Pneumocystis jirovecii Pneumonia in Patients with Solid Malignancies: A Retrospective Study in Two Hospitals
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of PJP Patients
2.2. PJP Clinical Characteristics and Laboratory Findings
2.3. Treatment Courses of PJP
2.4. ALC Changes before and after PJP Onset
3. Discussion
4. Materials and Methods
4.1. Study Design and Settings
4.2. Study Outcomes
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, E.H.; Kim, E.Y.; Lee, S.H.; Roh, Y.H.; Leem, A.Y.; Song, J.H.; Kim, S.Y.; Chung, K.S.; Jung, J.Y.; Kang, Y.A.; et al. Risk factors and clinical characteristics of Pneumocystis jirovecii pneumonia in lung cancer. Sci. Rep. 2019, 9, 2094. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Yakushijin, Y. Pneumocystis jirovecii pneumonia prophylaxis for cancer patients during chemotherapy. Pathogens 2021, 10, 237. [Google Scholar] [CrossRef]
- Takeda, K.; Harada, S.; Hayama, B.; Hoashi, K.; Enokida, T.; Sasaki, T.; Okamoto, K.; Nakano, K.; Ohkushi, D. Clinical characteristics and risk factors associated with Pneumocystis jirovecii infection in patients with solid tumors: Study of thirteen-year medical records of a large cancer center. BMC Cancer 2021, 21, 987. [Google Scholar] [CrossRef]
- Sepkowitz, K.A.; Brown, A.E.; Telzak, E.E.; Gottlieb, S.; Armstrong, D. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA 1992, 267, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Gentilotti, E.; Coppola, L.; Maffongelli, G.; Cerva, C.; Malagnino, V.; Mari, A.; Di Veroli, A.; Berrilli, F.; Apice, F.; et al. Infectious disease ward admission positively influences P. jiroveci pneumonia (PjP) outcome: A retrospective analysis of 116 HIV-positive and HIV-negative immunocompromised patients. PLoS ONE 2017, 12, e0176881. [Google Scholar] [CrossRef] [PubMed]
- Schoovaerts, K.; Dirix, L.; Rutten, A.; Van Schaeren, J.; Van Herendael, B.; Van Grieken, S.; Heytens, L. Pneumocystis jiroveci pneumonia (PJP) in non-HIV immunocompromised individuals. Acta Clin. Belg. 2017, 72, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Assal, M.; Lambert, J.; Chow-Chine, L.; Bisbal, M.; Servan, L.; Gonzalez, F.; de Guibert, J.M.; Faucher, M.; Vey, N.; Sannini, A.; et al. Prognostic impact of early adjunctive corticosteroid therapy in non-HIV oncology or haematology patients with Pneumocystis jirovecii pneumonia: A propensity score analysis. PLoS ONE 2021, 16, e0250611. [Google Scholar] [CrossRef] [PubMed]
