection in Pigs Mimics Human Seasonal Influenza Infections. PLoS ONE 2019, 14, e0222943. [Google Scholar] [CrossRef] [Green Version]
- Cuypers, F.; Schäfer, A.; Skorka, S.B.; Surabhi, S.; Tölken, L.A.; Paulikat, A.D.; Kohler, T.P.; Otto, S.A.; Mettenleiter, T.C.; Hammerschmidt, S.; et al. Innate Immune Responses at the Asymptomatic Stage of Influenza A Viral Infections of Streptococcus Pneumoniae Colonized and Non-Colonized Mice. Sci. Rep. 2021, 11, 20609. [Google Scholar] [CrossRef]
- Kovarik, P.; Castiglia, V.; Ivin, M.; Ebner, F. Type I Interferons in Bacterial Infections: A Balancing Act. Front. Immunol. 2016, 7, 652. [Google Scholar] [CrossRef] [Green Version]
- Castiglia, V.; Piersigilli, A.; Ebner, F.; Janos, M.; Goldmann, O.; Damböck, U.; Kröger, A.; Weiss, S.; Knapp, S.; Jamieson, A.M.; et al. Type I Interferon Signaling Prevents IL-1β-Driven Lethal Systemic Hyperinflammation during Invasive Bacterial Infection of Soft Tissue. Cell Host Microbe 2016, 19, 375–387. [Google Scholar] [CrossRef] [Green Version]
- LeMessurier, K.S.; Häcker, H.; Chi, L.; Tuomanen, E.; Redecke, V. Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung. PLoS Pathog. 2013, 9, e1003727. [Google Scholar] [CrossRef] [Green Version]
- Maier, B.B.; Hladik, A.; Lakovits, K.; Korosec, A.; Martins, R.; Kral, J.B.; Mesteri, I.; Strobl, B.; Müller, M.; Kalinke, U.; et al. Type I Interferon Promotes Alveolar Epithelial Type II Cell Survival during Pulmonary Streptococcus Pneumoniae Infection and Sterile Lung Injury in Mice. Eur. J. Immunol. 2016, 46, 2175–2186. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, L.R.V.; Gigliotti Rothfuchs, A.; Gonçalves, R.; Roffê, E.; Cheever, A.W.; Bafica, A.; Salazar, A.M.; Feng, C.G.; Sher, A. Intranasal Poly-IC Treatment Exacerbates Tuberculosis in Mice through the Pulmonary Recruitment of a Pathogen-Permissive Monocyte/Macrophage Population. J. Clin. Investig. 2010, 120, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Leiner, I.; Dorothee, G.; Brandl, K.; Pamer, E.G. MyD88 and Type I Interferon Receptor-Mediated Chemokine Induction and Monocyte Recruitment during Listeria Monocytogenes Infection. J. Immunol. 2009, 183, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerer, J.M.; Lesinski, G.B.; Radmacher, M.D.; Ruppert, A.; Carson, W.E. STAT1-Dependent and STAT1-Independent Gene Expression in Murine Immune Cells Following Stimulation with Interferon-Alpha. Cancer Immunol. Immunother. 2007, 56, 1845–1852. [Google Scholar] [CrossRef]
- Zhang, Z.; Clarke, T.B.; Weiser, J.N. Cellular Effectors Mediating Th17-Dependent Clearance of Pneumococcal Colonization in Mice. J. Clin. Investig. 2009, 119, 1899–1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, K.M.; Nakamura, S.; Weiser, J.N. Nod2 Sensing of Lysozyme-Digested Peptidoglycan Promotes Macrophage Recruitment and Clearance of S. Pneumoniae Colonization in Mice. J. Clin. Investig. 2011, 121, 3666–3676. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.-J.; Law, S.K.A.; Moestrup, S.K. Identification of the Haemoglobin Scavenger Receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Pulford, K.; Micklem, K.; McCarthy, S.; Cordell, J.; Jones, M.; Mason, D.Y. A Monocyte/Macrophage Antigen Recognized by the Four Antibodies GHI/61, Ber-MAC3, Ki-M8 and SM4. Immunology 1992, 75, 588–595. [Google Scholar]
