Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis
Abstract
1. Introduction
2. Materials and Methods
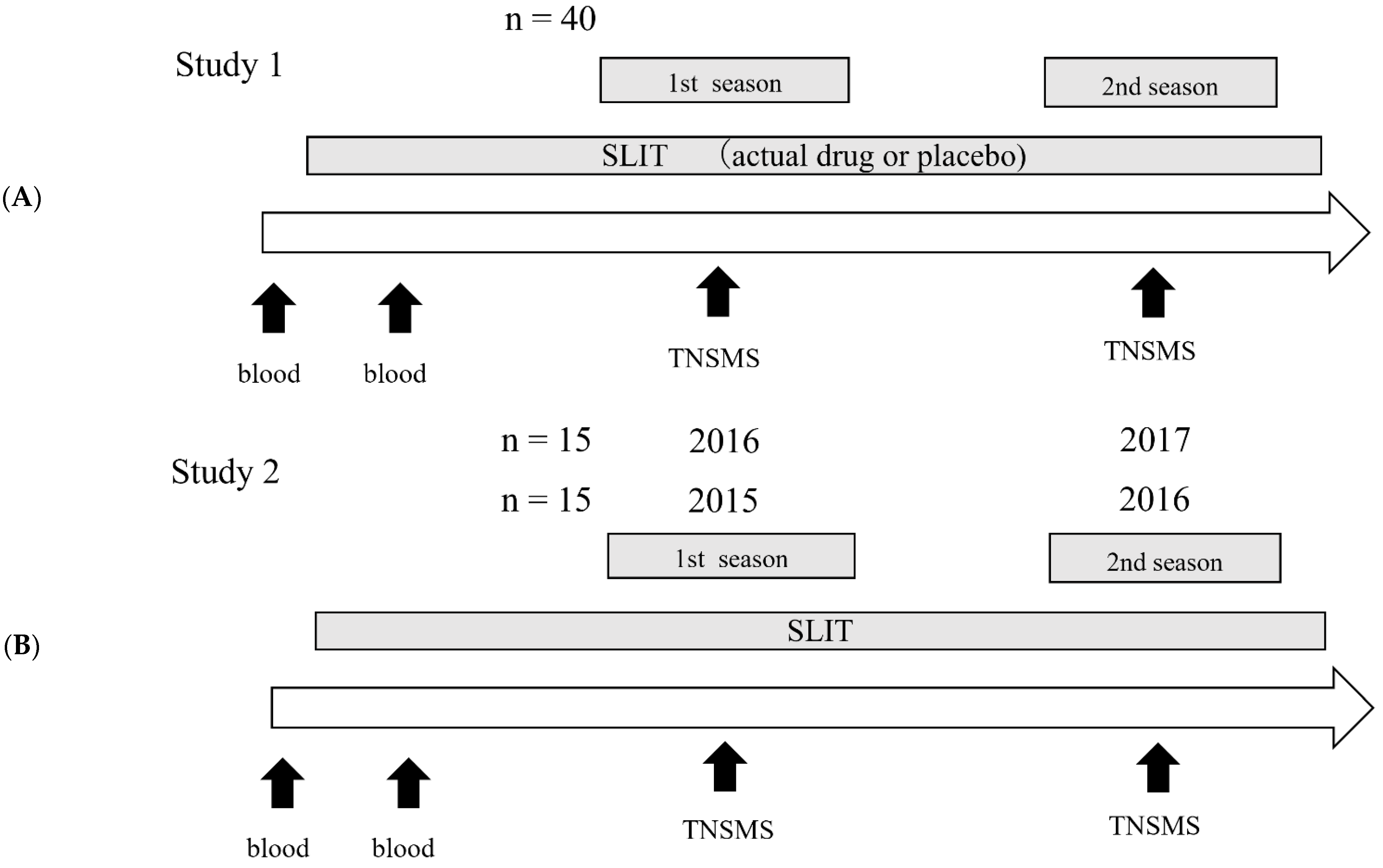
2.1. Study Design
2.2. Determination of Treatment Efficacy
2.2.1. Study 1
2.2.2. Study 2
2.3. Microarray
2.4. Real-Time PCR
2.5. Statistical Analysis
3. Results
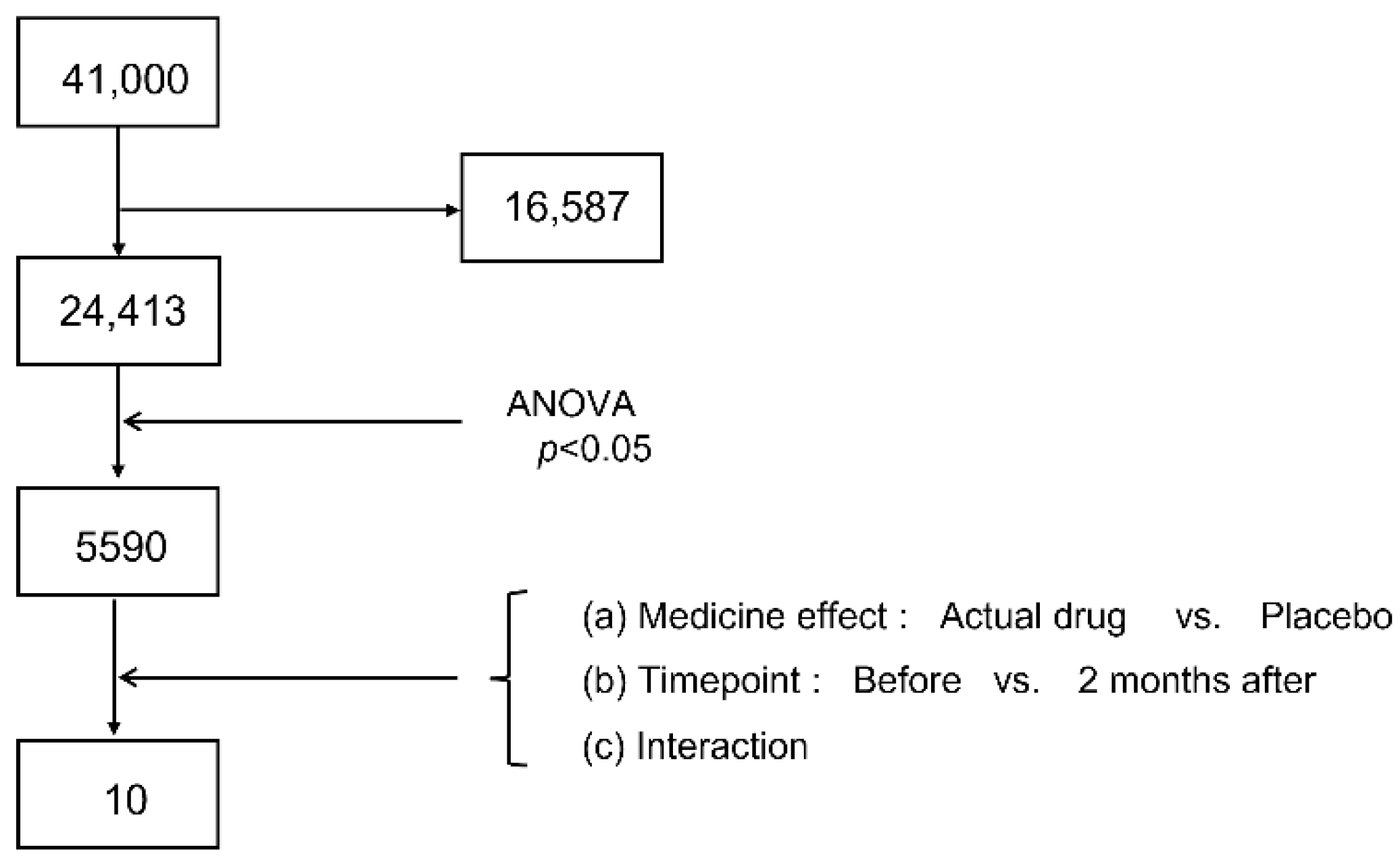
3.1. Candidate Narrowing by Microarray
3.2. Candidate Narrowing by Real-Time PCR
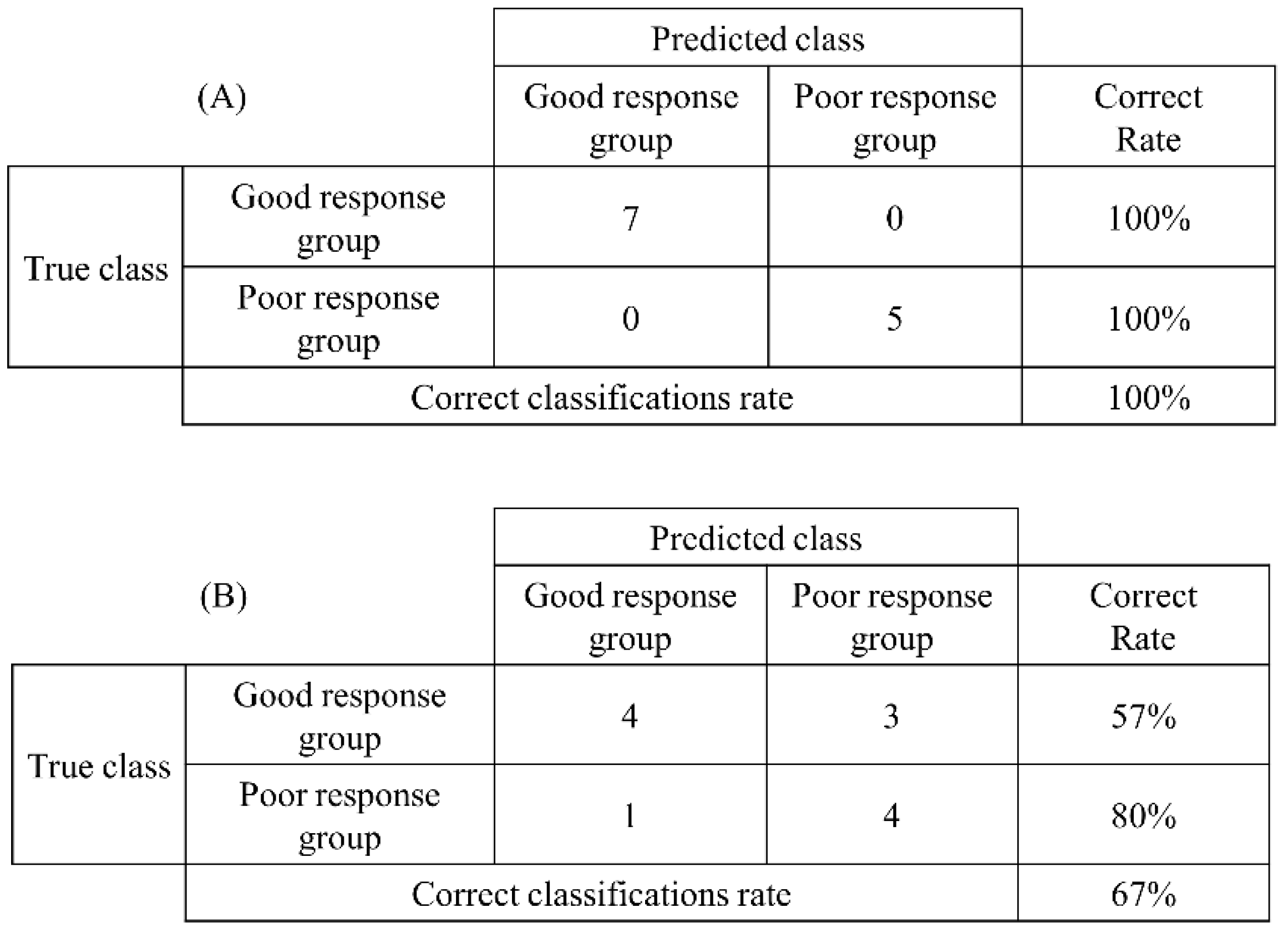
3.3. Evaluation of Prediction Accuracy by Discriminant Analysis
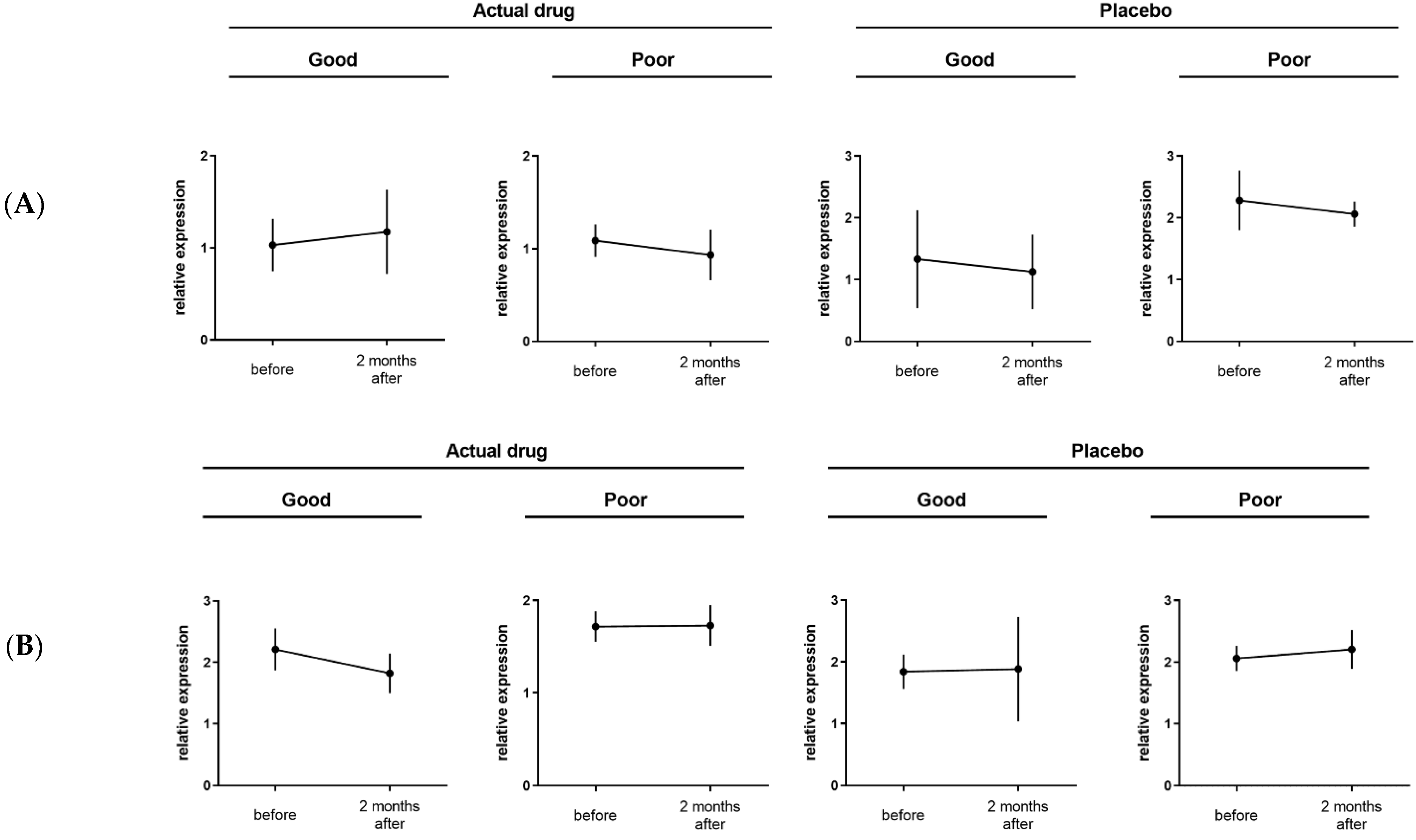
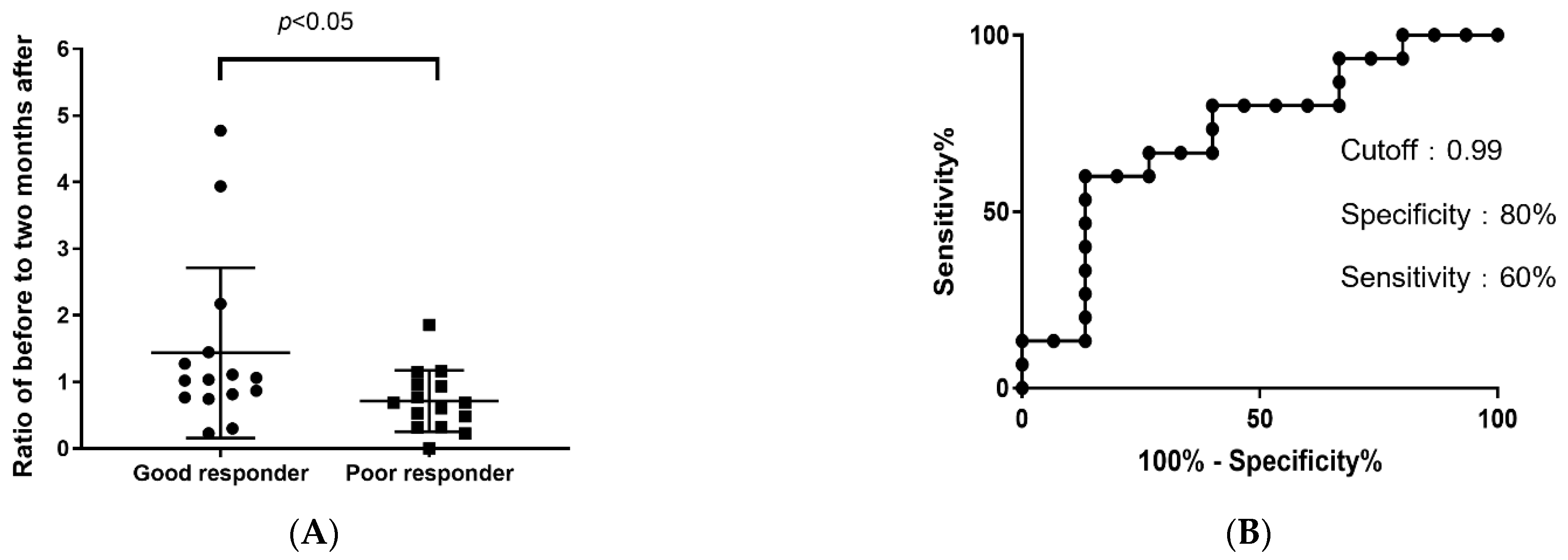
3.4. Efficacy of CFH as a Biomarker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese Society of Allergology. Japanese guidelines for allergic rhinitis 2020. Allergol. Int. 2020, 69, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Saito, H.; Fujieda, S. Present state of Japanese cedar pollinosis: The national affliction. J. Allergy Clin. Immunol. 2014, 133, 632–639.e5. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, S.; Okamoto, Y.; Yonekura, S.; Okawa, T.; Yamamoto, H.; Kunii, N.; Sakurai, D.; Fujimura, T.; Nakazawa, K.; Yasueda, H. A randomized controlled trial of sublingual immunotherapy for Japanese cedar pollinosis. Int. Arch. Allergy Immunol. 2008, 146, 76–84. [Google Scholar] [CrossRef]
