Factors Affecting Post-Challenge Survival of Flavobacterium psychrophilum in Susceptible Rainbow Trout from the Literature
Abstract
:1. Introduction
2. Results
2.1. Data Collection
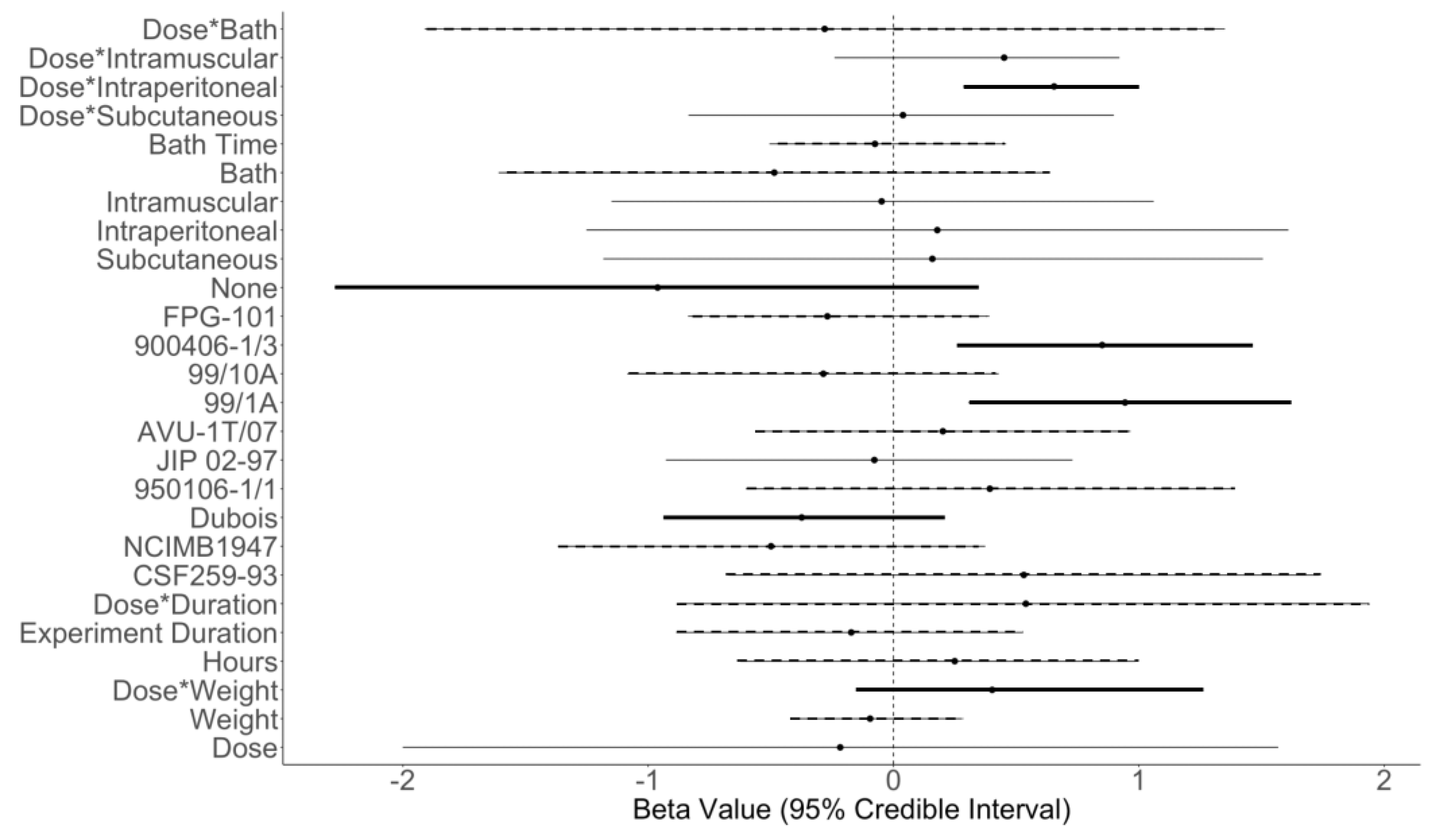
2.2. Regression
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Regression
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daszak, P.; Berger, L.; Cunningham, A.A.; Hyatt, A.D.; Green, D.E.; Speare, R. Emerging Infectious Diseases and Amphibian Population Declines. Emerg. Infect. Dis. 1999, 5, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Muths, E.; Corn, P.S.; Pessier, A.P.; Green, D.E. Evidence for disease-related amphibian decline in Colorado. Biol. Conserv. 2003, 110, 357–365. [Google Scholar] [CrossRef]
- Robinson, R.A.; Lawson, B.; Toms, M.P.; Peck, K.M.; Kirkwood, J.K.; Chantrey, J.; Clatworthy, I.R.; Evans, A.D.; Hughes, L.A.; Hutchinson, O.C.; et al. Emerging Infectious Disease Leads to Rapid Population Declines of Common British Birds. PLoS ONE 2010, 5, e12215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skerratt, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R.; Phillott, A.D.; Hines, H.B.; Kenyon, N. Spread of chytridomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 2007, 4, 125–134. [Google Scholar] [CrossRef]
- Edmunds, D.R.; Kauffman, M.J.; Schumaker, B.A.; Lindzey, F.G.; Cook, W.E.; Kreeger, T.J.; Grogan, R.G.; Cornish, T.E. Chronic Wasting Disease Drives Population Decline of White-Tailed Deer. PLoS ONE 2016, 11, e0161127. [Google Scholar] [CrossRef] [Green Version]
- Vincent, E.R. Whirling disease and wild trout: The Montana experience. Fisheries 1996, 21, 32–34. [Google Scholar]
- Klesius, P.H.; Pridgeon, J.W. Live attenuated bacterial vaccines in aquaculture. In Proceedings of the 9th International Symposium on Tilapia in Aquaculture, Shanghai, China, 22–23 April 2011; pp. 18–26. [Google Scholar]
- Pridgeon, J.W.; Klesius, P.H. Major bacterial diseases in aquaculture and their vaccine development. CAB Rev. 2012, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Subasinghe, R.P.; Bondad-Reantaso, M.G.; McGladdery, S.E. Aquaculture Development, Health and Wealth. Technical Proceedings of the Conference on Aquaculture in the Third Millennium Bangkok: NACA and FAO. 2001, pp. 167–191. Available online: http://www.fao.org/docrep/003/ab412e/ab412e09.htm (accessed on 1 November 2020).
