Multiplex PCR and Sequence Analysis to Investigate Genetic Diversity of Fasciola Isolates from Cattle and Sheep in Turkey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Adult Fasciola spp. Samples
2.2. Genomic DNA Isolation
2.3. Amplification of Pepck by Multiplex PCR
2.4. Amplification of nad1 by PCR
2.5. DNA Sequence Analysis
2.6. Haplotype Analysis
3. Results
3.1. Multiplex PCR Data
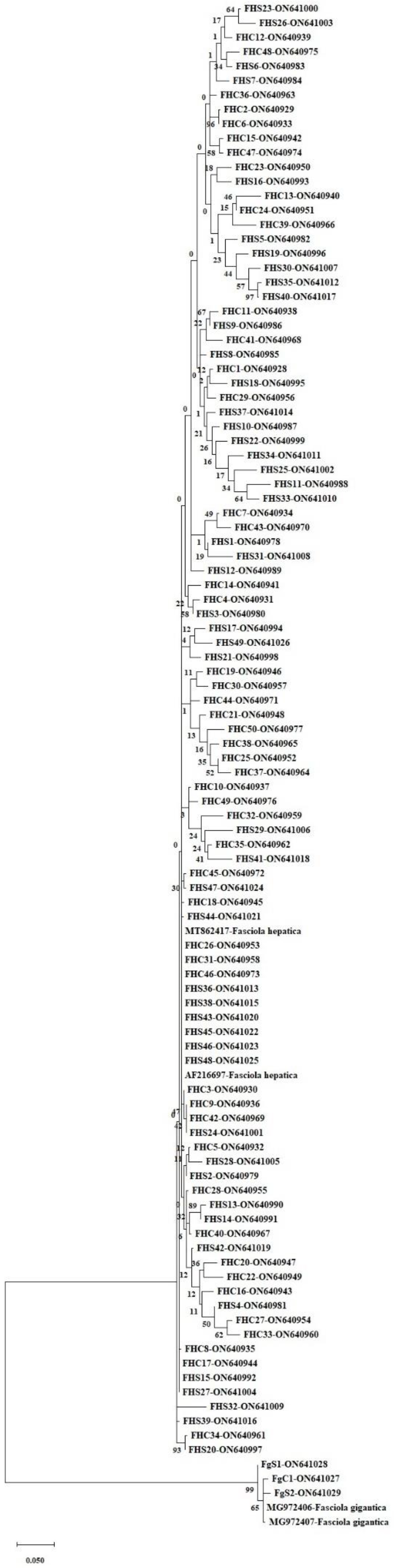
3.2. nad1 Sequence Analysis
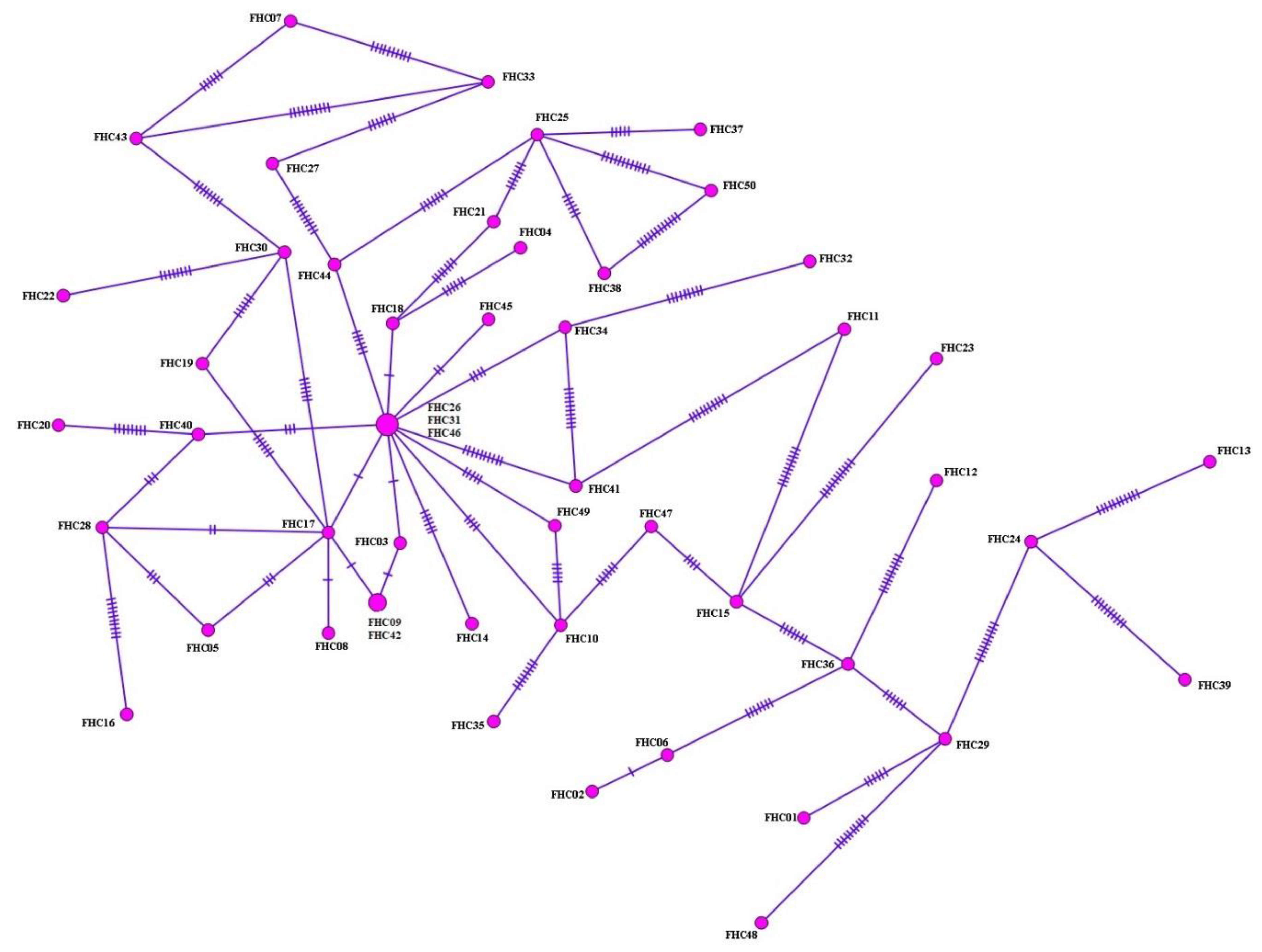
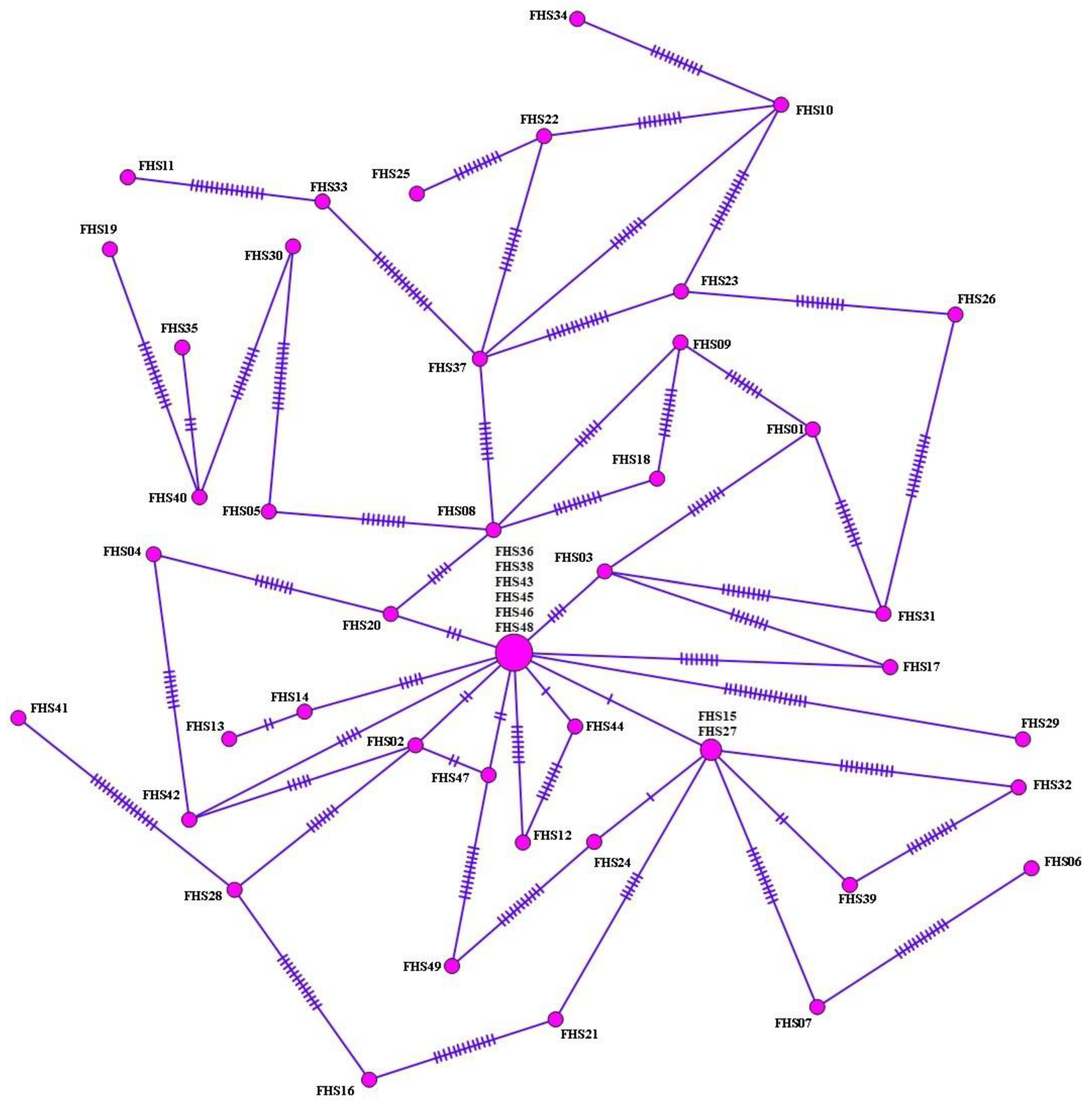
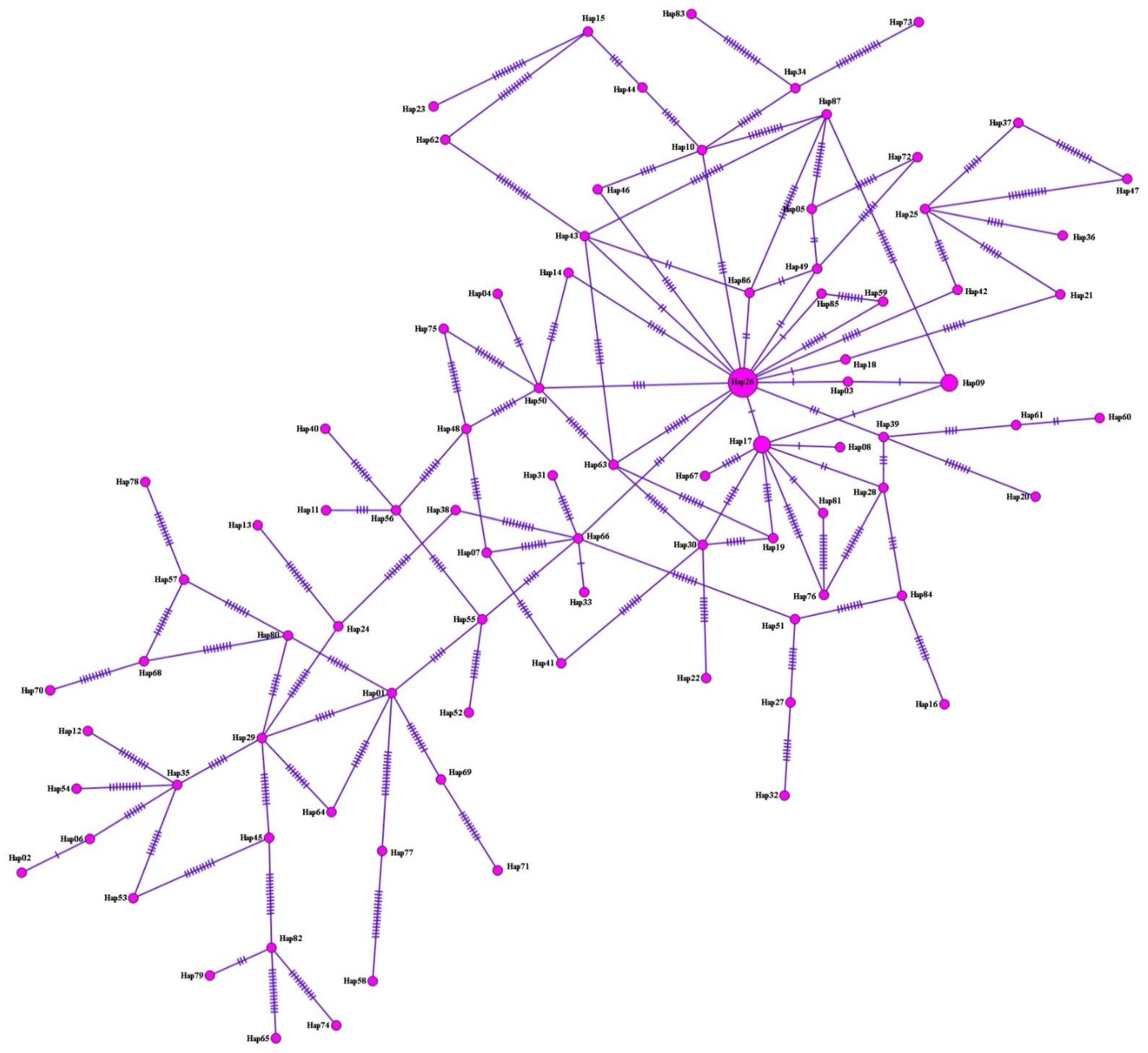
3.3. Haplotype Analysis, Nucleotide Polymorphism, and Diversity and Neutrality Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mas-Coma, S.; Valero, M.; Bargues, M. Fascioliasis. Adv. Exp. Med. Biol. 2019, 1154, 71–103. [Google Scholar] [PubMed]
- Amiri, S.; Shemshadi, B.; Shirali, S.; Kheirandish, F.; Fallahi, S. Accurate and rapid detection of Fasciola hepatica copro-DNA in sheep using loop-mediated isothermal amplification (LAMP) technique. Vet. Med. Sci. 2021, 7, 1316–1324. [Google Scholar] [CrossRef]
- Carnevale, S.; Malandrini, J.B.; Pantano, M.L.; Soria, C.C.; Rodrigues-Silva, R.; Machado-Silva, J.R.; Velásquez, J.N.; Kamenetzky, L. First genetic characterization of Fasciola hepatica in Argentina by nuclear and mitochondrial gene markers. Vet. Parasitol. 2017, 245, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Bozorgomid, A.; Rouhani, S.; Harandi, M.F.; Ichikawa-Seki, M.; Raeghi, S. Genetic diversity and distribution of Fasciola hepatica haplotypes in Iran: Molecular and phylogenetic studies. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100359. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, K.; Itagaki, T.; Shibahara, T.; Noda, Y.; Moriyama-Gonda, N. Comparative study of the reproductive organs of Fasciola groups by optical microscope. J. Vet. Med. Sci. 2001, 63, 