- Grønseth, S.; Rogne, T.; Hannula, R.; Åsvold, B.O.; Afset, J.E.; Damås, J.K. Epidemiological and clinical characteristics of immunocompromised patients infected with Pneumocystis jirovecii in a twelve-year retrospective study from Norway. BMC Infect. Dis. 2021, 21, 659. [Google Scholar] [CrossRef] [PubMed]
- Shiiba, R.; Himeji, D.; Matsumoto, R.; Tanaka, G.I.; Otomo, N. Pneumocystis jirovecii pneumonia in three patients with breast cancer receiving neoadjuvant dose-dense chemotherapy. Cureus 2022, 14, e21812. [Google Scholar] [CrossRef] [PubMed]
- Cooley, L.; Dendle, C.; Wolf, J.; Teh, B.W.; Chen, S.C.; Boutlis, C.; Thursky, K.A. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern. Med. J. 2014, 44, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Mansharamani, N.G.; Balachandran, D.; Vernovsky, I.; Garland, R.; Koziel, H. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest 2000, 118, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Saimi, M.; Huang, X.; Sun, T.; Fan, G.; Zhan, Q. Risk factors of mortality from Pneumocystis pneumonia in non-HIV patients: A meta-analysis. Front. Public Health 2021, 9, 680108. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Chen, S.; Kauffman, C.; Steinbach, W.; Baddley, J.; Verweij, P.; Clancy, C.; Wingard, J.; Lockhart, S.; Groll, A.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
| 30-Day Survival Group (n = 20) | 30-Day Mortality Group (n = 27) | Total (N = 47) | p-Value | |
|---|---|---|---|---|
| Age, years (median (range)) | 65.5 (46–81) | 71 (52–86) | 69 (46–86) | 0.785 |
| Sex (male; n, (%)) | 10 (50) | 22 (81.5) | 32 (68) | 0.024 |
| Comorbidity (n, (%)) | ||||
| Hypertension | 8 (40.0) | 9 (33.3) | 17 (36.2) | 0.870 |
| Diabetes | 6 (30.0) | 8 (29.6) | 14 (29.8) | 0.978 |
| Chronic hepatitis | 2 (10.0) | 3 (11.1) | 5 (10.6) | 0.903 |
| Chronic kidney disease | 2 (10.0) | 3 (11.1) | 5 (10.6) | 1.000 |
| Type of solid tumor (n, (%)) | ||||
| Lung cancer | 3 (15.0) | 11 (40.7) | 14 (29.8) | 0.056 |
| Breast cancer | 4 (20) | 2 (7.4) | 6 (12.8) | 0.201 |
| Gastric cancer | 0 | 5 (18.5) | 5 (10.6) | 0.063 |
| Colon cancer | 4 (20) | 0 (0) | 4 (8.5) | 0.027 |
| Periampullary cancer | 1 (5.0) | 3 (11.1) | 4 (8.5) | 0.626 |
| Esophageal cancer | 2 (2.0) | 1 (3.7) | 3 (6.4) | 0.567 |
| Prostate cancer | 1 (5.0) | 2 (7.4) | 3 (6.4) | 1.000 |