- Piper, R.G.; McElwain, I.B.; Orme, L.E.; McCaren, J.P.; Fowler, L.G.; Leonard, J.R. Fish Hatchery Management (No. 2175), 1st ed.; U.S. Fish and Wildlife Service: Washington, DC, USA, 1982.
- Cipriano, R.C.; Holt, R.A. Flavobacterium psychrophilum, Cause of Bacterial Cold-Water Disease and Rainbow Trout Fry Syndrome; Fish Disease Leaflet No. 86; United States Department of Interior, U.S. Geological Service, National Fish Health Research Laboratory: Kearneysvlle, WV, USA, 2005.
- LaFrentz, B.R.; Cain, K.D. Bacterial Coldwater Disease. An Extension Bulletin for the Western Regional Aquaculture Center; University of Idaho: Moscow, Russia, 2004. [Google Scholar]
- Borg, A.F. Studies on myxobacteria associated with diseases in salmonid fishes. J. Wildl. Dis. 1960, 8, 1–85. [Google Scholar]
- Rucker, R.R.; Earp, B.J.; Ordal, E.J. Infectious Diseases of Pacific Salmon. Trans. Am. Fish. Soc. 1954, 83, 297–312. [Google Scholar] [CrossRef]
- Michel, C.; Antonio, D.; Hedrick, R.P. Production of viable cultures of Flavobacterium psychrophilum: Approach and control. Res. Microbiol. 1999, 150, 351–358. [Google Scholar] [CrossRef]
- Barnes, M.E.; Brown, M.L. A review of Flavobacterium psychrophilum biology, clinical signs, and bacterial cold water disease prevention and treatment. Open Fish Sci. J. 2011, 4, 1–9. [Google Scholar] [CrossRef]
- Nicolas, P.; Mondot, S.; Achaz, G.; Bouchenot, C.; Bernardet, J.-F.; Duchaud, E. Population Structure of the Fish-Pathogenic Bacterium Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2008, 74, 3702–3709. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, H.; Olsen, A.B.; Vaagnes, O.; Hellberg, H.; Bottolfsen, K.; Skjelstad, H.; Colquhoun, D.J. Systemic Flavobacterium psychrophilum infection in rainbow trout, Oncorhynchus mykiss (Walbaum), farmed in fresh and brackish water in Norway. J. Fish Dis. 2011, 34, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Decostere, A.; D’Haese, E.; Lammens, M.; Nelis, H.; Haesebrouck, F. In vivo study of phagocytosis, intracellular survival and multiplication of Flavobacterium psychrophilum in rainbow trout, Oncorhynchus mykiss (Walbaum), spleen phagocytes. J. Fish Dis. 2001, 24, 481–487. [Google Scholar] [CrossRef]
- Wood, J.W. Diseases of Pacific Salmon: Their prevention and treatment, 2nd ed.; Washington Department of Fisheries: Olympia, WA, USA, 1974.