735–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itagaki, T.; Ichinomiya, M.; Fukuda, K.; Fusyuku, S.; Carmona, C. Hybridization experiments indicate incomplete reproductive isolating mechanism between Fasciola hepatica and Fasciola gigantica. Parasitology 2011, 138, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Amor, N.; Farjallah, S.; Merella, P.; Alagaili, A.N.; Mohammed, O.B. Multilocus approach reveals discordant molecular markers and corridors for gene flow between North African populations of Fasciola hepatica. Vet. Parasitol. 2020, 278, 109035. [Google Scholar] [CrossRef]
- Moazeni, M.; Sharifiyazdi, H.; Izadpanah, A. Characterization of Fasciola hepatica genotypes from cattle and sheep in Iran using cytochrome C oxidase gene (CO1). Parasitol. Res. 2012, 110, 2379–2384. [Google Scholar] [CrossRef]
- Ashrafi, K.; Bargues, M.D.; O’Neill, S.; Mas-Coma, S. Fascioliasis: A worldwide parasitic disease of importance in travel medicine. Travel Med. Infect. Dis. 2014, 12, 636–649. [Google Scholar] [CrossRef]
- Itagaki, T.; Kikawa, M.; Sakaguchi, K.; Shimo, J.; Terasaki, K.; Shibahara, T.; Fukuda, K. Genetic characterization of parthenogenic Fasciola sp. in Japan on the basis of the sequences of ribosomal and mitochondrial DNA. Parasitology 2005, 131, 679–685. [Google Scholar] [CrossRef]
- Itagaki, T.; Kikawa, M.; Terasaki, K.; Shibahara, T.; Fukuda, K. Molecular characterization of parthenogenic Fasciola sp. in Korea on the basis of DNA sequences of ribosomal ITS1 and mitochondrial NDI gene. J. Vet. Med. Sci. 2005, 67, 1115–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itagaki, T.; Sakaguchi, K.; Terasaki, K.; Sasaki, O.; Yoshihara, S.; Van Dung, T. Occurrence of spermic diploid and aspermic triploid forms of Fasciola in Vietnam and their molecular characterization based on nuclear and mitochondrial DNA. Parasitol. Int. 2009, 58, 81–85. [Google Scholar] [CrossRef]
