Systematic Review and Meta-Analysis on Human African Trypanocide Resistance
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Article Screening on Inclusion and Exclusion
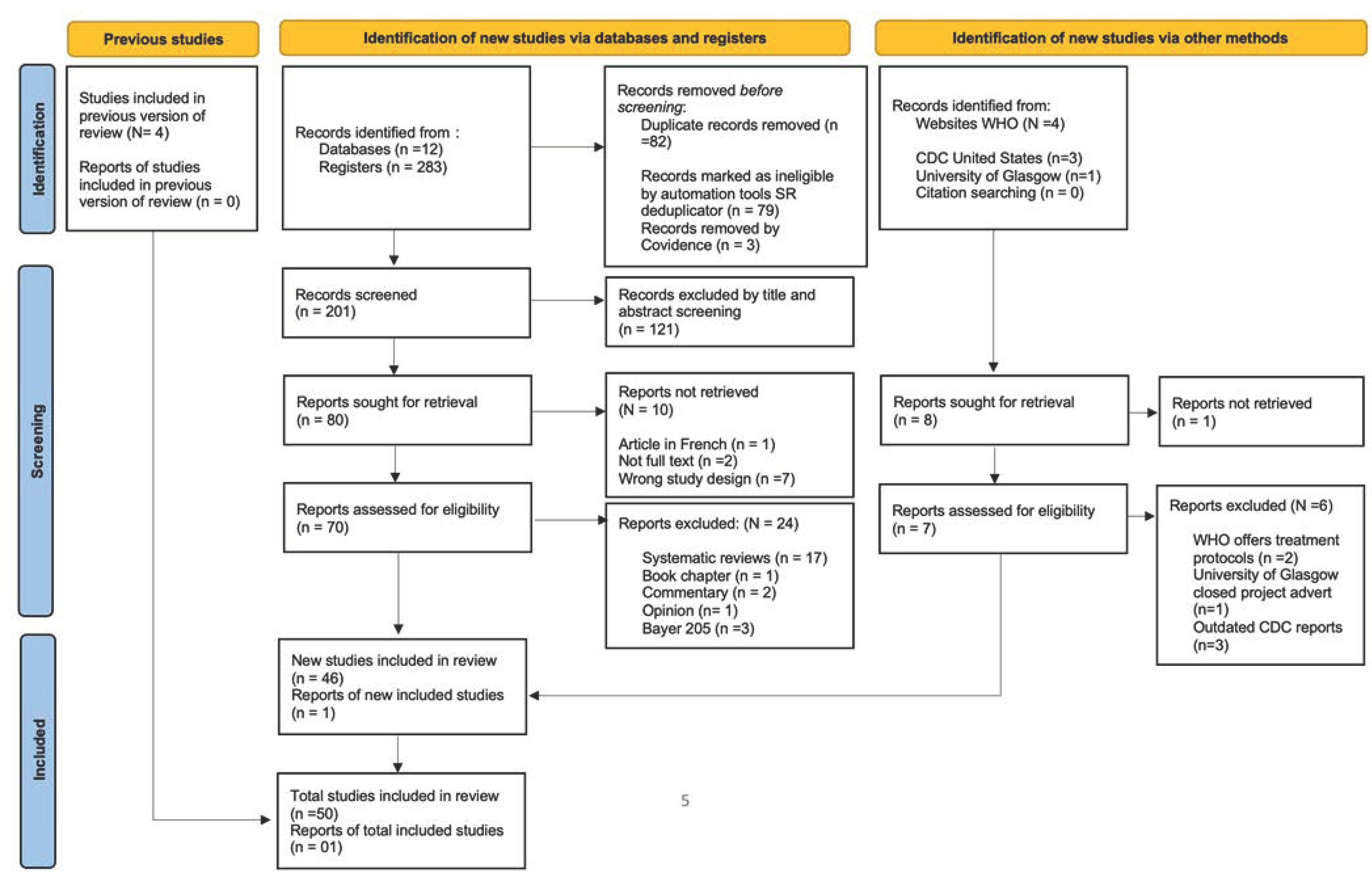
2.3. Statistical Analysis
3. Results and Discussion
3.1. Description of Human African Trypanocide Resistance in the Study
| Study | Ref | Study Population | Source of Pathogen | Intervention/Drugs Used | Gene Targets for Resistance |
|---|---|---|---|---|---|
| Bernhard 2007 | [13] | Mice | TBR | Pentamidine-melarsoprol | TbAT1 loss indicated cross-resistance on both compounds |
| Carter 2020 | [14] | T brucei ORFeome | Parasite library | Melarsoprol | Genes encoding trypanothione Mitochondrial and flagellar gene expression (post translational activator XAC1). |
| Scott 1997 | [15] | Procyclics | TBB | Cross resistance with arsenical-melarsoprol-pentamidine and diminazene aceturate | Melarsoprol can enter parasite through another route than TbAT1 |
| Jeacock 2017 | [16] | Mice | TBB/TBG | AQP2 disrupts glycerol transport | AQPs important for viability and osmoregulation |
| Graf 2013 | [17] | Procyclics | TBR and TBG isolates from 7 African countries | Pentamidine and melarsoprol | TbAT1 loss leads to a loss of transporter activity Aquaglyceroprotein (TbAQP2) |
| Graf 2016 | [18] | Procyclics | TBR from male patient in Tanzania | Pentamidine and melarsoprol | Loss of transporter genes TbAQP2/3 Point mutation renders TbAT1 useless (lacks HAPMT = high affinity pentamidine-melarsoprol transporter) |
| Graf 2015 | [19] | Procyclics | TBG | Pentamidine and melarsoprol | TbAQP2 reintroduced reversed resistance |
| Matovu 2003 | [20] | Procyclics/mice | TBB | Loss of TbAT1/P2 in pentamidine and diamidine uptake | HAPT1 and LAPT1 responsible for residual uptake of melaminophenyl arsenical |
| Lusher 2006 | [21] | Procyclics | TBB | Melarsoprol resistance | TbAT1 and TbMRPA when both present lead to significant decrease in drug influx |
| Sokolova 2010 | [29] | Procyclics | TB strain * | Nifurtimox-resistant cell lines | Nifurtimox metabolized fast and metabolites not effective on pathogen |
| Geiser 2005 | [30] | Procyclics | TBB strain BS 221 | Adenosine metabolites | P1/P2 TbAT1 loss. Conditions other than drugs themselves may favor loss of P1 to increase pathogen survival in bloodstream form of the parasite. |
| Burkard 2011 | [42] | RNAi induction | RNAi library | NA | Loss of TbAT1 leads to melarsoprol resistance. Loss of AAT6 leads to increased eflornithine resistance. |
| Vincent 2010 | [43] | Procyclics | T. brucei strain 427 wildtype | Eflornithine resistance | Ornithine decarboxylase unaltered in parasite. Deletion of TbAAT6 |
| Baker 2011 | [44] | Procyclics | TBR | NECT | Loss of amino acid transporter (AAT6) and nitroreductase (NTR) induces resistance |
| Wyllie 2016 | [38] | Procyclic/mice | Non-specific trypanosome used | Nifurtimox | NTR resistance determinants |
| Bridges 2007 | [31] | Rats | TBG | Arsenical and diamidine | High-affinity pentamidine transporter (HAPT) loss for cross-resistance |
| Lanteri 2006 | [32] | Procyclics/mice | TBB | 2,5-BIS(4-amidinophenyl) furan (DB75) (diamidine) | Loss of TbAT1 leads to loss in uptake of DB75. |
| Scott 1996 | [33] | Mice | TBB from Tanzania TBG from man in Ivory cost | Cross-resistance to MelCy and suramin | Differences in in vivo and in vitro results indicated alteration in surface adenosine transporters. |
| Matovu 1997 | [34] | Humans and livestock | TBR in Uganda | Resistant to ISM, DA | Cross-species resistance |
| Foucher 2006 | [35] | Procyclics | TBG clones | Cymelarsan | Putative NAC isoform loss. Alterations in the activity of the enzyme that generates protein translation modifiers. |
| Wiedemar 2019 | [45] | Procyclics | TBB | VSG expression has impact on suramin sensitivity and uptake | Decrease specific receptor-mediated endocytosis |
| Zeelen 2021 | [41] | Procyclics | TBR | VSG-suramin binding interactions | Resistance phenotype dependent on suramin binding with VSGsur |