| Mesothelioma | 0 | 2 (7.4) | 2 (4.3) | 0.500 |
| Renal cell carcinoma | 2 (100) | 0 (0) | 2 (4.3) | 0.176 |
| Bladder cancer | 0 | 1 (3.7) | 1(2.1) | 1.000 |
| Hepatocellular carcinoma | 1 (5.0) | 0 | 1 (2.1) | 0.426 |
| Tonsillar cancer | 1 (5.0) | 0 | 1 (2.1) | 0.426 |
| Thyroid cancer | 1 (5) | 0 | 1 (2.1) | 0.426 |
| ECOG* score of 3 or more (n (%)) | 2 (10.0) | 6 (22.8) | 8 (17.0) | 0.242 |
| Presence of metastasis (n (%)) | 13 (65) | 16 (59.3) | 29 (61.7) | 0.463 |
| Total chemotherapy duration (days) (median (range)) | 51 (0–1335) | 62 (0–370) | 56 (0–1335) | 0.871 |
| Chemotherapy within 3 months before pjp (n (%)) | 9 (45) | 18 (66.7) | 27 (57.4) | 0.871 |
| Radiation therapy (n (%)) | 7 (14.9) | 14 (51.9) | 21 (44.7) | 0.251 |
| Prednisolone 20 mg or more daily for ≥2 weeks within 3 months (n (%)) | 5 (25.0) | 11 (40.7) | 16 (34) | 0.260 |
| Pjp prophylaxis (n (%)) | 1 (5) | 1(2.2) | 2 (4.3) | 0.849 |
| 30-Day Survival Group (n = 20) | 30-Day Mortality Group (n = 27) | Total (N = 47) | p-Value | |
|---|---|---|---|---|
| Symptoms (n (%)) | ||||
| Dyspnea | 16 (80.0) | 25 (92.6) | 41 (87.2) | 0.379 |
| Cough | 9 (45.0) | 14 (51.9) | 23 (48.9) | 0.643 |
| Sputum | 7 (35.0) | 15 (55.6) | 22 (46.8) | 0.238 |
| Fever | 14 (70.0) | 17 (63.0) | 31 (66.0) | 0.758 |
| Increase in oxygen demand (n (%)) | 12 (60.0) | 25 (92.6) | 37 (78.7) | 0.011 |
| Types of respiratory specimen (n (%)) | ||||
| Induced sputum | 12 (60.0) | 21 (77.8) | 33 (70.2) | 0.280 |
| Bronchial washing | 1 (5.0) | 0 (0.0) | 1 (2.1) | 0.426 |
| Bronchoalveolar lavage | 7 (35.0) | 6 (22.2) | 13 (27.7) | 0.511 |
| Positive PJP PCR* (n (%)) | 19 (95.0) | 27 (100.0) | 46 (97.9) | 0.426 |
| 30-Day Survival Group (n = 20) | 30-Day Mortality Group (n = 27) | Total (N = 47) | p-Value | |
|---|---|---|---|---|
| Initial Laboratory Test at PJP Onset; Median (range) | ||||
| WBCs * (/µL) | 6990 (1900–15,030) | 9800 (2030–27,240) | 9180 (1900–27,240) | 0.084 |
| ANCs † (/µL) | 5530 (1460–12,410) | 8730 (1190–25,000) | 8030 (1190–25,000) | 0.019 |
| ALCs ‡ (/µL) | 635 (172–2016) | 710 (51–1884) | 680 (51–2016) | 0.561 |
| Hemoglobin (g/dL) | 9.5 (6.0–13.6) | 9.9 (7.5–14.3) | 9.7 (6.0–14.3) | 0.170 |
| Platelet (/µL) | 216 (21–683) | 164 (24–627) | 186 (21–683) | 0.449 |
| AST § (IU/L) | 37 (16–137) | 33 (15–235) | 35 (15–235) | 0.685 |
| ALT ∥ (IU/L) | 17.5 (8–201) | 24 (8–77) | 20 (8–101) | 0.563 |