- Okubo, K.; Gotoh, M.; Fujieda, S.; Okano, M.; Yoshida, H.; Morikawa, H.; Masuyama, K.; Okamoto, Y.; Kobayashi, M. A randomized double-blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergol. Int. 2008, 57, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Yonekura, S.; Horiguchi, S.; Taniguchi, Y.; Saito, A.; Yasueda, H.; Inamine, A.; Nakayama, T.; Takemori, T.; Taniguchi, M.; et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin. Immunol. 2011, 139, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Okubo, K.; Yonekura, S.; Hashiguchi, K.; Goto, M.; Otsuka, T.; Murata, T.; Nakao, Y.; Kanazawa, C.; Nagakura, H.; et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int. Arch. Allergy Immunol. 2015, 166, 177–188. [Google Scholar] [CrossRef]
- Yonekura, S.; Gotoh, M.; Kaneko, S.; Kanazawa, K.; Takeuji, Y.; Okubo, K.; Okamoto, Y. Treatment duration-dependent efficacy of Japanese cedar pollen sublingual immunotherapy: Evaluation of a phase II/III trial over three pollen dispersal seasons. Allergol. Int. 2019, 68, 494–505. [Google Scholar] [CrossRef]
- Sakurai, D.; Yonekura, S.; Iinuma, T.; Sakurai, T.; Morimoto, Y.; Mita, Y.; Arai, T.; Suzuki, S.; Okuma, Y.; Kaneko, S.; et al. Sublingual immunotherapy for allergic rhinitis: Subjective versus objective tools to evaluate its success. Rhinology 2016, 54, 221–230. [Google Scholar] [CrossRef]