- Peng, M.; Ichinomiya, M.; Ohtori, M.; Ichikawa, M.; Shibahara, T.; Itagaki, T. Molecular characterization of Fasciola hepatica, Fasciola gigantica, and aspermic Fasciola sp. in China based on nuclear and mitochondrial DNA. Parasitol. Res. 2009, 105, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Kobayashi, T. Visualization of the dynamic behavior of ribosomal RNA gene repeats in living yeast cells. Gen. Cells 2011, 16, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Shoriki, T.; Ichikawa-Seki, M.; Suganuma, K.; Naito, I.; Hayashi, K.; Nakao, M.; Aita, J.; Mohanta, U.K.; Inoue, N.; Murakami, K. Novel methods for the molecular discrimination of Fasciola spp. on the basis of nuclear protein-coding genes. Parasitol. Int. 2016, 65, 180–183. [Google Scholar] [CrossRef]
- Thang, T.N.; Vázquez-Prieto, S.; Vilas, R.; Paniagua, E.; Ubeira, F.M.; Ichikawa-Seki, M. Genetic diversity of Fasciola hepatica in Spain and Peru. Parasitol. Int. 2020, 76, 102100. [Google Scholar] [CrossRef]
- Giovanoli Evack, J.; Schmidt, R.S.; Boltryk, S.D.; Voss, T.S.; Batil, A.A.; Ngandolo, B.N.; Greter, H.; Utzinger, J.; Zinsstag, J.; Balmer, O. Molecular confirmation of a Fasciola gigantica× Fasciola hepatica hybrid in a Chadian bovine. J. Parasitol. 2020, 106, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Javanmard, E.; Ohari, Y.; Sadeghi, A.; Cheraghipour, K.; Aghdaei, H.A.; Mirjalali, H.; Zali, M.R.; Itagaki, T. Multigene typing and phylogenetic analysis of Fasciola from endemic foci in Iran. Infect. Genet. Evol. 2020, 80, 104202. [Google Scholar] [CrossRef]
- Simsek, S.; Utuk, A.; Balkaya, I. Molecular differentiation of Turkey cattle isolates of Fasciola hepatica and Fasciola gigantica. Helminthologia 2011, 48, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Fu, Y.-X.; Li, W.-H. Statistical tests of neutrality of mutations. Genetics 1993, 133, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.