Clinical Impact of Colonization with Carbapenem-Resistant Gram-Negative Bacteria in Critically Ill Patients Admitted for Severe Trauma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Endpoints
2.2. Recruitment of Patients, Population, and Setting
2.3. Clinical and Microbiological Data Collection
2.4. Definitions
2.5. Ethics
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Cohort
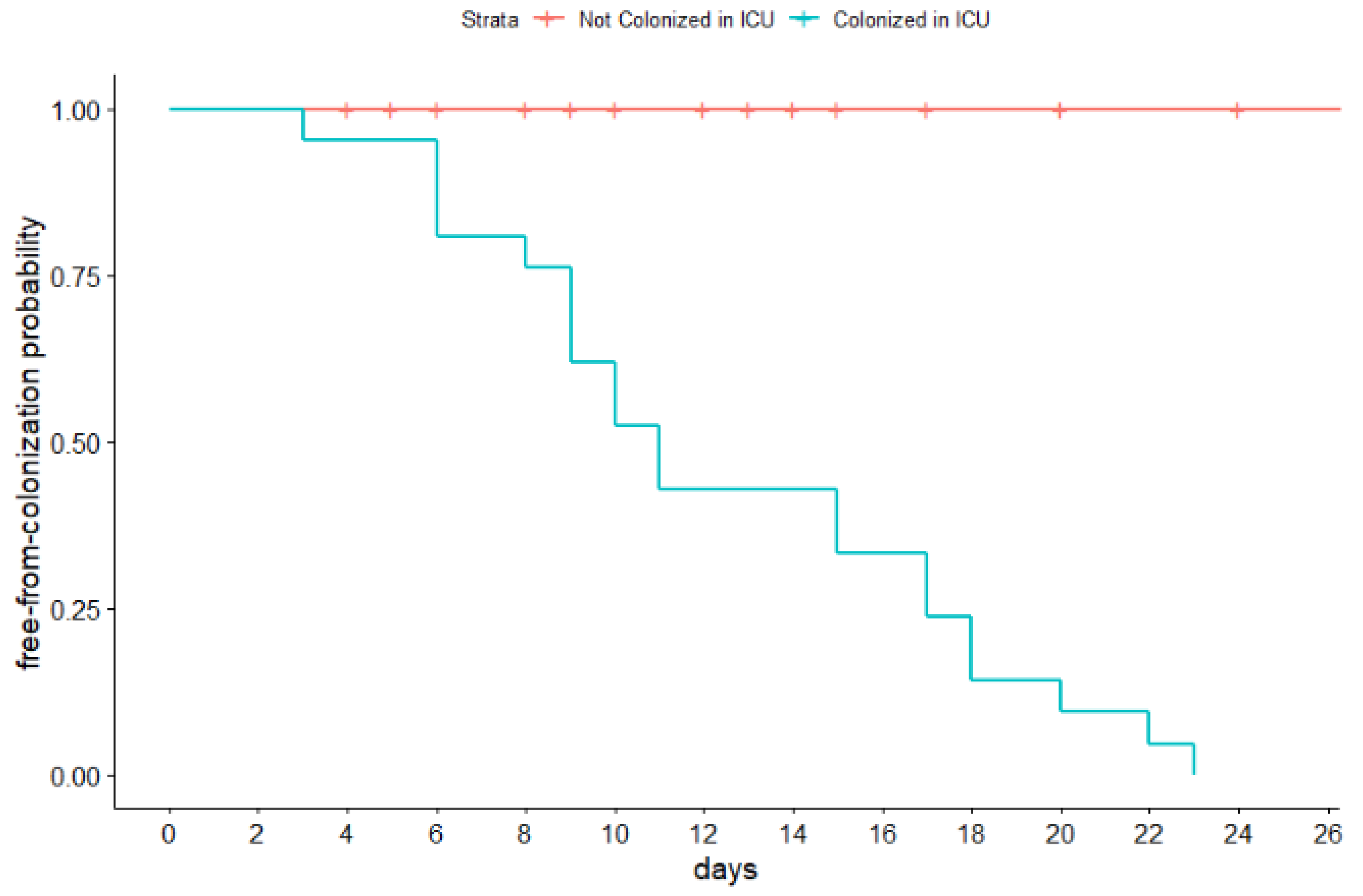
3.2. CR-GNB Colonizations
3.3. Infections Due to CR-GNB
3.4. In-ICU Related Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michetti, C.P.; Fakhry, S.M.; Fergunson, P.L.; Cook, A.; Moore, F.O.; Gross, R.; AAST Ventilator-Associated Pneumonia Investigators. Ventilator associated pneumonia rates at major trauma centers compared with a national benchmark: A multi-institutional study of AAST. J. Trauma 2012, 72, 1165–1173. [Google Scholar]
- Rodrigues Pires de Campos, L.; Farrel Côrtes, M.; Deo, B.; Rizek, C.; Santos, S.; Perdigão, L.; Costa, S.F. Risk factors for bloodstream infection by multidrug-resistant organisms in critically ill patients in a reference trauma hospital. Am. J. Infect. Control 2022, 50, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Papia, G.; McLellan, B.A.; El-Helou, P.; Louie, M.; Rachlis, A.; Szalai, J.P.; Simor, A.E. Infection in hospitalized trauma patients: Incidence, risk factors and complications. J. Trauma 1999, 47, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.S. Risk factors for infection in the trauma patient. J. Nat. Med. Assoc. 1992, 84, 1019–1023. [Google Scholar]
- Gilbert, L.J.; Li, P.; Murray, C.K.; Yun, H.C.; Aggarwal, D.; Weintrob, A.C.; Tribble, D.R.; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group. Multidrug-resistant Gram-negative bacilli colonization risk factors among trauma patients. Diagn. Microbiol. Infect. Dis. 2016, 84, 358–360. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Vital signs: Carbapenem-resistant Enterobacteriaceae. MMWR Morb. Mortal. Wkly Rep. 2013, 62, 165–170. [Google Scholar]
- Sharma, R.; Shultz, S.R.; Robinson, M.J.; Belli, A.; Hibbs, M.L.; O’Brien, T.J.; Semple, B.D. Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain Behav. Immun. 2019, 79, 63–74. [Google Scholar] [CrossRef]
- Flores, J.M.; Jiménez, P.I.; Rincón, M.D.; Márquez, J.A.; Navarro, H.; Arteta, D.; Murillo, F. Early risk factors for sepsis in patients with severe blunt trauma. Injury 2001, 32, 5–12. [Google Scholar] [CrossRef]