| Worthen 2010 | [39] | Mice | Modeling HATr resistance | Pentamidine, prostaglandin D2, quercetin, etoposide, camptothecin, tetrahydroquinoline | Defects in mitochondrial activity, ROS, cell cycle, and genome segregation. |
| Bacchi 1994 | [46] | Mice | TBR from Kenya | Combination of DFMO, eflornithine, and ornidyl | Cure rate in days |
| Bacchi 1993 | [47] | Mice | TBR from Kenya | DFMO resistance | S-adenosylmethionine metabolism increases resistance |
| Pati 2014 | [48] | Humans | TBG in DRC | Melarsoprol | Relapse following mutations in AQP2/3 |
| Matovu 2001 | [49] | Procyclics | TBG from northwestern Uganda | Melarsoprol | Elevated MIC |
| Brun 2001 | [50] | Humans and then mice | TBR KETRI and EATRO trypanosome isolates from Kenya STIB 241 and STIB 704 from Uganda | Melarsoprol | Cure rate |
| Hawking 1941 | [51] | Mice inoculated with patient blood/CSF | TBR | Tryparsamide | Relapse |
| Kagira 2007 | [52] | Mice | TBR in patients from Uganda and Kenya | Melarsoprol-pentamidine cross resistance (MPXR) | Relapse |
| Kibona 2006 | [53] | Mice | TBR from Tanzania | Melarsoprol resistance DA resistance at 14 mg/kg | High minimum inhibition concentrations (MIC) and IC50 |
| Maina 2007 | [54] | Humans/mice | TBG in South Sudan | Melarsoprol resistance | TBAT1/P2 loss |
| Mpia 2002 | [55] | Humans | TBG | Combination of eflornithine and melarsoprol | Cleared infection though toxicity concerns raised. |
| Munday 2014 | [56] | Procyclics | TBB | Pentamidine, melaminophenyl arsenic (PA) | TbAQP2 is HAPAT and source of resistance |
| Munday 2015 | [57] | Procyclics | TBB | TbAT1 | Residues F19, D140, and F316 interact with the TbAT1 substrate. |
| Mutuku 2021 | [58] | Mice | TBR in Busoga, Uganda | Suramin resistance | Differential pathogenicity in TBR strains |
| Nerima 2007 | [59] | Mice | TBG northwest Uganda | Detection of mutant P2/TbAT1 | Allele-specific PCR is cheaper than SfaN1 RFLP for screening of TbAT1 |
| Nnadi 2019 | [60] | Procyclics | T. congo TBB | Holarrhetine | TbAT1, AQP1-3 |
| Sanderson 2009 | [61] | Mice | TBB | Pentamidine | Blood–brain barrier via P-glycoprotein and multiple drug resistance-associated protein transporters. |
3.2. Human African Trypanocide Resistance in Clinical Studies
| Study | Ref | Study Population | Source of Pathogen | Intervention/Drugs Used | Marker for Resistance |
|---|---|---|---|---|---|
| Foulkes 1996 | [62] | Human | TBR in Zambia | Melarsoprol resistance Then given suramin | Melarsoprol refractory period/relapse |
| Pepin 1989 | [63] | Human | TBG in DRC | Nifurtimox for arseno-resistance | No relapse |
| Pepin 1992 | [64] | Human | TBG in DRC | Arsenic resistance | High-dose nifurtimox |
| Richardson 2016 | [65] | Human | TBG in DRC | Parasite regrowth leads to relapse not reinfection | |
| Matovu 2001 | [66] | Human | TBR in Uganda TBG from Angola | Melarsoprol | Mutated TbAT1 |
| Burri 2001 | [70] | Human | TBG in M’banza Congo, Angola | Melarsoprol | Cure rate in patients |
| Kazibwe 2009 | [71] | Human | TBG from northwestern Uganda | Melarsoprol withdrawal | TbAT1/P2 present in pathogen |
| Pyana 2015 | [72] | Human | TBG in DRC | Pentamidine melarsoprol resistance | Cure depends on patient factors such as nutrition, immunological and coinfections with other pathogens |
| Balasegaram 2006 | [73] | Human | TBG in DRC | Pentamidine | Relapse rate measured |
| Balasegaram 2006 | [67] | Human | TBG in DRC | Melarsoprol and eflornithine | In late HAT, more patients died with melarsoprol alone than eflornithine alone. |
| Pepin 2000 | [68] | Human | TBG in DRC | Eflornithine given to relapsing patients | 7-day treatment reduced relapse |
| WHO 2001 | [69] | Human | HAT | HATr | New drugs including DFMO, DB, trypanothione inhibitors, antagonists of polyamine metabolism, nitroimidazoles, combination therapy |
3.3. Evidence of Human African Trypanocide Resistance of TbAT1 in Clinical Studies
3.4. Human African Trypanocide Treatment Relapse Rates
3.5. Drug Sensitivity Profiles on HATr Using Resistance Profiling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Conflicts of Interest
References