-J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Hayashi, K.; Ichikawa-Seki, M.; Mohanta, U.K.; Shoriki, T.; Chaichanasak, P.; Itagaki, T. Hybrid origin of Asian aspermic Fasciola flukes is confirmed by analyzing two single-copy genes, pepck and pold. J. Vet. Med. Sci. 2017, 80, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Ichikawa-Seki, M.; Allamanda, P.; Wibowo, P.E.; Mohanta, U.K.; Guswanto, A.; Nishikawa, Y. Molecular characterization and phylogenetic analysis of Fasciola gigantica from western Java, Indonesia. Parasitol. Int. 2016, 65, 424–427. [Google Scholar] [CrossRef]
- Hayashi, K.; Mohanta, U.K.; Neeraja, T.; Itagaki, T. Molecular characterization of Fasciola gigantica in Delhi, India and its phylogenetic relation to the species from South Asian countries. J. Vet. Med. Sci. 2016, 78, 1529–1532. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, M.; Itagaki, T. Discrimination of the ITS1 types of Fasciola spp. based on a PCR–RFLP method. Parasitol. Res. 2010, 106, 757–761. [Google Scholar] [CrossRef]
- Ichikawa-Seki, M.; Peng, M.; Hayashi, K.; Shoriki, T.; Mohanta, U.K.; Shibahara, T.; Itagaki, T. Nuclear and mitochondrial DNA analysis reveals that hybridization between Fasciola hepatica and Fasciola gigantica occurred in China. Parasitology 2017, 144, 206–213. [Google Scholar] [CrossRef]
- Ichikawa, M.; Bawn, S.; Maw, N.N.; Htun, L.L.; Thein, M.; Gyi, A.; Sunn, K.; Katakura, K.; Itagaki, T. Characterization of Fasciola spp. in Myanmar on the basis of spermatogenesis status and nuclear and mitochondrial DNA markers. Parasitol. Int. 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Chaichanasak, P.; Ichikawa, M.; Sobhon, P.; Itagaki, T. Identification of Fasciola flukes in Thailand based on their spermatogenesis and nuclear ribosomal DNA, and their intraspecific relationships based on mitochondrial DNA. Parasitol. Int. 2012, 61, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Shoriki, T.; Ichikawa-Seki, M.; Devkota, B.; Rana, H.B.; Devkota, S.P.; Humagain, S.K.; Itagaki, T. Molecular phylogenetic identification of Fasciola flukes in Nepal. Parasitol. Int. 