Helicobacter trogontum Bacteremia and Lower Limb Skin Lesion in a Patient with X-Linked Agammaglobulinemia—A Case Report and Review of the Literature
Abstract
1. Introduction
1.1. Immunology
1.2. Microbiology
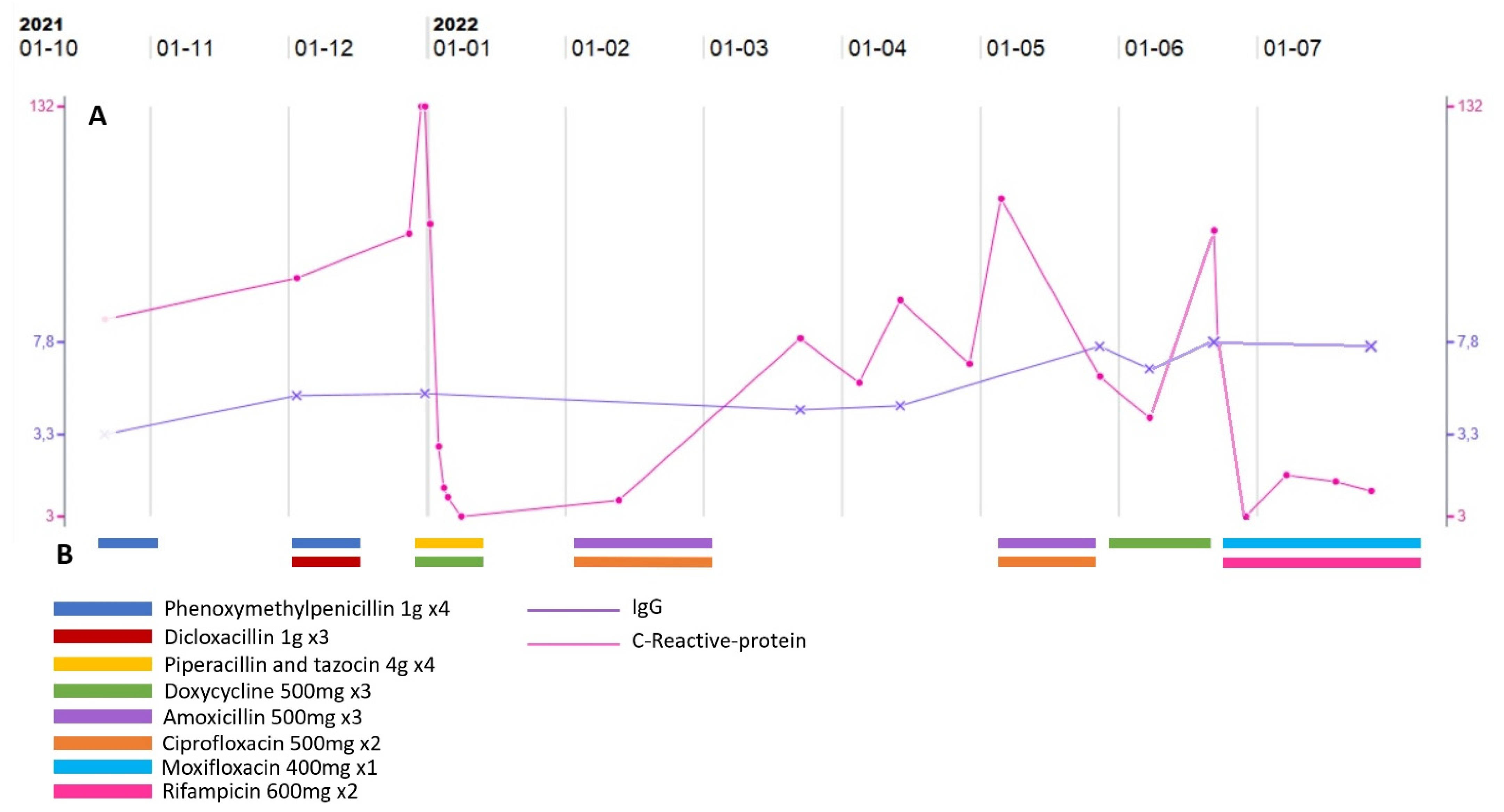
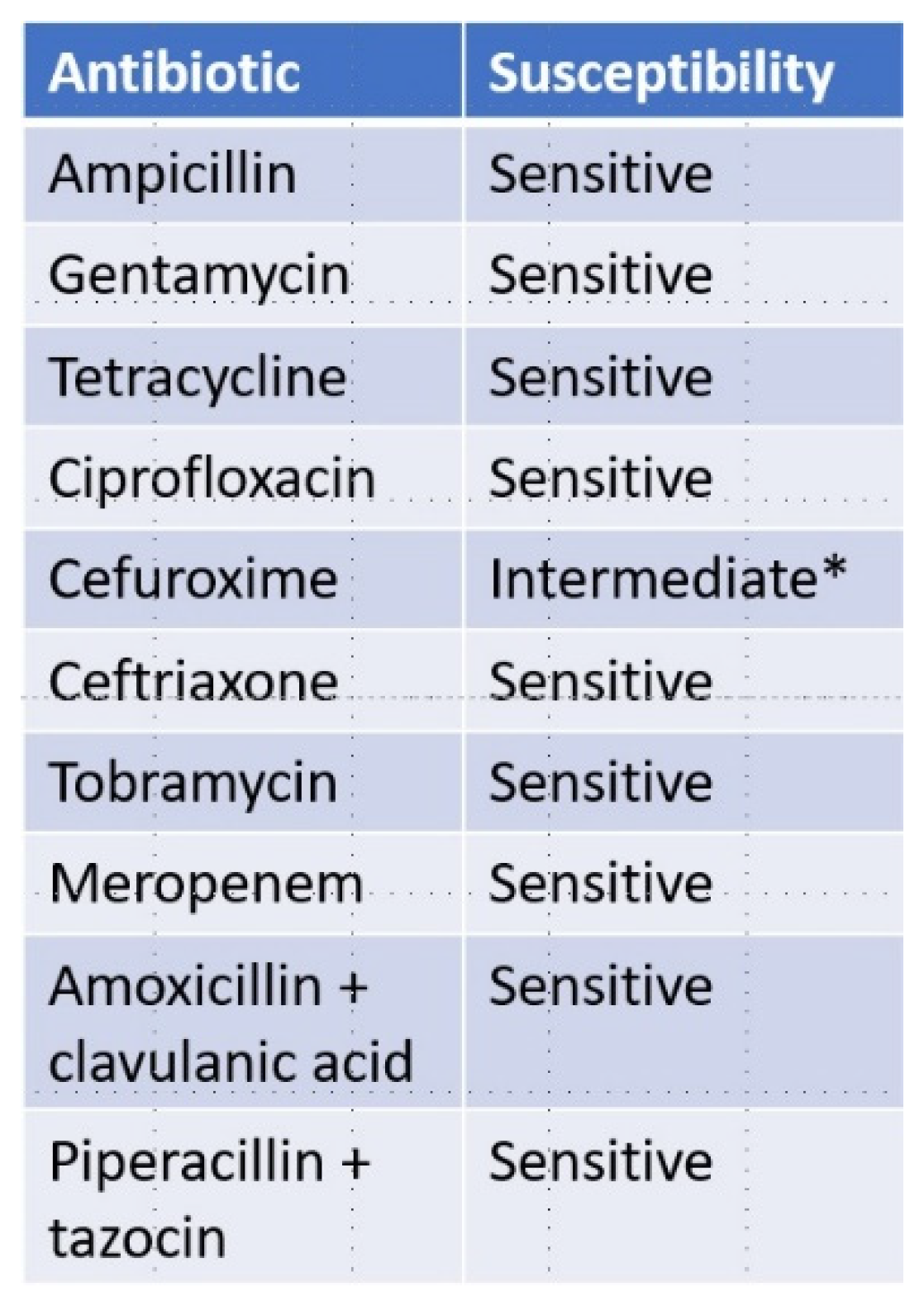
1.3. Case Presentation
2. Review of the Literature
3. Discussion
3.1. Why Do Patients with XLA Seem to Be Especially Susceptible to Infection with Enterohepatic Helicobacters?
3.2. What Is the Nature of the Skin Lesions?
3.3. Could Enterohepatic Helicobacters Be the Causative Agents of Other Diseases?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ochs, H.D.; Smith, E.C.I. X-linked agammaglobulinemia. A clinical and molecular analysis. Medicine 1996, 75, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.E.; Mathias, D.; Treadaway, J.; Minegishi, Y.; Rohrer, J. Mutations in Btk in patients with presumed X-linked agammaglobulinemia. Am. J. Hum. Genet. 1998, 62, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Groth, D.; Wright, H.; Bonilla, F.A.; Fuleihan, R.L.; Cunningham-Rundles, C.; Feuille, E. X-Linked Agammaglobulinemia: Infection Frequency and Infection-Related Mortality in the USIDNET Registry. J. Clin. Immunol. 2022, 42, 827–836. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Z.A.; Abramova, I.; Aldave, J.C.; Al-Herz, W.; Bezrodnik, L.; Boukari, R.; Sullivan, K.E. X-linked agammaglobulinemia (XLA): Phenotype, diagnosis, and therapeutic challenges around the world. World Allergy Organ. J. 2019, 12, 100018. [Google Scholar] [CrossRef]
- Perez, E.E.; Orange, J.S.; Bonilla, F.; Chinen, J.; Chinn, I.K.; Dorsey, M.; Ballow, M. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017, 139, S1–S46. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Ochoa, S.; Collado, L. Enterohepatic Helicobacter species–clinical importance, host range, and zoonotic potential. Crit. Rev. Microbiol. 2021, 47, 728–761. [Google Scholar] [CrossRef]
- Romero, S.; Archer, J.R.; Hamacher, M.E.; Bollogna, S.M.; Schell, R.F. Case report of an unclassified microaerophilic bacterium associated with gastroenteritis. J. Clin. Microbiol. 1988, 26, 142–143. [Google Scholar] [CrossRef]
- On, S.L.W. Identification methods for campylobacters, helicobacters, and related organisms. Clin. Microbiol. Rev. 1996, 9, 405–422. [Google Scholar] [CrossRef]