- Franco, J.R.; Cecchi, G.; Priotto, G.; Paone, M.; Diarra, A.; Grout, L.; Simarro, P.P.; Zhao, W.; Argaw, D. Monitoring the elimination of human African trypanosomiasis at continental and country level: Update to 2018. PLoS Negl. Trop. Dis. 2020, 14, e0008261. [Google Scholar] [CrossRef] [PubMed]
- Kasozi, K.I.; MacLeod, E.T.; Ntulume, I.; Welburn, S.C. An Update on African Trypanocide Pharmaceutics and Resistance. Front. Vet. Sci. 2022, 9, 828111. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.H.; Welburn, S.C. The Long Wait for a New Drug for Human African Trypanosomiasis. Trends Parasitol. 2018, 34, 818–827. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Lemke, T.L.; Williams, D. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 9781609133450. [Google Scholar]
- Barrett, M.P.; Vincent, I.M.; Burchmore, R.J.S.; Kazibwe, A.J.N.; Matovu, E. Drug resistance in human African trypanosomiasis. Future Microbiol. 2011, 6, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Bray, P.G.; Barrett, M.P.; Ward, S.A.; de Koning, H.P. Pentamidine uptake and resistance in pathogenic protozoa: Past, present and future. TRENDS Parasitol. 2003, 19, 232–239. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Horn, D. Melarsoprol resistance in African trypanosomiasis. Trends Parasitol. 2018, 34, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Priotto, G.; Kasparian, S.; Mutombo, W.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Baudin, E.; Buard, V.; Kazadi-Kyanza, S.; et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicentre, randomised, phase III, non-inferiority trial. Lancet 2009, 374, 56–64. [Google Scholar] [CrossRef]
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Mäser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.S.; Barrett, M.P. New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story. Trop. Med. Infect. Dis. 2020, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H. Fexinidazole for the treatment of human African trypanosomiasis. Drugs Today 2019, 55, 705. [Google Scholar] [CrossRef] [PubMed]
- Mesu, V.K.B.K.; Mutombo Kalonji, W.; Bardonneau, C.; Valverde Mordt, O.; Ngolo Tete, D.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Mahenzi Mbembo, H.; et al. Oral fexinidazole for stage 1 or early stage 2 African Trypanosoma brucei gambiense trypanosomiasis: A prospective, multicentre, open-label, cohort study. Lancet Glob. Health 2021, 9, e999–e1008. [Google Scholar] [CrossRef]
- Bernhard, S.C.; Nerima, B.; Maser, P.; Brun, R. Melarsoprol- and pentamidine-resistant Trypanosoma brucei rhodesiense populations and their cross-resistance. Int. J. Parasitol. 2007, 37, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; Gomez, S.; Gritz, S.; Larson, S.; Silva-Herzog, E.; Kim, H.; Schulz, D.; Hovel-Miner, G. A Trypanosoma brucei ORFeome-based gain-of-function library identifies genes that promote survival during melarsoprol treatment. mSphere 2020, 5, e00769-20. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.G.; Tait, A.; Turner, C.M.R. Trypanosoma brucei: Lack of cross-resistance to melarsoprol in vitro by cymelarsan-resistant parasites. Exp. Parasitol. 1997, 86, 181–190. [Google Scholar] [CrossRef]
- Jeacock, L.; Baker, N.; Wiedemar, N.; Maser, P.; Horn, D. Aquaglyceroporin-null trypanosomes display glycerol transport defects and respiratory-inhibitor sensitivity. PLoS Pathog. 2017, 13, e1006307. [Google Scholar] [CrossRef]
- Graf, F.E.; Ludin, P.; Wenzler, T.; Kaiser, M.; Brun, R.; Pyana, P.P.; Buscher, P.; de Koning, H.P.; Horn, D.; Maser, P. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl. Trop. Dis. 2013, 7, e2475. [Google Scholar] [CrossRef]
- Graf, F.E.; Ludin, P.; Arquint, C.; Schmidt, R.S.; Schaub, N.; Renggli, C.K.; Munday, J.C.; Krezdorn, J.; Baker, N.; Horn, D.; et al. Comparative genomics of drug resistance in Trypanosoma brucei rhodesiense. Cell. Mol. Life Sci. 2016, 73, 3387–3400. [Google Scholar] [CrossRef] [PubMed]