- Osborn, T.M.; Tracy, J.K.; Dunne, J.R.; Pasquale, M.; Napolitano, L.M. Epidemiology of sepsis in patients with traumatic injury. Crit. Care Med. 2004, 32, 2234–2240. [Google Scholar] [CrossRef]
- Eguia, E.; Cobb, A.N.; Baker, M.S.; Joyce, C.; Gilbert, E.; Gonzalez, R.; Afshar, M.; Churpek, M.M. Risk factors for infection and evaluation of Sepsis-3 in patients with trauma. Am. J. Surg. 2019, 218, 851–857. [Google Scholar] [CrossRef]
- De Belvis, A.; Pennisi, M.A.; Antonelli, M.; Franceschi, F.; Bocci, M.G.; Balducci, F.M.; Loconsole, L.; Ribaldi, S.; Cingolani, E. Major trauma critical pathway: Preliminary results from the monitoring system in the regional network and in a hub center in Rome metropolitan area. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7230–7239. [Google Scholar] [PubMed]
- Muntean, D.; Horhat, F.G.; Bădițoiu, L.; Dumitrașcu, V.; Bagiu, I.C.; Horhat, D.I.; Coșniță, D.A.; Krasta, A.; Dugăeşescu, D.; Licker, M. Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study of Trends in a Tertiary Healthcare Unit. Medicina 2018, 54, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, M.; Tiseo, G.; Dentali, F.; La Regina, M.; Foglia, E.; Gambacorta, M.; Garagiola, E.; Bonardi, G.; Clerici, P.; Colombo, F.; et al. Predicting Resistant Etiology in Hospitalized Patients with Blood Cultures Positive for Gram-Negative Bacilli. Eur. J. Intern. Med. 2018, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Europe and European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe, 2020 Data; Executive Summary; WHO Regional Office for Europe: Copenhagen, Denmark, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2020 (accessed on 12 August 2022).
- Italian Ministry of Labor, Health and Social Policies. Italian Version of ICD-9-CM. International Classification of Diseases, 9th Revision, Clinical Modification. 2007. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2251_allegato.pdf (accessed on 6 June 2022).
- Centers for Disease Control and Prevention (CDC) Facility Guidance for Control of Carbapenem Resistant Enterobacteriaceae (CRE) November 2015 Update. Available online: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf (accessed on 15 October 2022).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI supplement M100 (ISBN 978-1-68440-032-4 [Print]; ISBN 978-1-68440-033-1 [Electronic]). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA, 2019. Available online: https://clsi.org/media/2663/m100ed29_sample.pdf (accessed on 12 August 2022).