2014, 63, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, U.K.; Ichikawa-Seki, M.; Shoriki, T.; Katakura, K.; Itagaki, T. Characteristics and molecular phylogeny of Fasciola flukes from Bangladesh, determined based on spermatogenesis and nuclear and mitochondrial DNA analyses. Parasitol. Res. 2014, 113, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Thang, T.N.; Hakim, H.; Rahimi, R.R.; Ichikawa-Seki, M. Molecular analysis reveals expansion of Fasciola hepatica distribution from Afghanistan to China. Parasitol. Int. 2019, 72, 101930. [Google Scholar] [CrossRef]
- Tashiro, M.; Zokbi, Z.; Chibout, Y.; Megrane, S.; Mebarka, F.; Ichikawa-Seki, M. Molecular characterization and phylogenetic analysis of Fasciola hepatica from high-plateau and steppe areas in Algeria. Parasitol. Int. 2021, 80, 102234. [Google Scholar]
- Aydenizöz, M.; Yildiz, K. Kırıkkale’de kesilen koyunlarda karaciğer trematodlarının yaygınlığı. Türkiye Parazitol. Derg. 2002, 26, 317–319. [Google Scholar]
- Toparlak, M. Van ili belediye mezbahasinda kesilen koyunlarda karaciğer trematod enfeksiyonlari üzerinde araştirmalar. Ankara Üniv. Vet. Fak. Derg. 1988, 35, 270–274. [Google Scholar]
- Değer, S.; Akgül, Y.; Ağaoğlu, Z.T.; Tasçi, S. Van ve yöresinde Fasciola gigantica’dan ileri gelen fascioliasis enfeksiyonlarinin epidemiyolojisi ve ekolojisiüzerinde araştirmalar. Yüzüncü Yıl Üniv. Vet. Fak. Derg. 1992, 3, 133–140. [Google Scholar]
- Akyol, Ç.V. Bursa ortak girişim tesislerinde (ETBA) kesilen koyunlarda Distomatozis’ in yayılışı. J. Fac. Vet. Med. 2001, 20, 23–27. [Google Scholar]
- Balkaya, İ.; Şimşek, S. Erzurum’da kesilen sığırlarda Hidatidosis ve Fasciolosis’ in yaygınlığı ve ekonomik önemi. Kafkas Univ. Vet. Fak. Derg. 2010, 16, 793–797. [Google Scholar]
