Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates
Abstract
:1. Introduction
2. Results
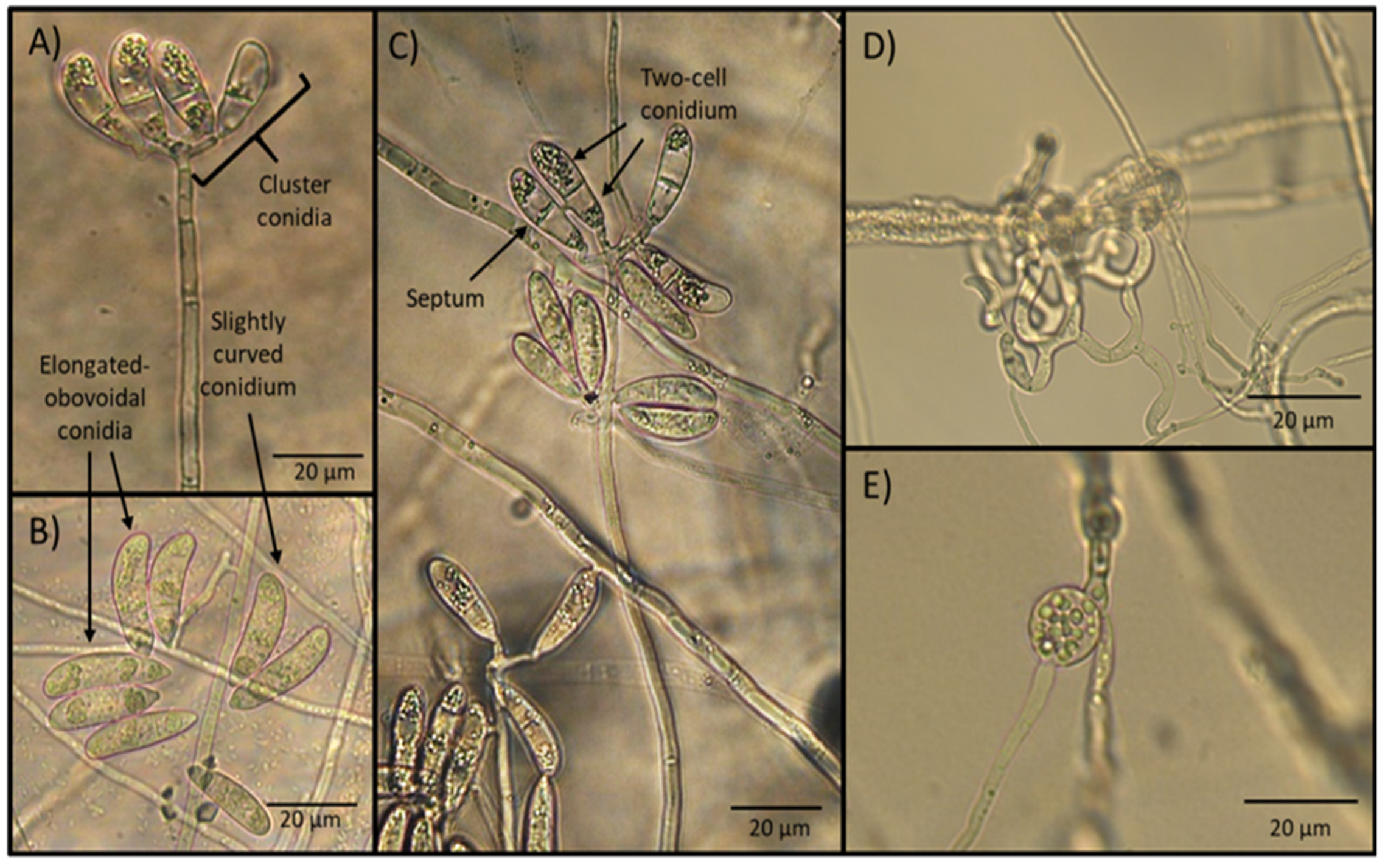
2.1. Traditional Taxonomy by Morphological Identification
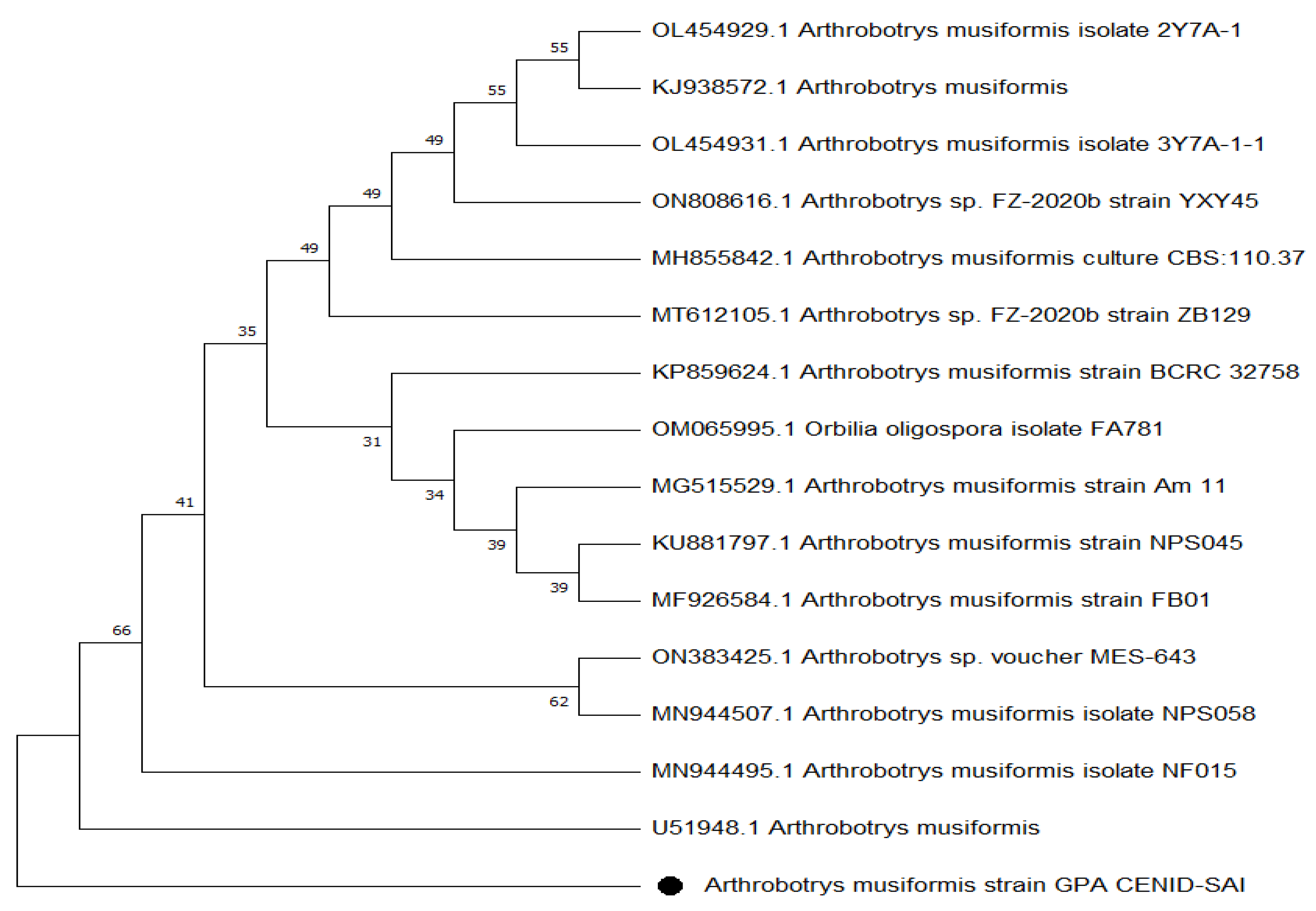
2.2. Molecular Taxonomy
2.3. Predatory Activity
2.4. Nematocidal Activity of the Culture Filtrates from A. musiformis against H. contortus Infective Larvae
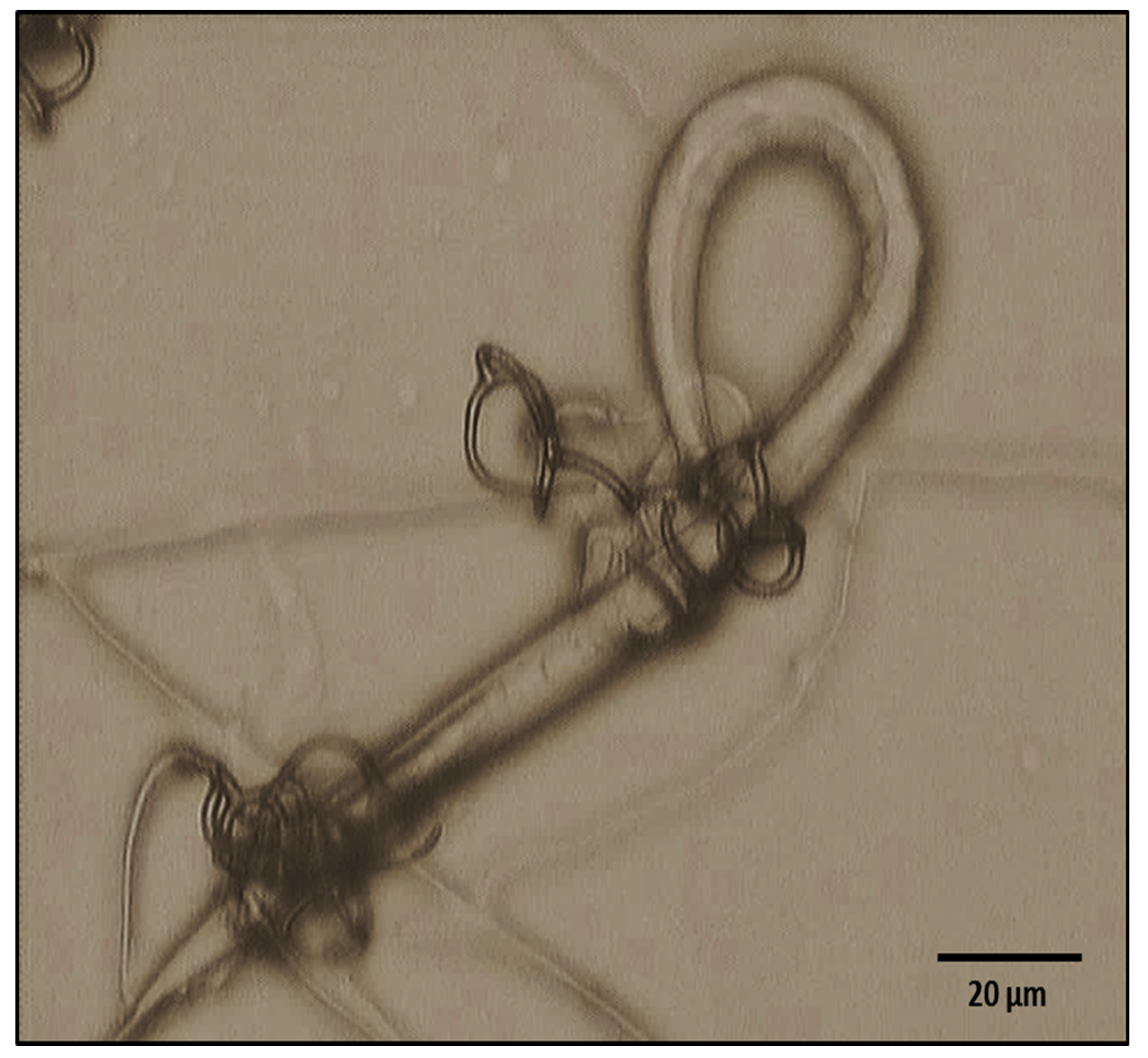
2.5. Microscopical Findings
2.6. Mycochemical Group Identification Profile
3. Discussion
3.1. Traditional Taxonomy
3.2. Molecular Taxonomy
3.3. Predatory Activity
3.4. Nematocidal Activity of Fungal Culture Filtrates
3.5. Photographic Analysis
3.6. Mycochemical Compound Group Identification
4. Materials and Methods
4.1. Allocation
4.2. Fungal Isolation
4.3. Traditional Morphological Taxonomy of Fungi
4.4. Molecular Taxonomy of Fungi
4.5. Nematodes
4.5.1. Panagrellus Redivivus
4.5.2. Haemonchus contortus Infective Larvae
4.6. Predatory Activity Assessment
4.7. Nematocidal Activity of A. musiformis Liquid Culture Filtrates
4.8. Statistical Analyses
4.8.1. Predation Assay
4.8.2. Fungal Culture Filtrate Assay
4.9. Microscopic Analysis
4.10. Myco-Qualitative Reagent Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, T.M. Gastrointestinal nematodes, diagnosis and control. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 185–199. [Google Scholar] [CrossRef]
- Zajac, A.M.; Garza, J. Biology, epidemiology, and control of gastrointestinal nematodes of small ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 73–87. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P. Soil-Borne nematodes: Impact in agriculture and livestock and sustainable strategies of prevention and control with special reference to the use of nematode natural enemies. Pathogens 2022, 11, 640. [Google Scholar] [CrossRef]
- Naeem, M.; Iqbal, Z.; Roohi, N. Ovine haemonchosis: A review. Trop. Anim. Health Prod. 2021, 53, 1–11. [Google Scholar] [CrossRef]
- Waghorn, T.S.; Miller, C.M.; Candy, P.; Carvalho, L.; Meban, J.; Green, P.; Leathwick, D.M. The production costs of Haemonchus contortus and other nematode parasites in pre-weaned beef calves in New Zealand. Vet. Parasitol. Reg. Stud. Rep. 2022, 30, 100718. [Google Scholar] [CrossRef]
- Cruz-Tamayo, A.A.; López-Arellano, M.E.; González-Garduño, R.; Torres-Hernández, G.; De la Mora-Valle, A.; Becerril-Pérez, C.; Hernández-Mendo, O.; Ramírez-Bribiesca, E.; Huchin-Cab, M. Haemonchus contortus infection induces a variable immune response in resistant and susceptible Pelibuey sheep. Vet. Immunol. Immunopathol. 2021, 234, 110218. [Google Scholar] [CrossRef]