- Graf, F.E.; Baker, N.; Munday, J.C.; de Koning, H.P.; Horn, D.; Maser, P. Chimerization at the AQP2-AQP3 locus is the genetic basis of melarsoprol-pentamidine cross-resistance in clinical Trypanosoma brucei gambiense isolates. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Matovu, E.; Stewart, M.L.; Geiser, F.; Brun, R.; Maser, P.; Wallace, L.J.; Burchmore, R.J.; Enyaru, J.C.; Barrett, M.P.; Kaminsky, R.; et al. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot.Cell 2003, 2, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Luscher, A.; Nerima, B.; Maser, P. Combined contribution of TbAT1 and TbMRPA to drug resistance in Trypanosoma brucei. Mol. Biochem. Parasitol. 2006, 150, 364–366. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.P. Ever-increasing complexities of diamidine and arsenical crossresistance in African trypanosomes. Trends Parasitol. 2008, 24, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Matovu, E.; Seebeck, T.; Enyaru, J.C.K.; Kaminsky, R. Drug resistance in Trypanosoma brucei spp., the causative agents of sleeping sickness in man and nagana in cattle. Microbes Infect. 2001, 3, 763–770. [Google Scholar] [CrossRef]
- Barrett, M.P. Veterinary link to drug resistance in human African trypanosomiasis? Lancet 2001, 358, 603–604. [Google Scholar] [CrossRef]
- Hamill, L.C.; Kaare, M.T.; Welburn, S.C.; Picozzi, K. Domestic pigs as potential reservoirs of human and animal trypanosomiasis in Northern Tanzania. Parasit. Vectors 2013, 6, 322. [Google Scholar] [CrossRef] [PubMed]
- Kasozi, K.I.; Namayanja, M.; Gaithuma, A.K.; Mahero, M.; Matovu, E.; Yamagishi, J.; Sugimoto, C.; MacLeod, E. Prevalence of hemoprotozoan parasites in small ruminants along a human-livestock-wildlife interface in western Uganda. Vet. Parasitol. Reg. Stud. Rep. 2019, 87, 100309. [Google Scholar] [CrossRef] [PubMed]
- Fèvre, E.M.; Coleman, P.G.; Odiit, M.; Magona, J.W.; Welburn, S.C.; Woolhouse, M.E.J. The origins of a new Trypanosoma brucei rhodesiense sleeping sickness outbreak in eastern Uganda. Lancet 2001, 358, 625–628. [Google Scholar] [CrossRef]
- Fyfe, J.; Picozzi, K.; Waiswa, C.; Bardosh, K.L.; Welburn, S.C. Impact of mass chemotherapy in domestic livestock for control of zoonotic T. b. rhodesiense human African trypanosomiasis in Eastern Uganda. Acta Trop. 2017, 165, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.Y.; Wyllie, S.; Patterson, S.; Oza, S.L.; Read, K.D.; Fairlamb, A.H. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Geiser, F.; Luscher, A.; de Koning, H.P.; Seebeck, T.; Maser, P. Molecular pharmacology of adenosine transport in Trypanosoma brucei: P1/P2 revisited. Mol. Pharmacol. 2005, 68, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Bridges, D.J.; Gould, M.K.; Nerima, B.; Maser, P.; Burchmore, R.J.S.; Koning, H.P. de Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol. Pharmacol. 2007, 71, 1098–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanteri, C.A.; Stewart, M.L.; Brock, J.M.; Alibu, V.P.; Meshnick, S.R.; Tidwell, R.R.; Barrett, M.P. Roles for the Trypanosoma brucei P2 transporter in DB75 uptake and resistance. Mol. Pharmacol. 2006, 70, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.G.; Tait, A.; Turner, C.M.R. Characterisation of cloned lines of Trypanosoma brucei expressing stable resistance to MelCy and suramin. Acta Trop. 1996, 60, 251–262. [Google Scholar] [CrossRef]
- Matovu, E.; Iten, M.; Enyaru, J.C.K.; Schmid, C.; Lubega, G.W.; Brun, R.; Kaminsky, R. Susceptibility of Ugandan Trypanosoma brucei rhodesiense isolated from man and animal reservoirs to diminazene, isometamidium and melarsoprol. Trop. Med. Int. Health 1997, 2, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Foucher, A.L.; McIntosh, A.; Douce, G.; Wastling, J.; Tait, A.; Turner, C.M.R. A proteomic analysis of arsenical drug resistance in Trypanosoma brucei. Proteomics 2006, 6, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Delespaux, V.; de Koning, H.P. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Update 1368, 10, 30–50. [Google Scholar] [CrossRef]
- De Koning, H.P. Transporters in African trypanosomes: Role in drug action and resistance. Int. J. Parasitol. 2001, 31, 512–522. [Google Scholar] [CrossRef]