- Akhlaghi, E.; Mohammadi, M.A.; Ziaali, N.; Baneshi, M.R.; Nasibi, S.; Kamyabi, H.; Rostami, S.; Harandi, M.F. Morphometric and molecular study of Fasciola isolates from ruminants in Iran. Türkiye Parazitol. Derg. 2017, 41, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghayan, S.; Gevorgian, H.; Ebi, D.; Atoyan, H.A.; Addy, F.; Mackenstedt, U.; Romig, T.; Wassermann, M. Fasciola spp. in Armenia: Genetic diversity in a global context. Vet. Parasitol. 2019, 268, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; Dalton, J.P.; Dufresne, P.J.; La Course, J.; Williams, D.J.; Hodgkinson, J.; Paterson, S. The Fasciola hepatica genome: Gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol. 2015, 16, 71. [Google Scholar] [CrossRef] [PubMed]
| n | hn | hd ± SD | πd ± SD | Tajima’s D | p Value | Fu’s Fs | p Value | FLD | p Value | FLF | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 47 | 0.997 ± 0.005 | 0.02423 ± 0.00159 | −0.43557 | p > 0.10 | −35.537 | 0.000 | 0.76537 | p > 0.10 | 0.38471 | p > 0.10 |
| n | hn | hd ± SD | πd ± SD | Tajima’s D | p Value | Fu’s Fs | p Value | FLD | p Value | FLF | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 49 | 43 | 0.986 ± 0.011 | 0.02810 ± 0.00220 | −0.62751 | p > 0.10 | −22.700 | 0.000 | 1.23614 | p > 0.10 | 0.65299 | p > 0.10 |
| Number | Haplotype Name | Isolate No. | Isolate Codes (Accession No.) |
|---|---|---|---|
| 1 | Hap01 | 1 | [FHC01-ON640928] |
| 2 | Hap02 | 1 | [FHC02-ON640929] |
| 3 | Hap03 | 1 | [FHC03-ON640930] |
| 4 | Hap04 | 1 | [FHC04-ON640931] |
| 5 | Hap05 | 1 | [FHC05-ON640932] |
| 6 | Hap06 | 1 | [FHC06-ON640933] |
| 7 | Hap07 | 1 | [FHC07-ON640934] |
| 8 | Hap08 | 1 | [FHC08-ON640935] |
| 9 | Hap09 | 3 | [FHC09-ON640936 FHC42-ON640969 FHS24-ON641001] |
| 10 | Hap10 | 1 | [FHC10-ON640937] |
| 11 | Hap11 | 1 | [FHC11-ON640938] |
| 12 | Hap12 | 1 | [FHC12-ON640939] |
| 13 | Hap13 | 1 | [FHC13-ON640940] |
| 14 | Hap14 | 1 | [FHC14-ON640941] |
| 15 | Hap15 | 1 | [FHC15-ON640942] |
| 16 | Hap16 | 1 | [FHC16-ON640943] |
| 17 | Hap17 | 1 | [FHC17-ON640944 FHS15-ON640992 FHS27-ON641004] |
| 18 | Hap18 | 1 | [FHC18-ON640945] |
| 19 | Hap19 | 1 | [FHC19-ON640946] |