- Flay, K.J.; Hill, F.I.; Muguiro, D.H. A Review: Haemonchus contortus infection in pasture-based sheep production systems, with a focus on the pathogenesis of anaemia and changes in haematological parameters. Animals 2022, 12, 1238. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Grisi, L.; Pérez de León, A.A.; Villela, H.S.; Torres-Acosta, J.F.D.J.; Fragoso Sánchez, H.; Romero Salas, D.; Rosario Cruz, R.; Saldierna, F.; García Carrasco, D. Potential economic impact assessment for cattle parasites in Mexico. Review. Rev. Mex. Cienc. Pecu. 2017, 8, 61–74. [Google Scholar] [CrossRef]
- Selemon, M. Review on Control of Haemonchus contortus in Sheep and Goat. J. Vet. Med. Res. 2018, 5, 1139. [Google Scholar]
- Bull, K.; Glover, M.J.; Rose Vineer, H.; Morgan, E.R. Increasing resistance to multiple anthelmintic classes in gastrointestinal nematodes on sheep farms in southwest England. Vet. Rec. 2022, 190, e1531. [Google Scholar] [CrossRef]
- Charlier, J.; Bartley, D.J.; Sotiraki, S.; Martínez-Valladares, M.; Claerebout, E.; von Samson-Himmelstjerna, G.; Thamsborg, S.M.; Hoste, H.; Morgan, E.R.; Rinaldi, L. Anthelmintic resistance in ruminants: Challenges and solutions. Adv. Parasitol. 2022, 115, 171–227. [Google Scholar] [CrossRef]
- Soares, V.M.; Pereira, J.G.; Barreto, F.; Jank, L.; Rau, R.B.; Dias Ribeiro, C.B.; Dos Santos Castilhos, T.; Tomaszewski, C.A.; Hillesheim, D.R.; Mondadori, R.G. Residues of veterinary drugs in animal products commercialized in the border region of Brazil, Argentina, and Uruguay. J. Food Prot. 2022, 85, 980–986. [Google Scholar] [CrossRef]
- Sands, B.; Noll, M. Toxicity of ivermectin residues in aged farmyard manure to terrestrial and freshwater invertebrates. Insect Conserv. Divers. 2022, 15, 9–18. [Google Scholar] [CrossRef]
- Wang, B.-B.; Wang, F.-H.; Xu, Q.; Wang, K.-Y.; Xue, Y.-J.; Ren, R.; Zeng, J.-Q.; Liu, Y.; Zhang, H.-Y.; Wang, H.-Y.; et al. In vitro and in vivo studies of the native isolates of nematophagous fungi from China against the larvae of trichostrongylides. J. Basic Microbiol. 2016, 57, 265–275. [Google Scholar] [CrossRef]
- Canhão-Dias, M.; Paz-Silva, A.; Madeira de Carvalho, L.M. The efficacy of predatory fungi on the control of gastrointestinal parasites in domestic and wild animals—A systematic review. Vet. Parasitol. 2020, 283, 109173. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef]
- Cao, X.; Xu, Q.; Wan, X.-M.; Yang, X.-C.; Jia, X.-Y.; Xue, X.-J.; Du, J.-L.; Cai, K.-Z. Isolation, identification, and in vitro predatory activity of nematophagous fungus Arthrobotrys musiformis and its interaction with nematodes using scanning electron microscopy. Biocontrol Sci. Technol. 2018, 28, 94–104. [Google Scholar] [CrossRef]
- Júnior, A.D.; Ferreira, V.M.; de Carvalho, L.M.; Álvares, F.B.V.; Vilela, V.L.R.; Ferraz, C.M.; Veloso, F.B.R.; Lima, T.F.; Braga, F.R.; de Araújo, J.V. Association of the nematophagous fungi Arthrobotrys musiformis and Monacrosporium sinense in vitro and in vivo for biological control of equine cyathostomins. Braz. J. Vet. Med. 2021, 43, e003021. [Google Scholar] [CrossRef]