- Antico, A. Long-term adherence to sublingual therapy: Literature review and suggestions for management strategies based on patients’ needs and preferences. Clin. Exp. Allergy 2014, 44, 1314–1326. [Google Scholar] [CrossRef]
- Shamji, M.H.; Kappen, J.H.; Akdis, M.; Jensen-Jarolim, E.; Knol, E.F.; Kleine-Tebbe, J.; Bohle, B.; Chaker, A.M.; Till, S.J.; Valenta, R.; et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI Position Paper. Allergy 2017, 72, 1156–1173. [Google Scholar] [CrossRef]
- Piconi, S.; Trabattoni, D.; Rainone, V.; Borgonovo, L.; Passerini, S.; Rizzardini, G.; Frati, F.; Iemoli, E.; Clerici, M. Immunological effects of sublingual immunotherapy: Clinical efficacy is associated with modulation of programmed cell death ligand 1, IL-10, and IgG4. J. Immunol. 2010, 185, 7723–7730. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.W.; Shamji, M.H.; Jacobson, M.R.; Lee, D.I.; Wilson, D.; Lima, M.T.; Pitkin, L.; Pilette, C.; Nouri-Aria, K.; Durham, S.R. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin. Exp. Allergy 2010, 40, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020–January 18, 2021. JAMA 2021, 325, 1101–1102. [Google Scholar] [CrossRef]