- Wyllie, S.; Foth, B.J.; Kelner, A.; Sokolova, A.Y.; Berriman, M.; Fairlamb, A.H. Nitroheterocyclic drug resistance mechanisms in Trypanosoma brucei. J. Antimicrob. Chemother. 2016, 71, 625–634. [Google Scholar] [CrossRef]
- Worthen, C.; Jensen, B.C.; Parsons, M. Diverse Effects on Mitochondrial and Nuclear Functions Elicited by Drugs and Genetic Knockdowns in Bloodstream Stage Trypanosoma brucei. PLoS Negl. Trop. Dis. 2010, 4, e678. [Google Scholar] [CrossRef]
- Wiedemar, N.; Graf, F.E.; Zwyer, M.; Ndomba, E.; Kunz Renggli, C.; Cal, M.; Schmidt, R.S.; Wenzler, T.; Mäser, P. Beyond immune escape: A variant surface glycoprotein causes suramin resistance in Trypanosoma brucei. Mol. Microbiol. 2018, 107, 57–67. [Google Scholar] [CrossRef]
- Zeelen, J.; van Straaten, M.; Verdi, J.; Hempelmann, A.; Hashemi, H.; Perez, K.; Jeffrey, P.D.; Halg, S.; Wiedemar, N.; Maser, P.; et al. Structure of trypanosome coat protein VSGsur and function in suramin resistance. Nat. Microbiol. 2021, 6, 392–400. [Google Scholar] [CrossRef]
- Burkard, G.S.; Jutzi, P.; Roditi, I. Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol. Biochem. Parasitol. 2011, 175, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.M.; Creek, D.; Watson, D.G.; Kamleh, M.A.; Woods, D.J.; Wong, P.; Burchmore, R.J.S.; Barrett, M.P. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog. 2010, 6, e1001204. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Alsford, S.; Horn, D. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol. Biochem. Parasitol. 2011, 176, 55–57. [Google Scholar] [CrossRef]
- Wiedemar, N.; Zwyer, M.; Zoltner, M.; Cal, M.; Field, M.C.; Maser, P. Expression of a specific variant surface glycoprotein has a major impact on suramin sensitivity and endocytosis in Trypanosoma brucei. FASEB BioAdv. 2019, 1, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Nathan, H.C.; Yarlett, N.; Goldberg, B.; McCann, P.P.; Sjoerdsma, A.; Saric, M.; Clarkson, A.B., Jr. Combination chemotherapy of drug-resistant Trypanosoma brucei rhodesiense infections in mice using DL-alpha-difluoromethylornithine and standard trypanocides. Antimicrob. Agents Chemother. 1994, 38, 563–569. [Google Scholar] [CrossRef]
- Bacchi, C.J.; Garofalo, J.; Ciminelli, M.; Rattendi, D.; Goldberg, B.; McCann, P.P.; Yarlett, N. Resistance to DL-a-difluoromethylornithine by clinical isolates of Trypanosoma brucei rhodesiense. Role of S-adenosylmethionine. Biochem. Pharmacol. 1993, 46, 471–481. [Google Scholar] [CrossRef]
- Pati, P.P.; van Reet, N.; Ngoyi, D.M.; Lukusa, I.N.; Shamamba, S.K.; Buscher, P. Melarsoprol sensitivity profile of Trypanosoma brucei gambiense isolates from cured and relapsed sleeping sickness patients from the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2014, 8, e3212. [Google Scholar] [CrossRef]
- Matovu, E.; Enyaru, J.C.K.; Legros, D.; Schmid, C.; Seebeck, T.; Kaminsky, R. Melarsoprol refractory T. b. gambiense from Omugo, north-western Uganda. Spec. Issue. Hum. Afr. Trypanos. Approaches Roll Back Sleep. Sick. 2001, 6, 407–411. [Google Scholar] [CrossRef]
- Brun, R.; Schumacher, R.; Schmid, C.; Kunz, C.; Burri, C. The phenomenon of treatment failures in human African trypanosomiasis. Trop. Med. Int. Health 2001, 6, 906–914. [Google Scholar] [CrossRef]
- Hawking, F. Drug Resistance acquired during the Treatment of Sleeping-Sickness with Tryparsamide and with Bayer 205. Am. J. Trop. Med. 1941, 21, 469–479. [Google Scholar] [CrossRef]
- Kagira, J.M.; Maina, N. Occurrence of multiple drug resistance in Trypanosoma brucei rhodesiense isolated from sleeping sickness patients. Onderstepoort J. Vet. Res. 2007, 74, 17–22. [Google Scholar] [PubMed]