- Aoki, M.; Kondo, M.; Kawai, K.; Oshima, S. Experimental bath infection with Flavobacterium psychrophilum, inducing typical signs of rainbow trout Oncorhynchus mykiss fry syndrome. Dis. Aquat. Org. 2005, 67, 73–79. [Google Scholar] [CrossRef]
- Bruce, T.J.; Ma, J.; Knupp Loch, T.P.; Faisal, M.; Cain, K.D. Cross-protection of a live-attenuated Flavobacterium psychrophilum immersion vaccine against novel Flavobacterium spp. and Chryseobacterium spp. strains. J Fish Dis. 2020, 43, 915–928. [Google Scholar] [CrossRef]
- Chettri, J.K.; Al-Jubury, A.; Dalsgaard, I.; Heegaard, P.M.H.; Buchmann, K. Experimental anal infection of rainbow trout with Flavobacterium psychrophilum: A novel challenge model. J. Fish Dis. 2018, 41, 1917–1919. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Furevik, A.; Gauthier, D.; Egenberg, M.; Paulsen, E.D.; Brudeseth, B. Intramuscular challenge of rainbow trout (Oncorhynchus mykiss) with two Norwegian field strains of Flavobacterium psychrophilum. Fish Shellfish Immunol. 2013, 35, 595–598. [Google Scholar] [CrossRef]
- Jarau, M.; Di Natale, A.; Huber, P.E.; MacInnes, J.I.; Lumsden, J.S. Virulence of Flavobacterium psychrophilum isolates in Rainbow Trout Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2018, 41, 1205–1514. [Google Scholar] [CrossRef]
- LaFrentz, B.; LaPatra, S.E.; Jones, G.R.; Cain, K.D. Passive immunization of rainbow trout, Oncorhynchus mykiss (Walbaum), against Flavobacterium psychrophilum, the causative agent of bacterial coldwater disease and rainbow trout fry syndrome. J. Fish Dis. 2003, 26, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Leeds, T.D.; Silverstein, J.T.; Weber, G.M.; Vallejo, R.L.; Palti, Y.; Rexroad, C.E., III; Evenhuis, J.; Hadidi, S.; Welch, T.J.; Wiens, G.D. Response to selection for bacterial cold water disease resistance in Rainbow Trout. J Anim Sci. 2010, 88, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Dalsgaard, I. Comparative studies of Danish Flavobacterium psychrophilum isolates: Ribotypes, plasmid profiles, serotypes and virulence. J. Fish Dis. 2000, 23, 211–218. [Google Scholar] [CrossRef]
- Wiens, G.D.; LaPatra, S.E.; Welch, T.J.; Evenhuis, J.P.; Rexroad, C.E., III; Leeds, T.D. On-farm performance of Rainbow Trout (Oncorhynchus mykiss) selectively bred for resistance to bacterial cold water disease: Effect of rearing environment on survival phenotype. Aquaculture 2013, 388–391, 128–136. [Google Scholar] [CrossRef]
- Garcia, C.; Pozet, F.; Michel, C. Standardization of experimental infection with Flavobacterium psychrophilum, the agent of rainbow trout Oncorhynchus mykiss fry syndrome. Dis. Aquat. Org. 2000, 42, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Madsen, L.; Dalsgaard, I. Reproducible methods for experimental infection with Flavobacterium psychrophilum in rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1999, 36, 169–176. [Google Scholar] [CrossRef] [Green Version]
- LaFrentz, B.R.; LaPatra, S.E.; Call, D.R.; Cain, K.D. Isolation of rifampicin resistant Flavobacterium psychrophilum strains and their potential as live attenuated vaccine candidates. Vaccine 2008, 26, 5582–5589. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Fehringer, T.R.; LaFrentz, B.R.; Call, D.R.; Cain, K.D. Development of a waterborne challenge model for Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2014, 359, 154–160. [Google Scholar] [CrossRef] [Green Version]