- Shamji, M.H.; Durham, S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J. Allergy Clin. Immunol. 2017, 140, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Yonekura, S.; Taniguchi, Y.; Horiguchi, S.; Saito, A.; Yasueda, H.; Nakayama, T.; Takemori, T.; Taniguchi, M.; Sakaguchi, M.; et al. The induced regulatory T cell level, defined as the proportion of IL-10+Foxp3+ cells among CD25+CD4+ leukocytes, is a potential therapeutic biomarker for sublingual immunotherapy: A preliminary report. Int. Arch. Allergy Immunol. 2010, 153, 378–387. [Google Scholar] [CrossRef]
- Makou, E.; Herbert, A.P.; Barlow, P.N. Functional anatomy of complement factor H. Biochemistry 2013, 52, 3949–3962. [Google Scholar] [CrossRef] [PubMed]
- Klos, A.; Tenner, A.J.; Johswich, K.O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef]
- Zhang, X.; Köhl, J. A complex role for complement in allergic asthma. Expert Rev. Clin. Immunol. 2010, 6, 269–277. [Google Scholar] [CrossRef]
- Sakashita, M.; Yamada, T.; Imoto, Y.; Hirota, T.; Tamari, M.; Ito, Y.; Kubo, S.; Osawa, Y.; Takahashi, N.; Fujieda, S. Long-term sublingual immunotherapy for Japanese cedar pollinosis and the levels of IL-17A and complement components 3a and 5a. Cytokine 2015, 75, 181–185. [Google Scholar] [CrossRef]