- Kibona, S.N.; Matemba, L.; Kaboya, J.S.; Lubega, G.W. Drug-resistance of Trypanosoma b. rhodesiense isolates from Tanzania. Trop. Med. Int. Health 2006, 11, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Maina, N.; Maina, K.J.; Maser, P.; Brun, R. Genotypic and phenotypic characterization of Trypanosoma brucei gambiense isolates from Ibba, South Sudan, an area of high melarsoprol treatment failure rate. Acta Trop. 2007, 104, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mpia, B.; Pepin, J. Combination of eflornithine and melarsoprol for melarsoprol-resistant Gambian trypanosomiasis. Trop. Med. Int. Health 2002, 7, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.C.; Eze, A.A.; Baker, N.; Glover, L.; Clucas, C.; Aguinaga Andrés, D.; Natto, M.J.; Teka, I.A.; McDonald, J.; Lee, R.S.; et al. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.C.; Tagoe, D.N.A.; Eze, A.A.; Krezdorn, J.A.M.; Rojas López, K.E.; Alkhaldi, A.A.M.; McDonald, F.; Still, J.; Alzahrani, K.J.; Settimo, L.; et al. Functional analysis of drug resistance-associated mutations in the T rypanosoma brucei adenosine transporter 1 (TbAT1) and the proposal of a structural model for the protein. Mol. Microbiol. 2015, 96, 887–900. [Google Scholar] [CrossRef]
- Mutuku, C.N.; Bateta, R.; Rono, M.K.; Njunge, J.M.; Awuoche, E.O.; Ndung’u, K.; Mang’era, C.M.; Akoth, M.O.; Adung’a, V.O.; Ondigo, B.N.; et al. Physiological and proteomic profiles of Trypanosoma brucei rhodesiense parasite isolated from suramin responsive and non-responsive HAT patients in Busoga, Uganda. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 57–67. [Google Scholar] [CrossRef]
- Nerima, B.; Matovu, E.; Lubega, G.W.; Enyaru, J.C.K. Detection of mutant P2 adenosine transporter (TbAT1) gene in Trypanosoma brucei gambiense isolates from northwest Uganda using allele-specific polymerase chain reaction. Trop. Med. Int. Health 2007, 12, 1361–1368. [Google Scholar] [CrossRef]
- Nnadi, C.O.; Ebiloma, G.U.; Black, J.A.; Nwodo, N.J.; Lemgruber, L.; Schmidt, T.J.; de Koning, H.P. Potent antitrypanosomal activities of 3-aminosteroids against African trypanosomes: Investigation of cellular effects and of cross-resistance with existing drugs. Molecules 2019, 24, 268. [Google Scholar] [CrossRef]
- Sanderson, L.; Dogruel, M.; Rodgers, J.; De Koning, H.P.; Thomas, S.A. Pentamidine Movement across the Murine Blood-Brain and Blood-Cerebrospinal Fluid Barriers: Effect of Trypanosome Infection, Combination Therapy, P-Glycoprotein, and Multidrug Resistance-Associated Protein. J. Pharmacol. Exp. Ther. 2009, 329, 967–977. [Google Scholar] [CrossRef]
- Foulkes, J.R. Metronidazole and suramin combination in the treatment of arsenical refractory Rhodesian sleeping sickness—A case study. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 422. [Google Scholar] [CrossRef]
- Pepin, J.; Milord, F.; Mpia, B.; Meurice, F.; Ethier, L.; DeGroof, D.; Bruneel, H. An open clinical trial of nifurtimox for arseno-resistant Trypanosoma brucei gambiense sleeping sickness in central Zaire. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 514–517. [Google Scholar] [CrossRef]
- Pepin, J.; Milord, F.; Meurice, F.; Ethier, L.; Loko, L.; Mpia, B. High-dose nifurtimox for arseno-resistant Trypanosoma brucei gambiense sleeping sickness: An open trial in central Zaire. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 254–256. [Google Scholar] [CrossRef]
- Richardson, J.B.; Evans, B.; Pyana, P.P.; van Reet, N.; Sistrom, M.; Buscher, P.; Aksoy, S.; Caccone, A. Whole genome sequencing shows sleeping sickness relapse is due to parasite regrowth and not reinfection. Evol. Appl. 2016, 9, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Matovu, E.; Geiser, F.; Schneider, V.; Maser, P.; Enyaru, J.C.K.; Kaminsky, R.; Gallati, S.; Seebeck, T. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 2001, 117, 73–81. [Google Scholar] [CrossRef]
- Balasegaram, M.; Harris, S.; Checchi, F.; Ghorashian, S.; Hamel, C.; Karunakara, U. Melarsoprol versus eflornithine for treating late-stage Gambian trypanosomiasis in the Republic of the Congo. Bull. World Health Organ. 2006, 84, 783–791. [Google Scholar] [CrossRef]
- Pepin, J.; Khonde, N.; Maiso, F.; Doua, F.; Jaffar, S.; Ngampo, S.; Mpia, B.; Mbulamberi, D.; Kuzoe, F. Short-course eflornithine in Gambian trypanosomiasis: A multicentre randomized controlled trial. Bull. World Health Organ. 2000, 78, 1284–1295. [Google Scholar]
- Scientific Working Group on African Trypanosomiasis. Proceedings of the Report of the Scientific Working Group meeting on African trypanosomiasis, Geneva, Switzerland, 4–8 June 2001; p. 96.