- Casadevall, A.; Pirofski, L.-A. Host–pathogen interactions: Basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 2000, 68, 6511–6518. [Google Scholar] [CrossRef] [Green Version]
- Center for Disease and Prevention Control National Healthcare Safety Network (NHSN) Overview Patient Safety Component Manual. 2022. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf (accessed on 12 August 2022).
- Migliara, G.; Di Paolo, C.; Barbato, D.; Baccolini, V.; Salerno, C.; Nardi, A.; Alessandri, F.; Giordano, A.; Tufi, D.; Marinelli, L.; et al. Multimodal surveillance of healthcare associated infections in an intensive care unit of a large teaching hospital. Ann. Ig. 2019, 31, 399–413. [Google Scholar]
- Russo, A.; Bassetti, M.; Ceccarelli, G.; Carannante, N.; Losito, A.R.; Bartoletti, M.; Corcione, S.; Granata, G.; Santoro, A.; Giacobbe, D.R.; et al. ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: Clinical features, therapy and outcome from a multicenter study. J. Infect. 2019, 79, 130–138. [Google Scholar]
- Falcone, M.; Russo, A.; Iacovelli, A.; Restuccia, G.; Ceccarelli, G.; Giordano, A.; Farcomeni, A.; Morelli, A.; Venditti, M. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin. Microbiol. Infect. 2016, 22, 444–450. [Google Scholar] [CrossRef]
- Açma, A.; Williams, A.; Repetto, E.; Cabral, S.; Sunyoto, T.; Woolley, S.C.; Mahama, G. Prevalence of MDR bacteria in an acute trauma hospital in Port-au-Prince, Haiti: A retrospective analysis from 2012 to 2018. JAC Antimicrob. Resist. 2021, 3, dlab140. [Google Scholar] [CrossRef]
- Barbadoro, P.; Dichiara, A.; Arsego, D.; Ponzio, E.; Savini, S.; Manso, E.; D’Errico, M.M.; The Mica-Net Collaborative Group. Spread of Carbapenem-Resistant Klebsiella pneumoniae in Hub and Spoke Connected Health-Care Networks: A Case Study from Italy. Microorganisms 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Wille, I.; Mayr, A.; Kreidl, P.; Brühwasser, C.; Hinterberger, G.; Fritz, A.; Posch, W.; Fuchs, S.; Obwegeser, A.; Orth-Höller, D.; et al. Cross-sectional point prevalence survey to study the environmental contamination of nosocomial pathogens in intensive care units under real-life conditions. J. Hosp. Infect. 2018, 98, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, X.; Luo, M.; Xu, X.; Su, K.; Chen, S.; Qing, Y.; Li, Y.; Qiu, J. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microb. Drug Resist. 2018, 24, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.; Harris, A.D.; Rock, C.; Johnson, J.K.; Pineles, L.; Bonomo, R.A.; Srinivasan, A.; Pettigrew, M.M.; Thom, K.A.; the CDC Epicenters Program. Risk Factors and Outcomes Associated with Multidrug-Resistant Acinetobacter baumannii upon Intensive Care Unit Admission. Antimicrob. Agents Chemother. 2017, 62, e01631-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Chen, B.; Liu, G.; Ran, J.; Lian, X.; Huang, X.; Wang, N.; Huang, Z. A multi-center study on the risk factors of infection caused by multi-drug resistant Acinetobacter baumannii. BMC Infect. Dis. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, S.E.; Watts, L.T.; Burmeister, D.M.; Merrill, D.; Scroggins, S.; Zou, Y.; Lai, Z.; Grandhi, R.; Lewis, A.M.; Newton, L.M.; et al. Moderate Traumatic Brain Injury Alters the Gastrointestinal Microbiome in a Time-Dependent Manner. Shock 2019, 52, 240–248. [Google Scholar] [CrossRef]