- Li, H.; Xu, E.; He, M. Cytokine responses to specific immunotherapy in house dust mite-induced allergic rhinitis patients. Inflammation 2015, 38, 2216–2223. [Google Scholar] [CrossRef]
- Yoshida, K.; Takabayashi, T.; Imoto, Y.; Sakashita, M.; Kato, Y.; Narita, N.; Fujieda, S. Increased thrombin-activatable fibrinolysis inhibitor in response to sublingual immunotherapy for allergic rhinitis. Laryngoscope 2021, 131, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
| Act/Good | Sex | Age | sIgE | First Season Severity | Second Season Severity |
|---|---|---|---|---|---|
| F | 60 | 50.9 | moderate | mild | |
| M | 61 | 31.4 | very severe | moderate | |
| M | 52 | 20.4 | very severe | moderate | |
| F | 43 | 16.0 | severe | mild | |
| F | 47 | 8.7 | moderate | mild | |
| F | 29 | 8.5 | moderate | mild | |
| F | 48 | 6.7 | severe | mild | |
| Act/Poor | Sex | Age | sIgE | First Season Severity | Second Season Severity |
| F | 55 | 100.0 | moderate | moderate | |
| F | 42 | 10.5 | moderate | moderate | |
| M | 48 | 8.9 | moderate | moderate | |
| F | 51 | 5.2 | moderate | severe | |
| Plc/Good | Sex | Age | sIgE | First Season Severity | Second Season Severity |
| F | 49 | 52.8 | very severe | moderate | |
| M | 43 | 19.5 | very severe | moderate | |
| F | 37 | 8.8 | severe | mild | |