- Wesley, I.V.; Wesley, R.D.; Cardella, M.; Dewhirst, F.E.; Paster, B.J. Oligodeoxynucleotide probes for Campylobacter fetus and Campylobacter hyointestinalis based on 16S rRNA sequences. J. Clin. Microbiol. 1991, 29, 1812–1817. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Fox, J.G.; Mendes, E.N.; Paster, B.J.; Gates, C.E.; Kirkbride, C.A.; Eaton, K.A. “Flexispira rappini” strains represent at least 10 Helicobacter taxa. Int. J. Syst. Evol. Microbiol. 2000, 50, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Dutasta, F.; Samaha, E.; Carayol, N.; Masse, J.M.; Bourillon, C.; Richaud, C.; Podglajen, I. Acute colitis caused by Helicobacter trogontum in immunocompetent patient. Emerg. Infect. Dis. 2016, 22, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Tee, W.; Leder, K.; Karroum, E.; Dyall-Smith, M. “Flexispira rappini” bacteremia in a child with pneumonia. J. Clin. Microbiol. 1998, 36, 1679–1682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sorlin, P.; Vandamme, P.; Nortier, J.; Hoste, B.; Rossi, C.; Pavlof, S.; Struelens, M.J. Recurrent “Flexispira rappini” bacteremia in an adult patient undergoing hemodialysis: Case report. J. Clin. Microbiol. 1999, 37, 1319–1323. [Google Scholar] [CrossRef][Green Version]
- Weir, S.; Cuccherini, B.; Whitney, A.M.; Ray, M.L.; MacGregor, J.P.; Steigerwalt, A.; Gill, V.J. Recurrent bacteremia caused by a ’Flexispira’-like organism in a patient with X-linked (Bruton’s) agammaglobulinemia. J. Clin. Microbiol. 1999, 37, 2439–2445. [Google Scholar] [CrossRef]
- Cuccherini, B.; Chua, K.; Gill, V.; Weir, S.; Wray, B.; Stewart, D.; Strober, W. Bacteremia and skin/bone infections in two patients with X-linked agammaglobulinemia caused by an unusual organism related to Flexispira/Helicobacter species. Clin. Immunol. 2000, 97, 121–129. [Google Scholar] [CrossRef]
- Han, S.R.; Schindel, C.; Genitsariotis, R.; Märker-Hermann, E.; Bhakdi, S.; Maeurer, M.J. Identification of a unique Helicobacter species by 16S rRNA gene analysis in an abdominal abscess from a patient with X-linked hypogammaglobulinemia. J. Clin. Microbiol. 2000, 38, 2740–2742. [Google Scholar] [CrossRef]
- Iten, A.; Graf, S.; Egger, M.; Täuber, M.; Graf, J. Helicobacter sp. Flexispira bacteremia in an immunocompetent young adult. J. Clin. Microbiol. 2001, 39, 1716–1720. [Google Scholar] [CrossRef]
- Gerrard, J.; Alfredson, D.; Smith, I. Recurrent bacteremia and multifocal lower limb cellulitis due to Helicobacter-like organisms in a patient with X-linked hypogammaglobulinemia. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 33, E116–E118. [Google Scholar] [CrossRef]