- Buechler, C.; Ritter, M.; Orsó, E.; Langmann, T.; Klucken, J.; Schmitz, G. Regulation of Scavenger Receptor CD163 Expression in Human Monocytes and Macrophages by Pro- and Antiinflammatory Stimuli. J. Leukoc. Biol. 2000, 67, 97–103. [Google Scholar] [CrossRef]
- Oliviero, S.; Cortese, R. The Human Haptoglobin Gene Promoter: Interleukin-6-Responsive Elements Interact with a DNA-Binding Protein Induced by Interleukin-6. EMBO J. 1989, 8, 1145–1151. [Google Scholar] [CrossRef]
- van den Heuvel, M.M.; Tensen, C.P.; van As, J.H.; van den Berg, T.K.; Fluitsma, D.M.; Dijkstra, C.D.; Döpp, E.A.; Droste, A.; van Gaalen, F.A.; Sorg, C.; et al. Regulation of CD163 on Human Macrophages: Cross-Linking of CD163 Induces Signaling and Activation. J. Leukoc. Biol. 1999, 66, 858–866. [Google Scholar] [CrossRef]
- Fabriek, B.O.; van Bruggen, R.; Deng, D.M.; Ligtenberg, A.J.M.; Nazmi, K.; Schornagel, K.; Vloet, R.P.M.; Dijkstra, C.D.; van den Berg, T.K. The Macrophage Scavenger Receptor CD163 Functions as an Innate Immune Sensor for Bacteria. Blood 2009, 113, 887–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneidl, J.; Löffler, B.; Erat, M.C.; Kalinka, J.; Peters, G.; Roth, J.; Barczyk, K. Soluble CD163 Promotes Recognition, Phagocytosis and Killing of Staphylococcus Aureus via Binding of Specific Fibronectin Peptides. Cell. Microbiol. 2012, 14, 914–936. [Google Scholar] [CrossRef] [PubMed]
- Reading, P.C.; Miller, J.L.; Anders, E.M. Involvement of the Mannose Receptor in Infection of Macrophages by Influenza Virus. J. Virol. 2000, 74, 5190–5197. [Google Scholar] [CrossRef]
- Pontow, S.E.; Kery, V.; Stahl, P.D. Mannose Receptor. Int. Rev. Cytol. 1993, 137, 221–244. [Google Scholar] [CrossRef]
- Sallusto, F.; Cella, M.; Danieli, C.; Lanzavecchia, A. Dendritic Cells Use Macropinocytosis and the Mannose Receptor to Concentrate Macromolecules in the Major Histocompatibility Complex Class II Compartment: Downregulation by Cytokines and Bacterial Products. J. Exp. Med. 1995, 182, 389–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, P.D. The Macrophage Mannose Receptor: Current Status. Am. J. Respir. Cell Mol. Biol. 1990, 2, 317–318. [Google Scholar] [CrossRef]
- Upham, J.P.; Pickett, D.; Irimura, T.; Anders, E.M.; Reading, P.C. Macrophage Receptors for Influenza A Virus: Role of the Macrophage Galactose-Type Lectin and Mannose Receptor in Viral Entry. J. Virol. 2010, 84, 3730–3737. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Zheng, N.-Y.; Clavijo, M.; Nussenzweig, M.C. Normal Host Defense during Systemic Candidiasis in Mannose Receptor-Deficient Mice. Infect. Immun. 2003, 71, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.D.; Lee, S.J.; Nussenzweig, M.C.; Harmsen, A.G. Absence of the Macrophage Mannose Receptor in Mice Does Not Increase Susceptibility to Pneumocystis Carinii Infection In Vivo. Infect. Immun. 2003, 71, 6213–6221. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine Metabolism: Nitric Oxide and Beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Starikova, E.A.; Sokolov, A.V.; Burova, L.A.; Golovin, A.S.; Lebedeva, A.M.; Vasilyev, V.B.; Freidlin, I.S. The Role of Arginine Deaminase from Streptococcus Pyogenes in Inhibition Macrophages Nitrogen Monooxide (NO) Synthesis. Russ. J. Infect. Immun. 2018, 8, 211–218. [Google Scholar] [CrossRef]