| LDH ¶ (U/L) | 635 (212–2100) | 623 (337–1670) | 628.5 (212–2100) | 0.979 |
| BUN ** (mg/dL) | 13 (7–27) | 20 (9–59) | 17.15 (7–59) | 0.011 |
| Creatinine (mg/dL) | 0.74 (0.26–2.36) | 0.66 (0.30–1.64) | 0.67 (0.26–2.36) | 0.907 |
| CRP †† (mg/dL) | 11.10 (2.11–24.91) | 9.53 (0.75–35.64) | 9.81 (0.75–35.64) | 0.732 |
| Follow-up Laboratory Test on Days 5–7 after PJP Treatment; Median (range) | ||||
| WBCs (/µL) | 13,710 (4050–36,060) | 13,615 (3070–30,420) | 13,710 (3070–36,060) | 0.826 |
| ANCs (/µL) | 11,145 (3230–33,540) | 12,545 (490–27,380) | 11,925 (490–33,540) | 0.599 |
| ALCs (/µL) | 1155 (120–3860) | 580 (0–1540) | 735 (0–3860) | 0.003 |
| Hemoglobin (g/dL) | 10.6 (7.0–12.8) | 10.15 (7.1–16.0) | 10.35 (7.0–16.0) | 0.784 |
| Platelet (/µL) | 246 (29–663) | 143.5(36–464) | 162 (29–663) | 0.994 |
| AST (IU/L) | 34.5 (15–141) | 27 (15–585) | 32 (15–585) | 0.060 |
| ALT (IU/L) | 21.5 (6–217) | 21.0 (7–688) | 21 (6–688) | 0.651 |
| LDH (U/L) | 579 (251–1519) | 780 (375–1784) | 588 (251–1784) | 0.479 |
| BUN (mg/dL) | 16.4 (6.6–154) | 26.5 (10.0–41.5) | 21.6 (6.6–154.0) | 0.897 |
| Creatinine (mg/dL) | 0.72 (0.25–2.96) | 0.80 (0.29–1.62) | 0.74 (0.25–2.96) | 0.887 |
| CRP (mg/dL) | 3.74 (0.26–14.76) | 5.05 (0.9–21.83) | 5.0 (0.26–21.83) | 0.171 |
| 30-Day Survival Group (n = 20) | 30-Day Mortality Group (n = 27) | Total (N = 47) | p-Value | |
|---|---|---|---|---|
| PJP Treatment (n, (%)) | 20 (100) | 25 (92.6) | 45 (95.7) | 0.500 |
| Treatment Duration (n (%)) | 20 (4–29) | 9.5 (1–25) | 12.5 (1–29) | <0.001 |
| Period from PJP Onset to Treatment Initiation (days) | 7.0 (1–53) | 8.0 (0–32) | 8.0 (0–53) | 0.878 |
| Adjuvant Steroid for PJP Treatment (n (%)) | 12 (60.0) | 20 (80.0) | 32 (71.1) | 0.141 |
| Coinfection with Bacterial Pathogen (n (%)) | 5 (25) | 3 (11.1) | 8 (17.0) | 0.258 |
| Adverse Reaction to TMP/SMX* (n (%)) | 13 (65.0) | 9 (33.3) | 22 (46.8) | 0.042 |
| Change to Second-Line Drug† of PJP (n (%)) | 4 (20.0) | 4 (16.0) | 8 (17.8) | 1.000 |
| Mechanical Ventilation (n (%)) | 7 (35.0) | 7 (25.9) | 14 (29.8) | 0.501 |
| Cause of Death | Patients (n = 27 (%)) |
|---|---|
| PJP aggravation | 19 (70.4) |
| Cancer progression | 4 (14.8) |
| Other infection | 2 (7.4) |
| Others | 2 (7.4) |
| 30-Day Survival | 30-Day Mortality | Total | ||||
|---|---|---|---|---|---|---|