- Acevedo-Ramírez, P.M.D.C.; Figueroa-Castillo, J.A.; Ulloa-Arvizú, R.; Martínez-García, L.G.; Guevara-Flores, A.; Rendón, J.L.; Valero-Cross, R.O.; Mendoza-de Gives, P.; Quiroz-Romero, H. Proteolytic activity of extracellular products from Arthrobotrys musiformis and their effect in vitro against Haemonchus contortus infective larvae. Vet. Rec. Open 2015, 2, e000103. [Google Scholar] [CrossRef]
- Kuo, T.H.; Yang, C.T.; Chang, H.Y.; Hsueh, Y.P.; Hsu, C.C. Nematode-trapping fungi produce diverse metabolites during predator–prey interaction. Metabolites 2020, 10, 117. [Google Scholar] [CrossRef]
- Drechsler, C. Some hyphomycetes that prey on free-living terricolous nematodes. Mycologia 1937, 29, 447–552. [Google Scholar] [CrossRef]
- Van Oorschot, C.A.N. A review of Arthrobotrys and allied genera. In Taxonomy of the Dactylaria Complex. Stud. Mycol. 1985, 26, 61–96. [Google Scholar]
- Zhang, F.; Boonmee, S.; Bhat, J.D.; Xiao, W.; Yang, X.Y. New Arthrobotrys nematode-trapping species (Orbiliaceae) from terrestrial soils and freshwater sediments in China. J. Fungi 2022, 8, 671. [Google Scholar] [CrossRef]
- Tzean, Y.; Chou, T.H.; Hsiao, C.C.; Shu, P.Y.; Walton, J.D.; Tzean, S.S. Cloning and characterization of cuticle-degrading serine protease from nematode-trapping fungus Arthrobotrys musiformis. Mycoscience 2016, 57, 136–143. [Google Scholar] [CrossRef]
- Liou, G.Y.; Tzean, S.S. Phylogeny of the genus Arthrobotrys and allied nematode-trapping fungi based on rDNA sequences. Mycologia 1997, 89, 876–884. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Gong, J.; Zhang, Y. Individual and combined application of nematophagous fungi as biological control agents against gastrointestinal nematodes in domestic animals. Pathogens 2022, 11, 172. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Olmedo-Juárez, A.; Mendoza-de Gives, P. Control and prevention of nematodiasis in small ruminants: Background, challenges and outlook in Mexico. Rev. Mex. Cienc. Pecu. 2021, 12, 186–204. [Google Scholar] [CrossRef]
- Cériac, S.; Archimède, H.; Feuillet, D.; Felicite, Y.; Giorgi, M.; Bambou, J.C. Supplementation with rumen-protected proteins induces resistance to Haemonchus contortus in goats. Sci. Rep. 2019, 9, 1237. [Google Scholar] [CrossRef]
- López-Leyva, Y.; González-Garduño, R.; Huerta-Bravo, M.; Ramírez-Valverde, R.; Torres-Hernández, G.; Arece-García, J.; López-Arellano, M.E. High energy levels in the diet reduce the parasitic effect of Haemonchus contortus in Pelibuey sheep. Heliyon 2020, 6, e05870. [Google Scholar] [CrossRef]
- Prasad, M.R.; Sundaram, S.M.; Gnanaraj, P.T.; Bandeswaran, C.; Harikrishnan, T.J.; Sivakumar, T.; Azhahiannambi, P. Influence of intensive rearing, continuous and rotational grazing systems of management on parasitic load of lambs. Vet. World 2019, 12, 1188. [Google Scholar] [CrossRef]