- Brachet-Castang, C.; Viallard, J.F.; Fourche, J.; Le Flèche, A.; Grimont, P.A. Bactériémie à Flexispira rappini et déficit immunitaire commun variable. Med. Mal. Infect. 2005, 35, 95–97. [Google Scholar] [CrossRef]
- Murray, P.; Lee, C.R.; Turner, M.L. Pyoderma Gangrenosum–Like Ulcer in a Patient With X-Linked Agammaglobulinemia. Arch. Dermatol. 2010, 146, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Romo-Gonzalez, C.; Bustamante-Ogando, J.C.; Yamazaki-Nakashimada, M.A.; Aviles-Jimenez, F.; Otero-Mendoza, F.; Espinosa-Rosales, F.J.; Lopez-Herrera, G. Infections with Enterohepatic Non-H. pylori Helicobacter Species in X-Linked Agammaglobulinemia: Clinical Cases and Review of the Literature. Front. Cell. Infect. Microbiol. 2022, 11, 1–12. [Google Scholar] [CrossRef]
- Araoka, H.; Baba, M.; Okada, C.; Kimura, M.; Sato, T.; Yatomi, Y.; Yoneyama, A. Risk factors for recurrent helicobacter cinaedi bacteremia and the efficacy of selective digestive decontamination with kanamycin to prevent recurrence. Clin. Infect. Dis. 2018, 67, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.E.; Leo, S.H.; Boos, A.; Deans, G.D.; Prendiville, J.; Crawford, R.I.; Morton, T.L. Successful approach to treatment of helicobacter bilis infection in x-linked agammaglobulinemia. J. Clin. Immunol. 2012, 32, 1404–1408. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Moyat, M.; Velin, D. Immune responses to Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 5583–5593. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.N.R.; Bittner, Z.; Liu, X.; Dang, T.M.; Radsak, M.P.; Brunner, C. Bruton’s tyrosine kinase: An emerging key player in innate immunity. Front. Immunol. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Shen, L.; Ogden, S.; Romero–Gallo, J.; Lapierre, L.A.; Israel, D.A.; Peek, R.M., Jr. Helicobacter pylori Dysregulation of Gastric Epithelial Tight Junctions by Urease-Mediated Myosin II Activation. Gastroenterology 2009, 136, 236–246. [Google Scholar] [CrossRef]
- Mannion, A.; Shen, Z.; Fox, J.G. Comparative genomics analysis to differentiate metabolic and virulence gene potential in gastric versus enterohepatic Helicobacter species 06 Biological Sciences 0604 Genetics. BMC Genom. 2018, 19, 1–19. [Google Scholar] [CrossRef]
- Rasmussen, S.L.; Ørsted, I.; Tarpgaard, I.H.; Nielsen, H.L. Helicobacter cinaedi bacteraemia secondary to enterocolitis in an immunocompetent patient. Gut Pathog. 2021, 13, 1–5. [Google Scholar] [CrossRef]