| 20 | Hap20 | 1 | [FHC20-ON640947] |
| 21 | Hap21 | 1 | [FHC21-ON640948] |
| 22 | Hap22 | 1 | [FHC22-ON640949] |
| 23 | Hap23 | 1 | [FHC23-ON640950] |
| 24 | Hap24 | 1 | [FHC24-ON640951] |
| 25 | Hap25 | 1 | FHC25-ON640952] |
| 26 | Hap26 | 9 | [FHC26-ON640953 FHC31-ON640958 FHC46-ON640973 FHS36-ON641013 FHS38-ON641015 FHS43-ON641020 FHS45-ON641022 FHS46-ON641023 FHS48-ON641025] |
| 27 | Hap27 | 1 | [FHC27-ON640954] |
| 28 | Hap28 | 1 | [FHC28-ON640955] |
| 29 | Hap29 | 1 | [FHC29-ON640956] |
| 30 | Hap30 | 1 | [FHC30-ON640957] |
| 31 | Hap31 | 1 | [FHC32-ON640959] |
| 32 | Hap32 | 1 | [FHC33-ON640960] |
| 33 | Hap33 | 1 | [FHC34-ON640961] |
| 34 | Hap34 | 1 | [FHC35-ON640962] |
| 35 | Hap35 | 1 | [FHC36-ON640963] |
| 36 | Hap36 | 1 | [FHC37-ON640964] |
| 37 | Hap37 | 1 | [FHC38-ON640965] |
| 38 | Hap38 | 1 | [FHC39-ON640966] |
| 39 | Hap39 | 1 | [FHC40-ON640967] |
| 40 | Hap40 | 1 | [FHC41-ON640968] |
| 41 | Hap41 | 1 | [FHC43-ON640970] |
| 42 | Hap42 | 1 | [FHC44-ON640971] |
| 43 | Hap43 | 1 | [FHC45-ON640972] |
| 44 | Hap44 | 1 | [FHC47-ON640974] |
| 45 | Hap45 | 1 | [FHC48-ON640975] |
| 46 | Hap46 | 1 | [FHC49-ON640976] |
| 47 | Hap47 | 1 | [FHC50-ON640977] |
| 48 | Hap48 | 1 | [FHS01-ON640978] |
| 49 | Hap49 | 1 | [FHS02-ON640979] |
| 50 | Hap50 | 1 | [FHS03-ON640980] |
| 51 | Hap51 | 1 | [FHS04-ON640981] |
| 52 | Hap52 | 1 | [FHS05-ON640982] |
| 53 | Hap53 | 1 | [FHS06-ON640983] |
| 54 | Hap54 | 1 | [FHS7-ON640984] |
| 55 | Hap55 | 1 | [FHS8-ON640985] |
| 56 | Hap56 | 1 | [FHS9-ON640986] |
| 57 | Hap57 | 1 | [FHS10-ON640987] |
| 58 | Hap58 | 1 | [FHS11-ON640988] |
| 59 | Hap59 | 1 | [FHS12-ON640989] |
| 60 | Hap60 | 1 | [FHS13-ON640990] |
| 61 | Hap61 | 1 | [FHS14-ON640991] |
| 62 | Hap62 | 1 | [FHS16-ON640993] |
| 63 | Hap63 | 1 | [FHS17-ON640994] |
| 64 | Hap64 | 1 | [FHS18-ON640995] |
| 65 | Hap65 | 1 | [FHS19-ON640996] |
| 66 | Hap66 | 1 | [FHS20-ON640997] |
| 67 | Hap67 | 1 | [FHS21-ON640998] |
| 68 | Hap68 | 1 | [FHS22-ON640999] |