| Absolute Lymphocyte Count Difference by Time | Z | p-Value | Z | p-Value | Z | p-Value |
| Period 2–Period 1 | −1.099 | 0.272 | −2.243 | 0.025 * | −2.473 | 0.013 |
| Period 3–Period 1 | −2.296 | 0.022 * | −3.027 | 0.002 ** | −3.382 | <0.001 *** |
| Period 4–Period 1 | −0.402 | 0.687 | −3.750 | <0.001 ** | −3.119 | 0.002 ** |
| Period 3–Period 2 | −0.724 | 0.469 | −0.955 | 0.339 | −1.257 | 0.209 |
| Period 4–Period 2 | −1.811 | 0.070 | −1.542 | 0.123 | −0.152 | 0.879 |
| Period 4–Period 3 | −2.213 | 0.027* | −1.114 | 0.265 | −0.978 | 0.328 |
| Period 1 | Period 2 | Period 3 | Period 4 | p-Value | ||
|---|---|---|---|---|---|---|
| 1–3 Months before PJP | 1–2 Weeks before PJP | From PJP Onset to Treatment | 5–7 Days after PJP Treatment | |||
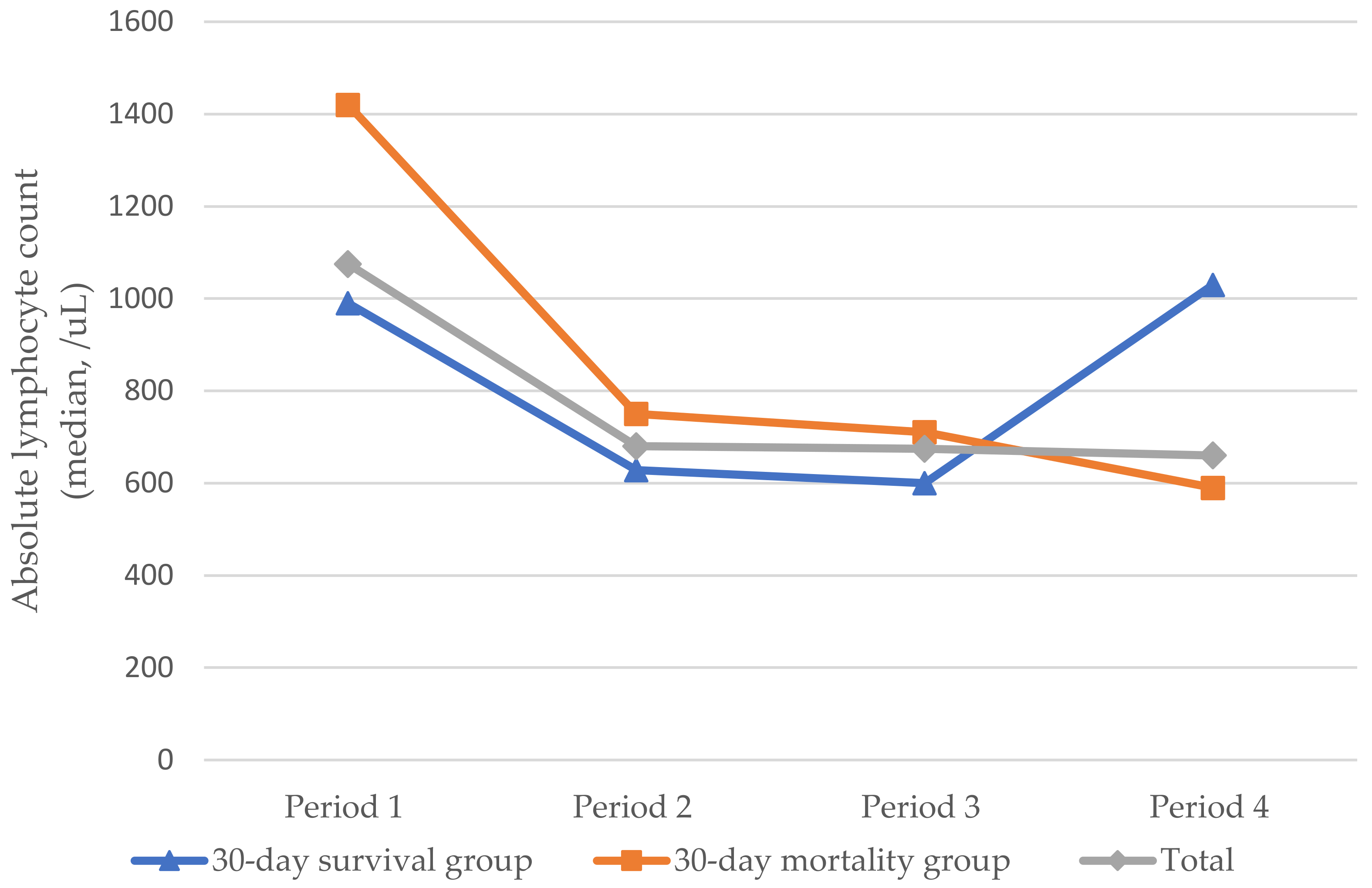
| 30-Day Survival Group (n = 15) | Median (range) | 990.0 (350.0–2370.0) | 628.0 (380.0–1630.0) | 600.0 (180.0–2016.0) | 1030.0 (120.0–3860.0) | 0.163 |
| Average rank | 3.10 | 2.50 | 2.08 | 2.32 | ||
| 30-Day Mortality Group (n = 21) | Median (range) | 1420.00 (141.0–3690.0) | 750.0 (96.8–2370) | 710.0 (51.0–1884.0) | 590.0 (0–1540) | 0.002 ** |
| Average rank | 3.33 | 2.57 | 2.19 | 1.90 | ||
| Total (n = 36) | Median (range) | 1075.00 (141.0–3690.0) | 679.8 (96.8–2370) | 674.50 (51–2016) | 660.0 (0–3860) | 0.007 ** |
| Average rank | 3.10 | 2.50 | 2.08 | 2.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, C.-H.; Kim, S.-H.; Kim, S.; Bae, M.; Lee, S.-J.; Lim, S. Pneumocystis jirovecii Pneumonia in Patients with Solid Malignancies: A Retrospective Study in Two Hospitals. Pathogens 2022, 11, 1169. https://doi.org/10.3390/pathogens11101169
Jeon C-H, Kim S-H, Kim S, Bae M, Lee S-J, Lim S. Pneumocystis jirovecii Pneumonia in Patients with Solid Malignancies: A Retrospective Study in Two Hospitals. Pathogens. 2022; 11(10):1169. https://doi.org/10.3390/pathogens11101169
Chicago/Turabian StyleJeon, Cheon-Hoo, Si-Ho Kim, Seulki Kim, Moonsuk Bae, Su-Jin Lee, and Seungjin Lim. 2022. "Pneumocystis jirovecii Pneumonia in Patients with Solid Malignancies: A Retrospective Study in Two Hospitals" Pathogens 11, no. 10: 1169. https://doi.org/10.3390/pathogens11101169
APA StyleJeon, C.-H., Kim, S.-H., Kim, S., Bae, M., Lee, S.-J., & Lim, S. (2022). Pneumocystis jirovecii Pneumonia in Patients with Solid Malignancies: A Retrospective Study in Two Hospitals. Pathogens, 11(10), 1169. https://doi.org/10.3390/pathogens11101169