| Total n = 54 (100%) | Not Colonized n = 26 (48.1%) | Colonized n = 28 (51.9%) | p-Value | |
|---|---|---|---|---|
| Sex M—n (%) | 44 (81.49) | 21 (80.77) | 23 (82.14) | 1 |
| Age—mean (sd) | 47.72 (20.25) | 43.38 (19.69) | 51.75 (20.27) | 0.13 |
| Charlson comorbidity index— median (min–max) | 1 (0—5) | 0 (0–5) | 1 (0–4) | 0.634 |
| ISS—mean (sd) | 31.42 (10.87) | 32 (12.4) | 30.9 (9.42) | 0.697 |
| Head and neck AIS— median (min–max) | 3 (0–5) | 3 (0–5) | 3 (0–5) | 0.254 |
| Face AIS—median (min–max) | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0.67 |
| Chest AIS—median (min–max) | 3 (0–5) | 3 (0–5) | 4 (2–5) | 0.647 |
| Abdomen AIS—median (min–max) | 2 (0–5) | 2 (0–5) | 1 (0–5) | 0.273 |
| Extremity—median (min–max) | 2 (0–5) | 2 (0–5) | 2 (0–5) | 0.422 |
| External—median (min–max) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0.892 |
| Pre-ICU days since trauma—median (min–max) | 1 (0–14) | 1 (0–5) | 1 (0–14) | 0.556 |
| SAPS-II—mean (sd) | 43.59 (13.16) | 41.46 (14.03) | 45.57 (12.21) | 0.258 |
| Died in ICU—n (%) | 16 (29.63) | 7 (26.92) | 9 (32.14) | 0.903 |
| ICU length of stay—mean (sd) | 26.98 (23.23) | 14.81 (9.10) | 38.28 (26.6) | <0.001 |
| Mechanical ventilation—n (%) | 52 (96.3) | 25 (96.15) | 27 (96.43) | 1 |
| Mechanical ventilation—mean (sd) | 16.81 (16.19) | 8.46 (7.67) | 24.57 (18.18) | <0.001 |
| CRRT—n (%) | 11 (20.37) | 3 (11.54) | 8 (28.5) | 0.224 |
| Vasopressors—n (%) | 40 (74.07) | 17 (65.38) | 23 (82.14) | 0.274 |
| Vasopressors—mean (sd) | 4.09 (6.67) | 2.92 (5.04) | 5.18 (7.83) | 0.211 |
| Transfusion—n (%) | 45 (83.33) | 20 (76.92) | 25 (89.28) | 0.394 |
| ECMO—n (%) | 2 (3.70) | 1 (3.85) | 1 (3.57) | 1 |
| Tracheostomy—n (%) | 25 (46.3) | 9 (34.61) | 16 (57.14) | 0.166 |
| Chest drain—n (%) | 30 (55.55) | 12 (46.15) | 18 (64.28) | 0.286 |
| Surgery—n (%) | 36 (66.67) | 18 (69.23) | 18 (64.28) | 0.923 |
| Chest—n (%) | 2 (3.7) | 1 (3.85) | 1 (3.57) | 1 |
| Abdomen—n (%) | 9 (16.67) | 6 (23.08) | 3 (10.71) | 0.441 |
| Neurosurgery—n (%) | 7 (12.96) | 3 (11.54) | 4 (12.28) | 1 |
| Head and neck—n (%) | 7 (12.96) | 4 (15.38) | 3 (10.71) | 1 |
| Spine—n (%) | 3 (5.55) | 0 (0) | 3 (10.71) | 0.228 |
| Arms—n (%) | 22 (40.74) | 11 (42.31) | 11 (39.28) | 1 |
| Plastic Surgery—n (%) | 1 (1.85) | 1 (3.85) | 1 (3.57) | 1 |
| Overall Colonized (n = 28) | p-Value | ||
|---|---|---|---|
| Not Infected n = 11 (39.3%) | Infected n = 17 (60.7%) | ||
| age—mean (sd) | 49.73 (25.45) | 53.06 (16.85) | 0.707 |
| Charlson comorbidity index—median (min–max) | 0 (0–4) | 1 (0–3) | 0.012 |
| ISS—mean (sd) | 28.72 (7.27) | 32.23 (10.56) | 0.308 |
| Head and neck—median (min–max) | 2 (0–4) | 3 (0–5) | 0.476 |
| Face—median (min–max) | 0 (0–2) | 0 (0–3) | 0.130 |
| Chest—median (min–max) | 3 (2–5) | 4 (3–5) | 0.997 |
| Abdomen—median (min–max) | 2 (0–5) | 0 (0–4) | 0.787 |
| Extremity—median (min–max) | 3 (0–4) | 0 (0–5) | 0.381 |
| External—median (min–max) | 0 (0–0) | 0 (0–1) | 0.421 |
| Pre-ICU days since trauma—median (min–max) | 1 ( 0–14) | 1 (0–4) | 0.058 |