| F | 48 | 8.8 | severe | mild | |
| F | 41 | 5.8 | moderate | mild | |
| Plc/Poor | Sex | Age | sIgE | First Season Severity | Second Season Severity |
| M | 58 | 38.1 | moderate | severe | |
| F | 52 | 27.1 | moderate | moderate | |
| M | 43 | 16.5 | severe | severe | |
| M | 58 | 15.6 | moderate | moderate | |
| F | 49 | 8.2 | moderate | moderate | |
| F | 28 | 5.2 | moderate | moderate | |
| F | 26 | 3.8 | moderate | moderate |
| Good | Sex | Age | sIgE | First Season TNSMS | Second Season TNSMS |
|---|---|---|---|---|---|
| F | 35 | 96.9 | 7.58 | 5.10 | |
| M | 43 | 85.7 | 5.94 | 4.35 | |
| F | 41 | 65.9 | 7.68 | 5.00 | |
| F | 62 | 58.4 | 5.71 | 4.32 | |
| M | 34 | 39.3 | 8.32 | 6.48 | |
| F | 18 | 37.8 | 8.43 | 5.05 | |
| F | 41 | 34.3 | 6.84 | 2.76 | |
| M | 21 | 32.6 | 3.00 | 1.43 | |
| F | 25 | 20.7 | 8.89 | 3.00 | |
| F | 63 | 19.2 | 4.43 | 3.26 | |
| F | 49 | 17.8 | 5.10 | 3.11 | |
| F | 61 | 10.1 | 7.16 | 5.67 | |
| M | 61 | 7.3 | 3.32 | 2.86 | |
| M | 27 | 5.2 | 5.05 | 3.71 | |
| F | 55 | 3.9 | 2.38 | 1.53 | |
| Poor | Sex | Age | sIgE | First Season TNSMS | Second Season TNSMS |
| F | 50 | 39.3 | 3.67 | 3.21 | |
| M | 24 | 21.3 | 3.11 | 3.38 | |
| M | 28 | 15.3 | 4.19 | 5.84 | |
| F | 30 | 11.1 | 3.16 | 3.00 | |
| F | 54 | 10.5 | 6.05 | 7.53 | |