- Ruiz-Huidobro, C.; Sagot, L.; Lugagne, S.; Huang, Y.; Milhes, M.; Bordes, L.; Prévot, F.; Grisez, C.; Gautier, D.; Valadier, C. Cell grazing and Haemonchus contortus control in sheep: Lessons from a two-year study in temperate Western Europe. Sci. Rep. 2019, 9, 12699. [Google Scholar] [CrossRef]
- Ehsan, M.; Hu, R.S.; Liang, Q.L.; Hou, J.L.; Song, X.; Yan, R.; Zhu, X.Q.; Li, X. Advances in the development of anti-Haemonchus contortus vaccines: Challenges, opportunities, and perspectives. Vaccines 2020, 8, 555. [Google Scholar] [CrossRef]
- Tian, X.; Lu, M.; Bu, Y.; Zhang, Y.; Aimulajiang, K.; Liang, M.; Li, C.; Yan, R.; Xu, L.; Song, X.; et al. Immunization with Recombinant Haemonchus contortus Y75B8A. 8 Partially Protects Local Crossbred Female Goats from Haemonchus contortus Infection. Front. Vet. Sci. 2022, 9, 765700. [Google Scholar] [CrossRef]
- Castillo-Mitre, G.F.; Rojo-Rubio, R.; Olmedo-Juarez, A.; Mendoza-de Gives, P.; Vázquez-Armijo, J.F.; Zamilpa, A.; Lee-Rangel, H.A.; Avendaño-Reyes, L.; Macias-Cruz, U. In vivo anthelmintic activity of Acacia cochliacantha leaves against Haemonchus contortus in Boer goat kids. Rev. Mex. Cienc. Pecu. 2021, 12, 138–150. [Google Scholar] [CrossRef]
- Torres-Fajardo, R.A.; Higuera-Piedrahita, R.I. Actividad antihelmíntica in vivo de terpenos y aceites esenciales en pequeños rumiantes. Rev. MVZ Córdoba 2021, 26, e2317. [Google Scholar] [CrossRef]
- Soares, A.C.F.; Sousa, C.D.S.; Coimbra, J.L.; Machado, G.D.S.; Garrido, M.D.S.; Almeida, N.D.S. Predatory ability of Arthrobotrys musiformis and Monacrosporium thaumasium on Scutellonema bradys. Sci. Agric. 2006, 63, 396–398. [Google Scholar] [CrossRef]
- Alfaro-Gutiérrez, I.C.; Mendoza-de Gives, P.; Liébano-Hernández, E.; López-Arellano, M.; Valero-Coss, R.O.; Hernández-Velázquez, V.M. Nematophagous fungi (Orbiliales) capturing, destroying and feeding on the histotrophic larvae of Haemonchus contortus (Nematoda: Trichostrongylidae). Rev. Mex. Micol. 2011, 33, 29–35. [Google Scholar]
- Da Silva, M.E.; Uriostegui, M.A.M.; Millán-Orozco, J.; Mendoza-de Gives, P.; Hernández, E.L.; Braga, F.R.; Araújo, J.V.D. Predatory activity of Butlerius nematodes and nematophagous fungi against Haemonchus contortus infective larvae. Rev. Parasitol. Vet. 2017, 26, 92–95. [Google Scholar] [CrossRef]
- Cai, K.Z.; Wang, B.B.; Xu, Q.; Liu, J.L.; Wang, K.Y.; Xue, Y.J.; Zhang, H.Y.; Wang, H.Y.; Cao, X.; Ma, Z.R. In vitro and in vivo studies of nematophagous fungi Arthrobotrys musiformis and Arthrobotrys robusta against the larvae of the trichostrongylides. Acta Parasitol. 2017, 62, 666–674. [Google Scholar] [CrossRef]