| SAPS II—mean (sd) | 44.36 (14.89) | 46.35 (10.55) | 0.705 |
| ICU length of stay—mean (sd) | 24.82 (16.77) | 47 (28.51) | 0.016 |
| Mechanical ventilation—n (%) | 10 (90.91) | 17 (100) | 0.823 |
| Mechanical ventilation—mean (sd) | 13.54 (15.84) | 31.7 (16.22) | 0.008 |
| CRRT—n (%) | 1 (9.09) | 7 (41.18) | 0.159 |
| Vasopressors—n (%) | 8 (72.73) | 15 (88.23) | 0.588 |
| Vasopressors—mean (sd) | 1.09 (1.14) | 7.82 (9.15) | 0.008 |
| Transfusion—n (%) | 10 (90.91) | 15 (88.23) | 1 |
| ECMO—n (%) | 0 (0) | 1 (5.88) | 1 |
| Tracheostomy—n (%) | 5 (45.45) | 11 (67.7) | 0.539 |
| Chest drain—n (%) | 6 (54.54) | 12 (70.59) | 0.644 |
| Surgery—n (%) | 8 (72.73) | 10 (58.82) | 0.729 |
| Time to colonization—mean (sd) days | 10.09 (7.37) | 8.94 (7.63) | 0.695 |
| Antibiotic before colonization—n (%) | 11 (100) | 17 (100) | N/A |
| Antibiotic before colonization—mean (sd) days | 8.36 (4.29) | 9.53 (4.58) | 0.502 |
| Piperacillin/tazobactam—n (%) | 5 (45.45) | 9 (52.94) | 1 |
| Pip. tazo—mean piperacillin/tazobactam (sd) days | 1.64 (2.01) | 3.12 (3.55) | 0.172 |
| Carbapenems—n (%) | 1 (9.09) | 5 (29.41) | 0.419 |
| Carbapenems—mean (sd) days | 0.09 (0.30) | 1.23 (2.11) | 0.042 |
| Cephalosporines—n (%) | 1 (9.09) | 1 (5.88) | 1 |
| Cephalosporines—mean (sd) days | 1.27 (4.22) | 0.29 (1.21) | 0.469 |
| Fluoroquinolones—n (%) | 0 (0) | 1 (5.88) | 1 |
| Fluoroquinolones—mean (sd) days | 0 (0) | 0.59 (2.42) | 0.332 |
| Glicopeptides—n (%) | 4 (36.36) | 7 (41.18) | 1 |
| Glicopeptides—mean (sd) days | 2 (3.19) | 1.65 (2.39) | 0.757 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, G.; Alessandri, F.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Fabris, S.; Russo, A.; Ruberto, F.; De Meo, D.; Ciccozzi, M.; et al. Clinical Impact of Colonization with Carbapenem-Resistant Gram-Negative Bacteria in Critically Ill Patients Admitted for Severe Trauma. Pathogens 2022, 11, 1295. https://doi.org/10.3390/pathogens11111295
Ceccarelli G, Alessandri F, Moretti S, Borsetti A, Maggiorella MT, Fabris S, Russo A, Ruberto F, De Meo D, Ciccozzi M, et al. Clinical Impact of Colonization with Carbapenem-Resistant Gram-Negative Bacteria in Critically Ill Patients Admitted for Severe Trauma. Pathogens. 2022; 11(11):1295. https://doi.org/10.3390/pathogens11111295
Chicago/Turabian StyleCeccarelli, Giancarlo, Francesco Alessandri, Sonia Moretti, Alessandra Borsetti, Maria Teresa Maggiorella, Silvia Fabris, Alessandro Russo, Franco Ruberto, Daniele De Meo, Massimo Ciccozzi, and et al. 2022. "Clinical Impact of Colonization with Carbapenem-Resistant Gram-Negative Bacteria in Critically Ill Patients Admitted for Severe Trauma" Pathogens 11, no. 11: 1295. https://doi.org/10.3390/pathogens11111295
APA StyleCeccarelli, G., Alessandri, F., Moretti, S., Borsetti, A., Maggiorella, M. T., Fabris, S., Russo, A., Ruberto, F., De Meo, D., Ciccozzi, M., Mastroianni, C. M., Venditti, M., Pugliese, F., & d’Ettorre, G. (2022). Clinical Impact of Colonization with Carbapenem-Resistant Gram-Negative Bacteria in Critically Ill Patients Admitted for Severe Trauma. Pathogens, 11(11), 1295. https://doi.org/10.3390/pathogens11111295