- Burri, C.; Keiser, J. Pharmacokinetic investigations in patients from northern Angola refractory to melarsoprol treatment. Spec. Issue. Hum. Afr. Trypanos. Approaches Roll Back Sleep. Sick. 2001, 6, 412–420. [Google Scholar] [CrossRef]
- Kazibwe, A.J.N.; Nerima, B.; de Koning, H.P.; Maser, P.; Barrett, M.P.; Matovu, E. Genotypic status of the TbAT1/P2 adenosine transporter of Trypanosoma brucei gambiense isolates from Northwestern Uganda following melarsoprol withdrawal. PLoS Negl. Trop. Dis. 2009, 3, e523. [Google Scholar] [CrossRef]
- Pyana, P.P.; Sere, M.; Kabore, J.; de Meeus, T.; MacLeod, A.; Bucheton, B.; van Reet, N.; Buscher, P.; Belem, A.M.G.; Jamonneau, V. Population genetics of Trypanosoma brucei gambiense in sleeping sickness patients with treatment failures in the focus of Mbuji-Mayi, Democratic Republic of the Congo. Infect. Genet. Evol. 2015, 30, 128–133. [Google Scholar] [CrossRef]
- Balasegaram, M.; Harris, S.; Checchi, F.; Hamel, C.; Karunakara, U. Treatment outcomes and risk factors for relapse in patients with early-stage human African trypanosomiasis (HAT) in the Republic of the Congo. Bull. World Health Organ. 2006, 84, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Lutje, V.; Seixas, J.; Kennedy, A. Chemotherapy for second-stage human African trypanosomiasis. Cochrane Database Syst. Rev. 2013, 2013, CD006201. [Google Scholar] [CrossRef] [PubMed]
- Begley, C.G.; Ioannidis, J.P.A. Reproducibility in science: Improving the standard for basic and preclinical research. Circ. Res. 2015, 116, 116–126. [Google Scholar] [CrossRef]
- Wolff, J.O. Laboratory studies with rodents: Facts or artifacts? Bioscience 2003, 53, 421–427. [Google Scholar] [CrossRef] [Green Version]
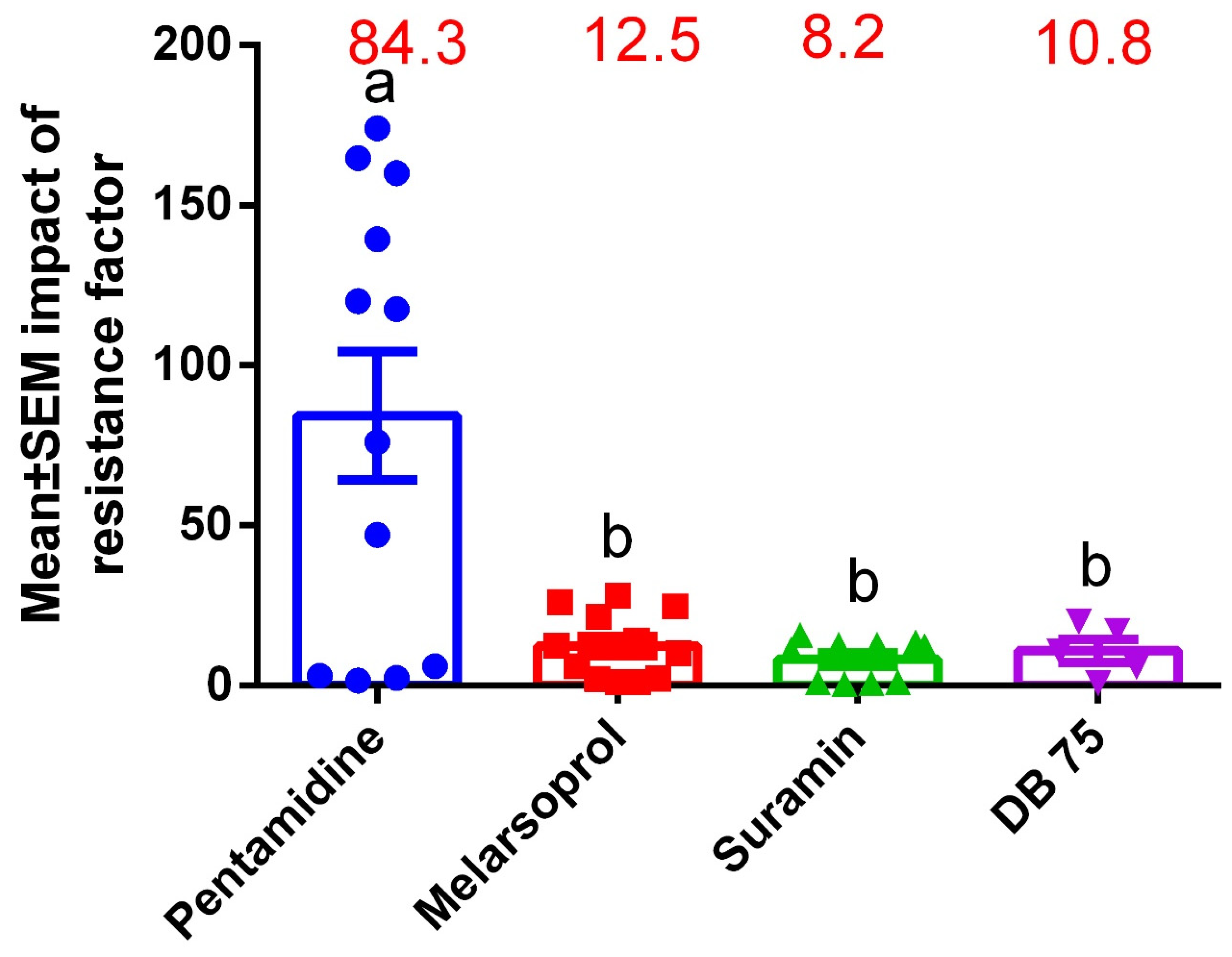
| Study | Ref | Sample size | Proportion (%) | 95% CI | Weight (%) | |
|---|---|---|---|---|---|---|
| Fixed | Random | |||||
| Kazibwe 2009 | [71] | 179 | 92.7 | 87.9–96.1 | 46.63 | 25.63 |
| Matovu 2001 | [66] | 68 | 55.9 | 43.3–67.9 | 17.88 | 25.04 |
| Nerima 2007 | [59] | 105 | 89.5 | 82.0–94.7 | 27.46 | 25.37 |
| Pati 2014 | [48] | 30 | 20.0 | 7.7–38.6 | 8.03 | 23.95 |
| Total (fixed effects) | 382 | 81.9 | 77.7–85.6 | 100 | 100 | |
| Total (random effects) | 382 | 68.0 | 38.0–91.6 | 100 | 100 | |
| Study | Ref | Pentamidine | Nifurtimox | Eflornithine | Melarsoprol | Combination Melarsoprol/Eflornithine | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relapse | Total | Relapse | Total | Relapse | Total | Relapse | Total | Relapse | Total | ||