- Ojeda-Robertos, N.F.; Aguilar-Marcelino, L.; Olmedo-Juárez, A.; Luna-Palomera, C.; Peralta-Torres, J.A.; López-Arellano, M.E.; Mendoza-de Gives, P. In vitro predatory activity of nematophagous fungi isolated from water buffalo feces and from soil in the Mexican southeastern. Rev. Bras. Parasitol. Vet. 2019, 28, 314–319. [Google Scholar] [CrossRef]
- Sánchez-Martínez, E.; Aguilar-Marcelino, L.; Hernández-Romano, J.; Castañeda-Ramírez, G.S.; Mendoza-de-Gives, P. Taxonomic and biological characterization and predatory activity of four nematophagous fungi isolates of Arthrobotrys species from Tapachula, Chiapas, Mexico. Arch. Agron. Soil Scie. 2021, 1–17. [Google Scholar] [CrossRef]
- Purba, R.T.T.; Fauzi, F.; Sari, R.W.; Naibaho, D.C.; Putri, Q.A.; Maulana, A.; Punnapayak, H. Arthrobotrys thaumasia and Arthrobotrys musiformis as biocontrol agents against Meloidogyne hapla on tomato plant. Biodiversitas 2022, 23, 3659–3666. [Google Scholar] [CrossRef]
- Pramer, D.; Stoll, N.R. Nemin: A morphogenic substance causing trap formation by predaceous fungi. Science 1959, 129, 966–967. [Google Scholar] [CrossRef]
- Nordbring-Hertz, B. Nematode-induced morphogenesis in the predacious fungus Arthrobotrys oligospora. Nematologica 1977, 23, 443–451. [Google Scholar] [CrossRef]
- Nordbring-Hertz, B. Morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora-an extensive plasticity of infection structures. Mycologist 2004, 18, 125–133. [Google Scholar] [CrossRef]
- Vidal-Diez de Ulzurrun, G.; Hsueh, Y.P. Predator-prey interactions of nematode-trapping fungi and nematodes: Both sides of the coin. Appl. Microbiol. Biotechnol. 2018, 102, 3939–3949. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.H.; Gharib, S.; Schroeder, F.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. eLife 2017, 6, e20023. [Google Scholar] [CrossRef]
- Tunlid, A.; Jansson, H.B.; Nordbring-Hertz, B. Fungal attachment to nematodes. Mycol. Res. 1992, 96, 401–412. [Google Scholar] [CrossRef]
- Olthof, T.H.; Estey, R.H. A nematotoxin produced by the nematophagous fungus Arthrobotrys oligospora Fresenius. Nature 1963, 197, 514–515. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P.; Behnke, J.M.; Davies, K.G. Extracellular enzyme production by nematophagous fungi in the presence and absence of nematodes. Int. J. Nematol. 2003, 13, 27–36. [Google Scholar]
- Huang, X.; Zhao, N.; Zhang, K. Extracellular enzymes serving as virulence factors in nematophagous fungi involved in infection of the host. Res. Microb. 2004, 155, 811–816. [Google Scholar] [CrossRef]
- Ocampo-Gutiérrez, A.Y.; Hernández-Velázquez, V.M.; Aguilar-Marcelino, L.; Cardoso-Taketa, A.; Zamilpa, A.; López-Arellano, M.E.; González-Cortázar, M.; Jesús Hernández-Romano, J.; Reyes-Estebanez, M.; Mendoza-de Gives, P. Morphological and molecular characterization, predatory behaviour and effect of organic extracts of four nematophagous fungi from Mexico. Fungal Ecol. 2021, 49, 101004. [Google Scholar] [CrossRef]