| F | 42 | 8.6 | 8.29 | 8.16 | |
| F | 52 | 8.0 | 5.00 | 8.05 | |
| M | 49 | 7.2 | 2.22 | 2.53 | |
| F | 57 | 7.1 | 4.16 | 3.95 | |
| F | 56 | 6.8 | 6.20 | 6.17 | |
| F | 45 | 5.9 | 2.81 | 3.42 | |
| F | 45 | 4.8 | 4.19 | 5.16 | |
| M | 48 | 3.5 | 2.70 | 3.26 | |
| F | 60 | 1.8 | 1.74 | 2.62 | |
| M | 31 | 1.1 | 3.21 | 3.10 |
| ANOVA | Lower Rank Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Time | Interaction | Act/Good | Act/Poor | Plc/Good | Plc/Poor | ||||||
| Gene Symbol | ANOVA Category | p-Value | p-Value | p-Value | FC | Reg. | FC | Reg. | FC | Reg. | FC | Reg. |
| CFH | a | 0.0239 | 0.7449 | 0.4867 | 1.23 | up | 1.08 | down | 1.10 | down | 1.02 | down |
| PRKCB | a | 0.0321 | 0.6997 | 0.1785 | 1.14 | down | 1.06 | up | 1.04 | up | 1.02 | up |
| SEPT7P2 | a | 0.0237 | 0.8003 | 0.2965 | 1.14 | down | 1.06 | up | 1.03 | up | 1.04 | up |
| GOLIM4 | b | 0.5854 | 0.0151 | 0.2764 | 1.50 | down | 1.03 | up | 1.14 | up | 1.24 | up |
| EIF2D | c | 0.5891 | 0.4864 | 0.0091 | 1.08 | up | 1.00 | down | 1.03 | down | 1.10 | down |
| FSCN1 | c | 0.5431 | 0.8282 | 0.0415 | 1.83 | up | 1.70 | down | 1.06 | down | 1.10 | down |
| DTX3 | c | 0.4475 | 0.0793 | 0.0097 | 1.13 | up | 1.32 | down | 1.01 | down | 1.17 | down |
| TTC39C | c | 0.2651 | 0.2308 | 0.0278 | 1.12 | up | 1.18 | down | 1.03 | down | 1.12 | down |