- LaFrentz, B.R.; LaPatra, S.E.; Call, D.R.; Cain, K.D. Immunization of rainbow trout Oncorhynchus mykiss (Walbaum) with a crude lipopolysaccharide extract from Flavobacterium psychrophilum. Aquac. Res. 2014, 45, 476–483. [Google Scholar] [CrossRef]
- Apablaza, P.; Loland, A.D.; Brevik, O.J.; Ilardi, P.; Battaglia Nylund, A. Genetic variation among Flavobacterium psychrophilum isolates from wild and farmed salmonids in Norway and Chile. J. Appl. Microbiol. 2013, 114, 943–946. [Google Scholar] [CrossRef]
- Siekoula-Nguedia, C.; Blanc, G.; Duchaud, E.; Calvez, S. Genetic diversity of Flavobacterium psychrophilum isolated from rainbow trout in France: Predominance of a clonal complex. Vet. Microbiol. 2012, 161, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Strepparava, N.; Nicolas, P.; Wahli, T.; Segner, H.; Petrini, O. Molecular epidemiology of Flavobacterium psychrophilum from Swiss fish farms. Dis. Aquat. Org. 2013, 105, 203–210. [Google Scholar] [CrossRef]
- Van Vliet, D.; Wiens, G.D.; Loch, T.P.; Nicolas, P.; Faisal, M. Genetic diversity of Flavobacterium psychrophilum isolates from three Oncorhynchus spp. in the United States, as revealed by multilocus sequence typing. J. Appl. Environ. Microbiol. 2016, 82, 3246–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFrentz, B.; LaPatra, S.; Jones, G.; Cain, K. Protective immunity in rainbow trout Oncorhynchus mykiss following immunization with distinct molecular mass fractions isolated from Flavobacterium psychrophilum. Dis. Aquat. Org. 2004, 59, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbank, D.R.; Shah, D.H.; LaPatra, S.E.; Fornshell, G.; Cain, K.D. Enhanced resistance to coldwater disease following feeding of probiotic bacterial strains to rainbow trout (Oncorhynchus mykiss). Aquaculture 2011, 321, 185–190. [Google Scholar] [CrossRef]
- Henriksen, M.M.M.; Madsen, L.; Dalsgaard, I. Effect of Hydrogen Peroxide on Immersion Challenge of Rainbow Trout Fry with Flavobacterium psychrophilum. PLoS ONE 2013, 8, e62590. [Google Scholar] [CrossRef] [Green Version]
- Glenn, R.A.; Gannam, A.L.; LaPatra, S.E. The lack of effectiveness of roemary oil on fish feed in controlling bacterial cold-water disease in Rainbow Trout. N. Am. J. Aquac. 2014, 76, 359–363. [Google Scholar] [CrossRef]
- Wagner, E.J.; Oplinger, R.W. Comparison of the Susceptibility of Four Rainbow Trout Strains to Cold-Water Disease. J. Aquat. Anim. Health 2014, 26, 160–167. [Google Scholar] [CrossRef]
- Schubiger, C.B.; Orfe, L.H.; Sudheesh, P.S.; Cain, K.D.; Shah, D.H.; Call, D.R. Entericidin is required for probiotic treatment (Enterobacter sp. Strain C6-6) to protect trout from cold-water disease challenge. J. Appl. Environ. Microbiol. 2015, 81, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.; Cain, K.D.; Nowak, B.F.; Bridle, A.R. Microencapsulation of a putative probiotic Enterobacter species, C6-6, to protect Rainbow Trout, Oncorhynchus mykiss (Walbaum), against bacterial coldwater disease. J Fish Dis. 2016, 39, 1–11. [Google Scholar] [CrossRef]