- Wan, J.; Dai, Z.; Zhang, K.; Li, G.; Zhao, P. Pathogenicity and metabolites of endoparasitic nematophagous fungus Drechmeria coniospora YMF1. 01759 against nematodes. Microorganisms 2021, 9, 1735. [Google Scholar] [CrossRef]
- Scholler, M.; Rubner, A. Predacious activity of the nematode-destroying fungus Arthrobotrys oligospora in dependence of the medium composition. Microbiol. Res. 1994, 149, 145–149. [Google Scholar] [CrossRef]
- Vidal, A.R.; Zaucedo-Zuñiga, A.L.; de Lorena Ramos-García, M. Propiedades nutrimentales del camote (Ipomoea batatas L.) y sus beneficios en la salud humana. Rev. Iberoam. Tecno. Postcosecha 2018, 19, 132–146. [Google Scholar]
- Montagner, C.; de Souza, S.M.; Groposo, C.; Delle Monache, F.; Smânia, E.F.; Smânia, A., Jr. Antifungal activity of coumarins. Z. Nat. C 2008, 63, 21–28. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Me Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A review on anti-tumor mechanisms of coumarins. Front. Oncol. 2020, 10, 2720. [Google Scholar] [CrossRef]
- Matos, M.J. Coumarin and its derivatives. Molecules 2021, 26, 6320. [Google Scholar] [CrossRef]
- von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Valles-de la Mora, B.; Mendoza-de Gives, P.; González-Cortazar, M.; Zamilpa, A. Anthelmintic effect of 2H-chromen-2-one isolated from Gliricidia sepium against Cooperia punctata. Exp. Parasitol. 2017, 178, 1–6. [Google Scholar] [CrossRef]
- Jasso-Díaz, G.; Torres-Hernández, G.; Zamilpa, A.; Becerril-Pérez, C.M.; Ramírez-Bribiesca, J.E.; Hernández-Mendo, O.; Sánchez-Arroyo, H.; González-Cortazar, M.; Mendoza-de Gives, P.M. In vitro assessment of Argemone mexicana, Taraxacum officinale, Ruta chalepensis and Tagetes filifolia against Haemonchus contortus nematode eggs and infective (L3) larvae. Microb. Pathog. 2017, 109, 162–168. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; Mendoza-de Gives, P.; Aguilar-Marcelino, L.; López-Arellano, M.E.; Hernández-Romano, J. Phylogenetic analysis of nucleotide sequences from the ITS Region and biological characterization of nematophagous fungi from Morelos, Mexico. J. Mycol. 2016, 2016, 8502629. [Google Scholar] [CrossRef]
- Haard, K. Taxonomic studies on the genus Arthrobotrys corda. Mycologia 1968, 60, 1140–1159. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appi. 1990, 18, 315–322. [Google Scholar]
- de Lara, R.; Castro, T.; Castro, J.; Castro, G. Cultivo del nematodo Panagrellus redivivus (Goodey, 1945) en un medio de avena enriquecida con Spirulina sp. Rev. Biol. Mar. Oceanogr. 2007, 42, 29–36. [Google Scholar] [CrossRef]
- Valcárcel-Sancho, F.; Rojo-Vázquez, F.A.; Olmeda-García, A.S.; Arribas-Novillo, B.; Márquez-Sopeña, L.; Fernando-Pat, N. Atlas de Parasitología Ovina. Editorial Servet. Zaragoza, España, 2009; 137p, ISBN 978-84-92569-05-2. Available online: http://www.servet.es/editorial.php?pg=ce&_pagi_pg=6 (accessed on 23 May 2022).