| Balasegaram 2006 | [73] | 33 | 692 | ||||||||
| Balasegaram 2006 | [67] | 11 | 136 | 36 | 258 | ||||||
| Brun 2001 | [50] | 8 | 36 | ||||||||
| Burri 2001 | [70] | 7 | 16 | ||||||||
| Kagira 2007 | [52] | 5 | 6 | ||||||||
| Kazibwe 2009 | [71] | 9 | 101 | ||||||||
| Matovu 2001 | [66] | 43 | 65 | ||||||||
| Mpia 2002 | [55] | 19 | 42 | 2 | 42 | ||||||
| Pati 2014 | [48] | 30 | 45 | ||||||||
| Pepin 1989 | [63] | 12 | 19 | ||||||||
| Pepin 1989 | [63] | 0 | 7 | ||||||||
| Pepin 1992 * | [64] | 0 | 9 | 9 | 30 | ||||||
| Pepin 2000 # in Côte d’Ivoire | [68] | 0 | 33 | 0 | 33 | ||||||
| Pepin 2000 # in DRC | [68] | 7 | 140 | ||||||||
| Pepin 2000 # in Uganda | [68] | 13 | 116 | ||||||||
| Total | 33 | 692 | 0 | 49 | 31 | 425 | 178 | 618 | 2 | 42 | |
| Total random effects | NA | 1.32 | 6.56 | 41.49 | NA | ||||||
| 95% CI | 0.043–6.17 | 3.057 to 11.252 | 24.944 to 59.094 | ||||||||
| Test for heterogeneity, Q(df), p value I2 (inconsistency), 95% CI | 0.34 (2), p = 0.85. I2 = 0.00%, 0.00 to 79.99 | 8.43 (3), 0.038 I2 = 64.43%, 0.00–87.95 | 150.68 (9), p < 0.0001; I2 = 94.03%, 90.93–96.06 | ||||||||
| Publication bias: Egger’s test intercept (95% CI, p value); Begg’s test Kendall’s Tau, p value | 1.036 (0.99–1.08, 0.002); Tau =1.00, p = 0.117) | −4.1621 (17.6527 to 9.3285, p = 0.3156); Tau = 0.0000, 1.0000 | 5.7053 (0.5442 to 10.8664), p = 0.0342; Tau = 0.2000, p = 0.4208 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasozi, K.I.; MacLeod, E.T.; Welburn, S.C. Systematic Review and Meta-Analysis on Human African Trypanocide Resistance. Pathogens 2022, 11, 1100. https://doi.org/10.3390/pathogens11101100
Kasozi KI, MacLeod ET, Welburn SC. Systematic Review and Meta-Analysis on Human African Trypanocide Resistance. Pathogens. 2022; 11(10):1100. https://doi.org/10.3390/pathogens11101100
Chicago/Turabian StyleKasozi, Keneth Iceland, Ewan Thomas MacLeod, and Susan Christina Welburn. 2022. "Systematic Review and Meta-Analysis on Human African Trypanocide Resistance" Pathogens 11, no. 10: 1100. https://doi.org/10.3390/pathogens11101100
APA StyleKasozi, K. I., MacLeod, E. T., & Welburn, S. C. (2022). Systematic Review and Meta-Analysis on Human African Trypanocide Resistance. Pathogens, 11(10), 1100. https://doi.org/10.3390/pathogens11101100