- Ryerse, I.A.; Hooft, J.M.; Bureau, D.P.; Hayes, M.A.; Lumsden, J.S. Diets containing corn naturally contaminated with deoxynivalenol reduces the susceptibility of Rainbow Trout (Oncorhynchus mykiss) to experimental Flavobacterium psychrophilum infection. Aquac Res. 2016, 47, 787–796. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; Cain, K.D. Optimization of efficacy of a live attenuated Flavobacterium psychrophilum immersion vaccine. Fish Shellfish Immunol. 2016, 56, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Hoare, R.; Ngo, T.P.H.; Bartie, K.L.; Adams, A. Efficacy of a polyvalent immersion vaccine against Flavobacterium psychrophilum and evaluation of immune response to vaccination in Rainbow Trout fry (Oncorhynchus mykiss L.). Vet. Res. 2017, 48, 1–13. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Sudheesh, P.S.; Knupp, C.; Loch, T.P.; Faisal, M.; Cain, K.D. Assessment of cross-protection to heterologous strains of Flavobacterium psychrophilum following vaccination with a live-attenuated coldwater disease immersion vaccine. J. Fish Dis. 2018, 42, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Sundell, K.; Landor, L.; Nicolas, P.; Jørgensen, J.; Castillo, D.; Middelboe, M.; Dalsgaard, I.; Donati, V.L.; Madsen, L.; Wiklund, T. Phenotypic and Genetic Predictors of Pathogenicity and Virulence in Flavobacterium psychrophilum. Front. Microbiol. 2019, 10, 1711. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bruce, T.J.; Oliver, L.P.; Cain, K.D. Co-infection of rainbow trout (Oncorhynchus mykiss) with infectious hematopoietic necrosis virus and Flavobacterium psychrophilum. J. Fish Dis. 2019, 42, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Avila, B.W.; Winkelman, D.L.; Fetherman, E.R. Dual Resistance to Flavobacterium psychrophilum and Myxobolus cerebralis in rainbow trout (Oncorhynchus mykiss, Walbaum). J. Fish Dis. 2022, 45, 801–813. [Google Scholar] [CrossRef]
- Silverstein, J.T.; Vallejo, R.L.; Palti, Y.; Leeds, T.D.; Rexorad, I.I.I.C.E.; Welch, T.J.; Wiens, G.D.; Ducrocq, V. Rainbow trout resistance to bacterial cold-water disease is moderately heritable and is not adversely correlated with growth. J. Anim. Sci. 2009, 87, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Wiens, G.D.; Palti, Y.; Leeds, T.D. Three generations of selective breeding improve rainbow trout (Oncorhynchus mykiss) disease resistance against natural challenge with Flavobacterium psychrophilum during early life-state rearing. Aquaculture 2018, 497, 414–421. [Google Scholar] [CrossRef]
- Decostere, A.; Lammens, M.; Haesebrouck, F. Difficulties in experimental infection studies with Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) using immersion, oral and anal challenges. Res. Vet. Sci. 2000, 69, 165–169. [Google Scholar] [CrossRef]
- Castillo, D.; Donati, V.; Jørgensen, J.; Sundell, K.; Dalsgaard, I.; Madsen, L.; Wiklund, T.; Middelboe, M. Comparative Genomic Analyses of Flavobacterium psychrophilum Isolates Reveals New Putative Genetic Determinants of Virulence Traits. Microorganisms 2021, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, N.T.; Hooten, M.B. Bayesian Models a Statistical Primer for Ecologists, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Avendaño-Herrera, R.; Houel, A.; Irgang, R.; Bernardet, J.