| TUBB3 | c | 0.5786 | 0.2574 | 0.0058 | 1.30 | down | 1.14 | up | 1.00 | up | 1.52 | up |
| ATP8B3 | c | 0.3515 | 0.1176 | 0.0114 | 1.18 | down | 1.13 | up | 1.17 | up | 1.23 | up |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneda, R.; Iinuma, T.; Sakurai, D.; Kurita, J.; Arai, T.; Sonobe, Y.; Yonekura, S.; Okamoto, Y.; Hanazawa, T. Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis. Pathogens 2022, 11, 1280. https://doi.org/10.3390/pathogens11111280
Yoneda R, Iinuma T, Sakurai D, Kurita J, Arai T, Sonobe Y, Yonekura S, Okamoto Y, Hanazawa T. Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis. Pathogens. 2022; 11(11):1280. https://doi.org/10.3390/pathogens11111280
Chicago/Turabian StyleYoneda, Riyo, Tomohisa Iinuma, Daiju Sakurai, Junya Kurita, Tomoyuki Arai, Yuri Sonobe, Syuji Yonekura, Yoshitaka Okamoto, and Toyoyuki Hanazawa. 2022. "Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis" Pathogens 11, no. 11: 1280. https://doi.org/10.3390/pathogens11111280
APA StyleYoneda, R., Iinuma, T., Sakurai, D., Kurita, J., Arai, T., Sonobe, Y., Yonekura, S., Okamoto, Y., & Hanazawa, T. (2022). Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis. Pathogens, 11(11), 1280. https://doi.org/10.3390/pathogens11111280