- Hesse, M.; Modolell, M.; La Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential Regulation of Nitric Oxide Synthase-2 and Arginase-1 by Type 1/Type 2 Cytokines In Vivo: Granulomatous Pathology Is Shaped by the Pattern of l-Arginine Metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef] [Green Version]
- Serbina, N.V.; Salazar-Mather, T.P.; Biron, C.A.; Kuziel, W.A.; Pamer, E.G. TNF/INOS-Producing Dendritic Cells Mediate Innate Immune Defense against Bacterial Infection. Immunity 2003, 19, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Tavares, L.P.; Teixeira, M.M.; Garcia, C.C. The Inflammatory Response Triggered by Influenza Virus: A Two Edged Sword. Inflamm. Res. 2017, 66, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Brouwer, S.; Schroder, K.; Walker, M.J. Inflammasome Activation and IL-1β Signalling in Group A Streptococcus Disease. Cell. Microbiol. 2021, 23, e13373. [Google Scholar] [CrossRef]
- Harder, J.; Franchi, L.; Munoz-Planillo, R.; Park, J.-H.; Reimer, T.; Nunez, G. Activation of the Nlrp3 Inflammasome by Streptococcus Pyogenes Requires Streptolysin O and NF- B Activation but Proceeds Independently of TLR Signaling and P2X7 Receptor. J. Immunol. 2009, 183, 5823–5829. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.E.; Beasley, F.C.; Keller, N.; Hollands, A.; Urbano, R.; Troemel, E.R.; Hoffman, H.M.; Nizet, V. A Group a Streptococcus ADP-Ribosyltransferase Toxin Stimulates a Protective Interleukin 1beta-Dependent Macrophage Immune Response. MBio 2015, 6, e00133. [Google Scholar] [CrossRef] [Green Version]
- Valderrama, J.A.; Riestra, A.M.; Gao, N.J.; LaRock, C.N.; Gupta, N.; Ali, S.R.; Hoffman, H.M.; Ghosh, P.; Nizet, V. Group A Streptococcal M Protein Activates the NLRP3 Inflammasome. Nat. Microbiol. 2017, 2, 1425–1434. [Google Scholar] [CrossRef] [Green Version]
- Stasakova, J.; Ferko, B.; Kittel, C.; Sereinig, S.; Romanova, J.; Katinger, H.; Egorov, A. Influenza A Mutant Viruses with Altered NS1 Protein Function Provoke Caspase-1 Activation in Primary Human Macrophages, Resulting in Fast Apoptosis and Release of High Levels of Interleukins 1β and 18. J. Gen. Virol. 2005, 86, 185–195. [Google Scholar] [CrossRef]
- Park, H.S.; Lu, Y.; Pandey, K.; Liu, G.Q.; Zhou, Y. NLRP3 Inflammasome Activation Enhanced by TRIM25 Is Targeted by the NS1 Protein of 2009 Pandemic Influenza A Virus. Front. Microbiol. 2021, 12, 778950. [Google Scholar] [CrossRef] [PubMed]
- Pothlichet, J.; Meunier, I.; Davis, B.K.; Ting, J.P.-Y.; Skamene, E.; von Messling, V.; Vidal, S.M. Type I IFN Triggers RIG-I/TLR3/NLRP3-Dependent Inflammasome Activation in Influenza A Virus Infected Cells. PLoS Pathog. 2013, 9, e1003256. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, L.A.; Tafforeau, L. How Influenza a Virus Ns1 Deals with the Ubiquitin System to Evade Innate Immunity. Viruses 2021, 13, 2309. [Google Scholar] [CrossRef]