-F.; Godoy, M.; Nicolas, P.; Duchaud, E. Introduction, expansion and coexistence of epidemic Flavobacterium psychrophilum lineages in Chilean fish farms. Vet. Microbiol. 2014, 170, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, H.; Sundell, K.; Duchaud, E.; Nicolas, P.; Dalsgaard, I.; Madsen, L.; Aspán, A.; Jansson, E.; Colquhoun, D.J.; Wiklund, T. Multilocus sequence typing identifies epidemic clones of Flavobacterium psychrophilum in Nordic countries. Appl. Environ. Microbiol. 2014, 80, 2728–2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekman, E.; Norrgren, L. Pathology and immunohistochemistry in three species of salmonids after experimental infection with Flavobacterium psychrophilum. J. Fish Dis. 2003, 26, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.N.; Furevik, A.; Olsen, R.H.; Gauthier, D.; Mendoza, J.; Norderhus, E.A. Virulence of Chilean field isolates of Flavobacterium psychrophilum in Atlantic salmon (Salmo salar L.) parr. Bull Eur Assoc Fish Pathol. 2016, 36, 67. [Google Scholar]
- Knupp, C.; Kiupel, M.; Brenden, T.O.; Loch, T.P. Host-specific preference of some Flavobacterium psychrophilum multilocus sequence typing genotypes determines their ability to cause bacterial coldwater disease in coho salmon (Oncorhynchus kisutch). J. Fish Dis. 2021, 44, 521–531. [Google Scholar] [CrossRef]
- Nagai, T.; Nakai, T. Growth of Flavobacterium psychrophilum in fish serum correlates with pathogenicity. J. Fish Dis. 2011, 34, 303–310. [Google Scholar] [CrossRef]
- Wang, L.; Fan, D.; Chen, W.; Terentjev, E. Bacterial growth, detachment and cell size control on polyethylene terephthalate surfaces. Sci. Rep. 2015, 5, srep15159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, M.; Kawai, K.; Yagyu, K.; Nakayama, K.; Kurohara, K.; Oshima, S. Changes in the cell structure of Flavobacterium psychrophilum with length of culture. Microbiol Immunol. 2001, 45, 813–818. [Google Scholar] [CrossRef]
- Avila, B.W. Bacterial Coldwater Disease Investigations. Ph.D. Dissertation, Colorado State University, Fort Collins, CO, USA, 2021. [Google Scholar]
- Holt, R.A.; Amandi, A.; Rohovec, J.S.; Fryer, J.L. Relation of Water Temperature to Bacterial Cold-Water Disease in Coho Salmon, Chinook Salmon, and Rainbow Trout. J. Aquat. Anim. Health 1989, 1, 94–101. [Google Scholar] [CrossRef]
- Fetherman, E.; Winkelman, D.; Schisler, G.; Antolin, M. Genetic basis of differences in myxospore count between whirling disease-resistant and -susceptible strains of rainbow trout. Dis. Aquat. Org. 2012, 102, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedrick, R.P.; McDowell, T.S.; Marty, G.D.; Fosgate, G.; Mukkatira, K.; Myklebust, K.; El-Matbouli, M. Susceptibility of two strains of rainbow trout (one with suspected resistance to whirling disease) to Myxobolus cerebralis infection. Dis. Aquat. Org. 2003, 55, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bernardet, J.F.; Segers, P.; Vancanneyt, M.; Berthe, F.; Kersters, K.; Vandamme, P. Cutting a Gordian knot: Emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (Basonym, Cytophaga aquatilis Strohl and Tait 1978). Int. J. Syst. Bacteriol. 1996, 46, 128–148. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 9 August 2022).