- Greuter, T.; Navarini, A.; Vavricka, S.R. Skin Manifestations of Inflammatory Bowel Disease. Clin. Rev. Allergy Immunol. 2017, 53, 413–427. [Google Scholar] [CrossRef]
- Shimizu, S.; Shimizu, H. Cutaneous manifestations of Helicobacter cinaedi: A review. Br. J. Dermatol. 2016, 175, 62–68. [Google Scholar] [CrossRef]
- Pérez-Garza, D.M.; Chavez-Alvarez, S.; Ocampo-Candiani, J.; Gomez-Flores, M. Erythema Nodosum: A Practical Approach and Diagnostic Algorithm. Am. J. Clin. Dermatol. 2021, 22, 367–378. [Google Scholar] [CrossRef]
- Flora, A.; Kozera, E.; Frew, J.W. Pyoderma gangrenosum: A systematic review of the molecular characteristics of disease. Exp. Dermatol. 2022, 31, 498–515. [Google Scholar] [CrossRef]
- Hansen, R.; Thomson, J.M.; Fox, J.G.; El-Omar, E.M.; Hold, G.L. Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol. Med. Microbiol. 2011, 61, 1–14. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, S.; Li, L.; Xiong, L.; Chao, K.; Zhong, B.; Chen, M. Enterohepatic Helicobacter Species as a Potential Causative Factor in Inflammatory Bowel Disease. Medicine 2015, 94, e1773. [Google Scholar] [CrossRef]
- Pac, M.; Bernatowska, E.A.; Kierkuś, J.; Ryżko, J.P.; Cielecka-Kuszyk, J.; Jackowska, T.; Mikołuć, B. Gastrointestinal disorders next to respiratory infections as leading symptoms of X-linked agammaglobulinemia in children—34-year experience of a single center. Arch. Med. Sci. 2017, 13, 412–417. [Google Scholar] [CrossRef]
- Staines Boone, A.T.; Torres Martínez, M.G.; López Herrera, G.; Portilla, J.O.D.L.; Padilla, S.E.E.; Rosales, F.J.E.; Reyes, S.O.L. Gastric adenocarcinoma in the context of X-linked agammaglobulinemia: Case report and review of the literature. J. Clin. Immunol. 2014, 34, 134–137. [Google Scholar] [CrossRef]


| Database | Search String | Hits | Relevant Hits |
|---|---|---|---|
| PubMed | Flexispira rappini and human infection | 161 | 9 |
| PMC | Flexispira rappini and human infection | 102 | 6 (these were also found in PubMed) |
| Embase | Flexispira rappini and human infection | 0 | 0 |
| Year | Author | Material | Found | Disease of the Patient | Disease as Cause of Infection |
|---|---|---|---|---|---|
| 1998 | Tee et al. [13] | Blood culture | Flexispira rappini | Appendectomy and recurrent chest infections over the last 2 years | Pneumonia/Bacteremia |
| 1999 | Sorlin et al. [14] | Blood culture | Flexispira rappini | End-stage renal failure, chronic pancreatitis and secondary diabetes mellitus | Bacteremia |