- Midiri, A.; Mancuso, G.; Beninati, C.; Gerace, E.; Biondo, C. The Relevance of Il-1-Signaling in the Protection against Gram-Positive Bacteria. Pathogens 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- LaRock, C.N.; Todd, J.; LaRock, D.L.; Olson, J.; O’Donoghue, A.J.; Robertson, A.A.B.; Cooper, M.A.; Hoffman, H.M.; Nizet, V. IL-1β Is an Innate Immune Sensor of Microbial Proteolysis. Sci. Immunol. 2016, 1, eaah3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ely, C.F. Influenza as Seen at the Puget Sound Navy Yard. J. Am. Med. Assoc. 1919, 72, 24. [Google Scholar] [CrossRef]
- Okamoto, S.; Kawabata, S.; Fujitaka, H.; Uehira, T.; Okuno, Y.; Hamada, S. Vaccination with Formalin-Inactivated Influenza Vaccine Protects Mice against Lethal Influenza Streptococcus Pyogenes Superinfection. Vaccine 2004, 22, 2887–2893. [Google Scholar] [CrossRef]
- Grabenstein, J.D. Immunization to Protect the US Armed Forces: Heritage, Current Practice, and Prospects. Epidemiol. Rev. 2006, 28, 3–26. [Google Scholar] [CrossRef]
- Ozgur, S.K.; Beyazova, U.; Kemaloglu, Y.K.; Maral, I.; Sahin, F.; Camurdan, A.D.; Kizil, Y.; Dinc, E.; Tuzun, H. Effectiveness of Inactivated Influenza Vaccine for Prevention of Otitis Media in Children. Pediatr. Infect. Dis. J. 2006, 25, 401–404. [Google Scholar] [CrossRef]
- Clements, D.A. Influenza A Vaccine Decreases the Incidence of Otitis Media in 6- to 30-Month-Old Children in Day Care. Arch. Pediatr. Adolesc. Med. 1995, 149, 1113. [Google Scholar] [CrossRef]



| Positive Cultures | GAS | GAS+IAV | IAV+GAS |
|---|---|---|---|
| blood | 20% (2/10) | 30% (3/10) | 50% (5/10) |
| knee joint capsule | 10% (1/10) | 30% (3/10) | 50% (5/10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleith, J.; Brendel, M.; Weipert, E.; Müller, M.; Schultz, D.; Ko-Infekt Study Group; Müller-Hilke, B. Influenza A Virus Exacerbates Group A Streptococcus Infection and Thwarts Anti-Bacterial Inflammatory Responses in Murine Macrophages. Pathogens 2022, 11, 1320. https://doi.org/10.3390/pathogens11111320
Aleith J, Brendel M, Weipert E, Müller M, Schultz D, Ko-Infekt Study Group, Müller-Hilke B. Influenza A Virus Exacerbates Group A Streptococcus Infection and Thwarts Anti-Bacterial Inflammatory Responses in Murine Macrophages. Pathogens. 2022; 11(11):1320. https://doi.org/10.3390/pathogens11111320
Chicago/Turabian StyleAleith, Johann, Maria Brendel, Erik Weipert, Michael Müller, Daniel Schultz, Ko-Infekt Study Group, and Brigitte Müller-Hilke. 2022. "Influenza A Virus Exacerbates Group A Streptococcus Infection and Thwarts Anti-Bacterial Inflammatory Responses in Murine Macrophages" Pathogens 11, no. 11: 1320. https://doi.org/10.3390/pathogens11111320
APA StyleAleith, J., Brendel, M., Weipert, E., Müller, M., Schultz, D., Ko-Infekt Study Group, & Müller-Hilke, B. (2022). Influenza A Virus Exacerbates Group A Streptococcus Infection and Thwarts Anti-Bacterial Inflammatory Responses in Murine Macrophages. Pathogens, 11(11), 1320. https://doi.org/10.3390/pathogens11111320