- Gelman, A.; Rubin, D.B. Inference from Iterative Simulation Using Multiple Sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
| Data Points | Dose Range | Exposure Type | Experiment Duration | Isolate | Weight (g) | Culture Hours | Bath Time | Manuscript | Author |
|---|---|---|---|---|---|---|---|---|---|
| 31 | 700–4.0 × 107 | IP | 28 | 950106-1/1, 99/1A, 99/10A, 900406-1/3 | 1.0–28 | 48 | NA | 1 | [32] |
| 11 | 4.2 × 103–4.2 × 107 | IP, IM | 29 | JIP 02-97 | 4.5 | 36 | NA | 2 | [31] |
| 6 | 1.0 × 106 | IP | 6 | Dubois | 1–300 | 72 | NA | 3 | [20] |
| 12 | 1.25 × 104–1.25 × 106 | Sub | 28 | CSF259-93 | 1.4 | 72 | NA | 4 | [27] |
| 3 | 6.25 × 106–6.25 × 107 | Sub | 28 | CSF259-93 | 7.5 | 72 | NA | 5 | [40] |
| 10 | 2.0 × 106–3.4 × 108 | Bath | 14 | NCIMB1947 | 1.1–6.4 | 18–66 | 1 | 6 | [22] |
| 6 | 6.25 × 106–6.25 × 107 | Sub | 28 | CSF259-93 | 4.6, 15 | 48 | NA | 7 | [33] |
| 8 | 3.0 × 105–7.0 × 106 | Sub | 28 | CSF259-93 | 5 | 72 | NA | 8 | [41] |
| 2 | 1.0 × 105–1.0 × 107 | Bath | 30 | 950106-1/1 | 0.77 | 24–48 | 24, 48 | 9 | [42] |
| 3 | 2.0 × 108–5.0 × 108 | Sub | 28 | CSF259-93 | 2.3 | 72 | NA | 10 | [43] |
| 6 | 5.0 × 107–1.20 × 108 | Sub | 28 | CSF259-93 | 5, 10 | 96 | NA | 11 | [35] |
| 4 | 7.9 × 108–8.9 × 108 | Bath | 28 | CSF259-93 | 0.3, 1.5 | 48 | 1 | 12 | [34] |
| 6 | 3.6 × 106–6.3 × 107 | IP | 21 | CSF259-93 | 6.8–9.5 | 72 | NA | 13 | [44] |
| 2 | 1.03 × 107 | Sub | 28 | CSF259-93 | 4 | 72 | NA | 14 | [45] |
| 2 | 1.2 × 107 | IP | 28 | CSF259-93 | 0.5 | 72 | NA | 15 | [46] |
| 4 | 3.03 × 107 | IP | 21 | FPG-101 | 7.5 | 72 | NA | 16 | [47] |
| 4 | 2.29 × 107–3.0 × 107 | IP | 28 | CSF259-93 | 3.08, 4.27 | 72 | NA | 17 | [48] |
| 2 | 1.0 × 108 | Bath | 28 | AVU-1T/07 | 12.46, 12.75 | 24 | 5 | 18 | [49] |
| 2 | 1.4 × 107 | IP | 28 | 950106-1/1 | 3.5 | 48 | NA | 19 | [24] |
| 1 | 4.7 × 107 | IM | 28 | CSF259-93 | 3.5 | 72 | NA | 20 | [50] |
| 12 | 1 × 103–1 × 107 | IM | 21 | NCIMB1947, 950106-1/1 | 5 | 48 | NA | 21 | [51] |
| 2 | 1.0 × 106 | Sub | 28 | CSF259-93 | 3.5 | 72 | NA | 22 | [52] |
| 3 | 1.34 × 105 | IM | 21 | CSF259-93 | 1.4–11.3 | 18–72 | NA | 23 | [23] |
| Parameter | Mean | Standard Deviation | Multiplicative Change | [Neg., Pos.] |
|---|---|---|---|---|
| SDose | −0.22 | 0.91 | 0.80 | [59%, 41%] |
| SWeight | −0.10 | 0.18 | 0.91 | [72%, 28%] |
| SDose*Weight | 0.40 | 0.33 | 1.50 | [8%, 92%] |
| SHours | 0.25 | 0.41 | 1.28 | [25%, 75%] |
| SExperiment Duration | −0.17 | 0.39 | 0.84 | [69%, 31%] |
| SDose*Duration | 0.54 | 0.72 | 1.72 | [23%, 77%] |
| SCSF259-93 | 0.53 | 0.61 | 1.70 | [19%, 81%] |
| SNCIMB1947 | −0.50 | 0.44 | 0.61 | [87%, 13%] |
| SDubois | −0.37 | 0.30 | 0.69 | [91%, 9%] |
| S950106-1/1 | 0.39 | 0.50 | 1.48 | [22%, 78%] |
| SJIP 02-97 | −0.078 | 0.42 | 0.93 | [57%, 43%] |
| SAVU-1T/07 | 0.20 | 0.38 | 1.22 | [29%, 71%] |
| S99/1A | 0.94 | 0.33 | 2.57 | [0.2%, 99.8%] |
| S99/10A | −0.29 | 0.39 | 0.75 | [77%, 23%] |
| S900406-1/3 | 0.85 | 0.31 | 234 | [0.3%, 99.7] |