| 69 | Hap69 | 1 | [FHS23-ON641000] |
| 70 | Hap70 | 1 | [FHS25-ON641002] |
| 71 | Hap71 | 1 | [FHS26-ON641003] |
| 72 | Hap72 | 1 | [FHS28-ON641005] |
| 73 | Hap73 | 1 | [FHS29-ON641006] |
| 74 | Hap74 | 1 | [FHS30-ON641007] |
| 75 | Hap75 | 1 | [FHS31-ON641008] |
| 76 | Hap76 | 1 | [FHS32-ON641009] |
| 77 | Hap77 | 1 | [FHS33-ON641010] |
| 78 | Hap78 | 1 | [FHS34-ON641011] |
| 79 | Hap79 | 1 | [FHS35-ON641012] |
| 80 | Hap80 | 1 | [FHS37-ON641014] |
| 81 | Hap81 | 1 | [FHS39-ON641016] |
| 82 | Hap82 | 1 | [FHS40-ON641017] |
| 83 | Hap83 | 1 | [FHS41-ON641018] |
| 84 | Hap84 | 1 | [FHS42-ON641019] |
| 85 | Hap85 | 1 | [FHS44-ON641021] |
| 86 | Hap86 | 1 | [FHS47-ON641024] |
| 87 | Hap87 | 1 | [FHS49-ON641026] |
| n | hn | hd ± SD | πd ± SD | Tajima’s D | p Value | Fu’s Fs | p Value | FLD | p Value | FLF | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 99 | 87 | 0.991 ± 0.005 | 0.02661 ± 0.00148 | −0.85844 | p > 0.10 | −33.441 | 0.000 | 1.00700 | p > 0.10 | 0.26059 | p > 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzun, V.; Celik, F.; Simsek, S.; Kesik, H.K.; Kilinc, S.G.; Zhang, X.; Ahmed, H.; Cao, J. Multiplex PCR and Sequence Analysis to Investigate Genetic Diversity of Fasciola Isolates from Cattle and Sheep in Turkey. Pathogens 2022, 11, 1235. https://doi.org/10.3390/pathogens11111235
Uzun V, Celik F, Simsek S, Kesik HK, Kilinc SG, Zhang X, Ahmed H, Cao J. Multiplex PCR and Sequence Analysis to Investigate Genetic Diversity of Fasciola Isolates from Cattle and Sheep in Turkey. Pathogens. 2022; 11(11):1235. https://doi.org/10.3390/pathogens11111235
Chicago/Turabian StyleUzun, Veysel, Figen Celik, Sami Simsek, Harun Kaya Kesik, Seyma Gunyakti Kilinc, Xiaocheng Zhang, Haroon Ahmed, and Jianping Cao. 2022. "Multiplex PCR and Sequence Analysis to Investigate Genetic Diversity of Fasciola Isolates from Cattle and Sheep in Turkey" Pathogens 11, no. 11: 1235. https://doi.org/10.3390/pathogens11111235