- Cedillo-Borda, M.; López-Arellano, M.E.; Reyes-Guerrero, D.E. In vitro assessment of ivermectin resistance and gene expression profiles of P-glycoprotein genes from Haemonchus contortus (L3). Bio-protocpl 2020, 10, e3851. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Delgado-Núñez, E.J.; Bahena-Vicencio, A.; Villa-Mancera, A.; Zamilpa, A.; González-Cortazar, M.; Rivero-Pérez, N.; Flores-Franco, G.; López-Arellano, M.E.; Mendoza-de Gives, P. In vitro nematocidal properties from two extracts: Lippia graveolens leaves and Delonix regia flowers against eggs and infective larvae of Haemonchus contortus. J. Med. Food 2022, 25, 329–337. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A.; Elsebai, M.F. Activity of Purpureocillium lilacinum filtrates on biochemical characteristics of Sclerotinia sclerotiorum and induction of defense responses in common bean. Eur. J. Plant Pathol. 2019, 155, 39–52. [Google Scholar] [CrossRef]

| Characteristic | Mean (µm) | Range (µm) |
|---|---|---|
| Conidia length | 36.16 | 30.11–40.08 |
| Conidia width | 8.99 | 7.66–10.29 |
| Conidiophore length | 240.8 | 166–407 |
| Chlamydospores | Present | |
| Type of traps | Adhesive nets | |
| Strain | Query Cover % | Similarity % | Gen Bank Accession Number |
|---|---|---|---|
| Arthrobotrys sp. FZ-2020b | 96 | 99.83 | MT612105.1 |
| A. musiformis CBS 110.37 | 96 | 99.83 | MH855842.1 |
| A. musiformis 3Y7A-1–1 | 96 | 99.83 | OL454931.1 |
| A. musiformis Am_11 | 96 | 99.66 | MG515529.1 |
| A. musiformis | 96 | 99.66 | KJ938572.1 |
| Group | Mean of Recovered Larvae ± SD | Reduction Larvae % |
|---|---|---|
| Group 1 Larvae/Fungus Interaction | 62 ± 41.67 | 71.54% |
| Group 2 Larvae (Control) | 217 ± 28.05 |
| Group | 100 mg/mL | 50 mg/mL | 25 mg/mL | |||
|---|---|---|---|---|---|---|
| Dead/Total | Mortality % | Dead/Total | Mortality % | Dead/Total | Mortality % | |
| CzDox- A. musiformis | 99/106 | 93.42 ± 10.49 a | 75/103 | 73.02 ± 16.02 a | 49/95 | 51.61 ± 19.41 a |
| SPDB- A. musiformis | 26/97 | 26.80 ± 2.76 b | 18/97 | 18.42 ± 8.98 b | 15/87 | 16.91 ± 2.37 b |
| CzDox- No Fungus | 4/98 | 4.45 ± 2.55 c | 3/98 | 3.48 ± 2.88 b | 5/74 | 6.76 ± 4.77 b |
| SPDB- No Fungus | 5/83 | 5.51 ± 3.97 c | 8/96 | 8.77 ± 2.01 b | 9/108 | 8.38 ± 1.07 b |
| PBS | 6/98 | 6.00 ± 2.29 c | 6/98 | 6.00 ± 2.29 b | 6/98 | 6.00 ± 2.29 b |
| Metabolite Group | Assay | Colourimetric Reaction | Fungus in Czapek–Dox Broth | Fungus in Sweet Potato Dextrose Broth |
|---|---|---|---|---|
| Alkaloids | Dragendorff | Change of colour to brown | + | ++ |
| Mayer | Change of colour to yellow | + | ++ | |
| Wagner | and formation of precipitate | + | ++ | |
| * Coumarins | Bornträger | Yellow fluorescence after 24 h (see in U.V) | + | − |