| SFPG-101 | −0.27 | 0.30 | 0.76 | [84%, 16%] |
| SNone | −0.96 | 0.67 | 0.38 | [93%, 7%] |
| SSubcutaneous | 0.16 | 0.69 | 1.17 | [40%, 60%] |
| SIntraperitoneal | 0.18 | 0.73 | 1.20 | [40%, 60%] |
| SIntramuscular | 0.048 | 0.56 | 0.95 | [54%, 46%] |
| SBath | −0.49 | 0.57 | 0.62 | [81%, 19%] |
| SBath Time | −0.076 | 0.25 | 0.93 | [66%, 34%] |
| SDose*Subcutaneous | 0.039 | 0.44 | 1.04 | [46%, 54%] |
| SDose*Intraperitoneal | 0.65 | 0.18 | 1.92 | [0.1%, 99.9%] |
| SDose*Intramuscular | 0.45 | 0.28 | 1.57 | [7%, 93%] |
| SDose*Bath | −0.28 | 0.83 | 0.76 | [65%, 35%] |
| Search Terms | |
|---|---|
| 1 | Flavobacterium psychrophilum |
| 2 | bacterial coldwater disease |
| 3 | Rainbow Trout Fry Syndrome |
| 4 | Flavobacterium psychrophilum AND challenge |
| 5 | bacterial coldwater disease AND challenge |
| 6 | Rainbow Trout Fry Syndrome AND challenge |
| 7 | Flavobacterium psychrophilum AND experiment |
| 8 | bacterial coldwater disease AND experiment |
| 9 | Rainbow Trout Fry Syndrome AND experiment |
| 10 | Flavobacterium psychrophilum AND exposure |
| 11 | bacterial coldwater disease AND exposure |
| 12 | Rainbow Trout Fry Syndrome AND exposure |
| 13 | Flavobacterium psychrophilum AND infection |
| 14 | bacterial coldwater disease AND infection |
| 15 | Rainbow Trout Fry Syndrome AND infection |
| 16 | Flavobacterium psychrophilum AND challenge OR experiment OR exposure OR infection |
| 17 | bacterial coldwater disease AND challenge OR experiment OR exposure OR infection |
| 18 | Rainbow Trout Fry Syndrome AND challenge OR experiment OR exposure OR infection |
| 19 | Flavobacterium psychrophilum OR bacterial coldwater disease OR Rainbow Trout Fry Syndrome AND challenge OR experiment OR exposure OR infection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication. (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, B.W.; Huyvaert, K.P.; Winkelman, D.L.; Fetherman, E.R. Factors Affecting Post-Challenge Survival of Flavobacterium psychrophilum in Susceptible Rainbow Trout from the Literature. Pathogens 2022, 11, 1318. https://doi.org/10.3390/pathogens11111318
Avila BW, Huyvaert KP, Winkelman DL, Fetherman ER. Factors Affecting Post-Challenge Survival of Flavobacterium psychrophilum in Susceptible Rainbow Trout from the Literature. Pathogens. 2022; 11(11):1318. https://doi.org/10.3390/pathogens11111318
Chicago/Turabian StyleAvila, Brian W., Kathryn P. Huyvaert, Dana L. Winkelman, and Eric R. Fetherman. 2022. "Factors Affecting Post-Challenge Survival of Flavobacterium psychrophilum in Susceptible Rainbow Trout from the Literature" Pathogens 11, no. 11: 1318. https://doi.org/10.3390/pathogens11111318
APA StyleAvila, B. W., Huyvaert, K. P., Winkelman, D. L., & Fetherman, E. R. (2022). Factors Affecting Post-Challenge Survival of Flavobacterium psychrophilum in Susceptible Rainbow Trout from the Literature. Pathogens, 11(11), 1318. https://doi.org/10.3390/pathogens11111318