| 1999 | Weir et al. [15] | Blood culture | Flexispira rappini (possible new taxon) | X-linked agammaglobulinemia | Persistent sepsis with leg swelling |
| 2000 | Cuccherini et al. [16] | Blood/skin | Flexispira rappini | X-linked agammaglobulinemia | Bacteremia, skin/bone infection |
| 2000 | Han et al. [17] | Pus from the abscess | Flexispira rappini, Helicobacter bilis, and Helicobacter sp. strain Mainz | X-linked agammaglobulinemia | Abdominal abscess |
| 2001 | Iten et al. [18] | Blood culture | Flexispira rappini taxon 8 | Healthy but skin lesion in relation to travelling | Sweats, chills, fever, diffuse arthralgias, and painful legs |
| 2001 | Gerrard et al. [19] | Blood samples | Flexispira rappini and Helicobacter canis | X-linked agammaglobulinemia | Recurrent bacteremia and multifocal lower limb cellulitis |
| 2005 | Brachet-Castang et al. [20] | Blood culture | Flexispira rappini taxon 8 | Common Variable Immunodeficiency | Bacteremia |
| 2010 | R. Murray et al. [21] | Blood culture | Flexispira rappini Taxon 8 | X-linked agammaglobulinemia | Chronic pyoderma gangrenosum-like leg ulcer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fjordside, L.; Herløv, C.; Drabe, C.H.; Andersen, L.P.; Katzenstein, T.L. Helicobacter trogontum Bacteremia and Lower Limb Skin Lesion in a Patient with X-Linked Agammaglobulinemia—A Case Report and Review of the Literature. Pathogens 2022, 11, 1247. https://doi.org/10.3390/pathogens11111247
Fjordside L, Herløv C, Drabe CH, Andersen LP, Katzenstein TL. Helicobacter trogontum Bacteremia and Lower Limb Skin Lesion in a Patient with X-Linked Agammaglobulinemia—A Case Report and Review of the Literature. Pathogens. 2022; 11(11):1247. https://doi.org/10.3390/pathogens11111247
Chicago/Turabian StyleFjordside, Lasse, Caroline Herløv, Camilla Heldbjerg Drabe, Leif Percival Andersen, and Terese L. Katzenstein. 2022. "Helicobacter trogontum Bacteremia and Lower Limb Skin Lesion in a Patient with X-Linked Agammaglobulinemia—A Case Report and Review of the Literature" Pathogens 11, no. 11: 1247. https://doi.org/10.3390/pathogens11111247
APA StyleFjordside, L., Herløv, C., Drabe, C. H., Andersen, L. P., & Katzenstein, T. L. (2022). Helicobacter trogontum Bacteremia and Lower Limb Skin Lesion in a Patient with X-Linked Agammaglobulinemia—A Case Report and Review of the Literature. Pathogens, 11(11), 1247. https://doi.org/10.3390/pathogens11111247