| Flavonoids | Mg2 + y HCL | Red, orange and violet colours | − | − |
| Tannins | Ferric chloride | Hydrolysable tannins (blue) | − | − |
| Condensed tannins (green) | − | − | ||
| Confirmation | White precipitate | − | − | |
| Solution of gelatin | ||||
| Gelatin and saline solution | ||||
| Saline solution | ||||
| Triterpenes/Sterols | Reaction of Liebermann- Buchard | Blue or blue-green (sterols) | − | − |
| Reaction of Salkowski | Red or purple (triterpenes) | − | − | |
| Saponins | Foam formation | Persistent foam formation | + | ++ |
| Blank Nematode | Host | Predatory Activity % | Authors |
|---|---|---|---|
| Scutellonema bradys | Yam tubers | 94.6% | [36] |
| H. contortus | Sheep | 97% | [37] |
| H. contortus | Small ruminants | 90.4% | [38] |
| Trichostrongylidae | Ruminants | 60.72–99.95% | [39] |
| Trichostrongylus colubriformis | Ruminants | 94.8% | [17] |
| H. contortus | Sheep | 100% | [40] |
| Panagrellus redivivus | A non-parasitic free-living nematode | 62.7–93.6% | [41] |
| Meloidogyne hapla | Tomatoes | 97% | [42] |
| Group | Medium | Fungus |
|---|---|---|
| Group 1 | Czapek–Dox broth | A. musiformis |
| Group 2 | Sweet potato dextrose broth | A. musiformis |
| Group 3 | Czapek–Dox broth | No fungus * |
| Group 4 | Sweet potato dextrose broth | No fungus * |
| Group 5 | PBS control | No fungus * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Anzúrez, G.; Olmedo-Juárez, A.; von-Son de Fernex, E.; Alonso-Díaz, M.Á.; Delgado-Núñez, E.J.; López-Arellano, M.E.; González-Cortázar, M.; Zamilpa, A.; Ocampo-Gutierrez, A.Y.; Paz-Silva, A.; et al. Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates. Pathogens 2022, 11, 1068. https://doi.org/10.3390/pathogens11101068
Pérez-Anzúrez G, Olmedo-Juárez A, von-Son de Fernex E, Alonso-Díaz MÁ, Delgado-Núñez EJ, López-Arellano ME, González-Cortázar M, Zamilpa A, Ocampo-Gutierrez AY, Paz-Silva A, et al. Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates. Pathogens. 2022; 11(10):1068. https://doi.org/10.3390/pathogens11101068
Chicago/Turabian StylePérez-Anzúrez, Gustavo, Agustín Olmedo-Juárez, Elke von-Son de Fernex, Miguel Ángel Alonso-Díaz, Edgar Jesús Delgado-Núñez, María Eugenia López-Arellano, Manasés González-Cortázar, Alejandro Zamilpa, Ana Yuridia Ocampo-Gutierrez, Adolfo Paz-Silva, and et al. 2022. "Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates" Pathogens 11, no. 10: 1068. https://doi.org/10.3390/pathogens11101068
APA StylePérez-Anzúrez, G., Olmedo-Juárez, A., von-Son de Fernex, E., Alonso-Díaz, M. Á., Delgado-Núñez, E. J., López-Arellano, M. E., González-Cortázar, M., Zamilpa, A., Ocampo-Gutierrez, A. Y., Paz-Silva, A., & Mendoza-de Gives, P. (2022). Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates. Pathogens, 11(10), 1068. https://doi.org/10.3390/pathogens11101068