A Comprehensive Evaluation of Enterobacteriaceae Primer Sets for Analysis of Host-Associated Microbiota
Abstract
1. Introduction
2. Results
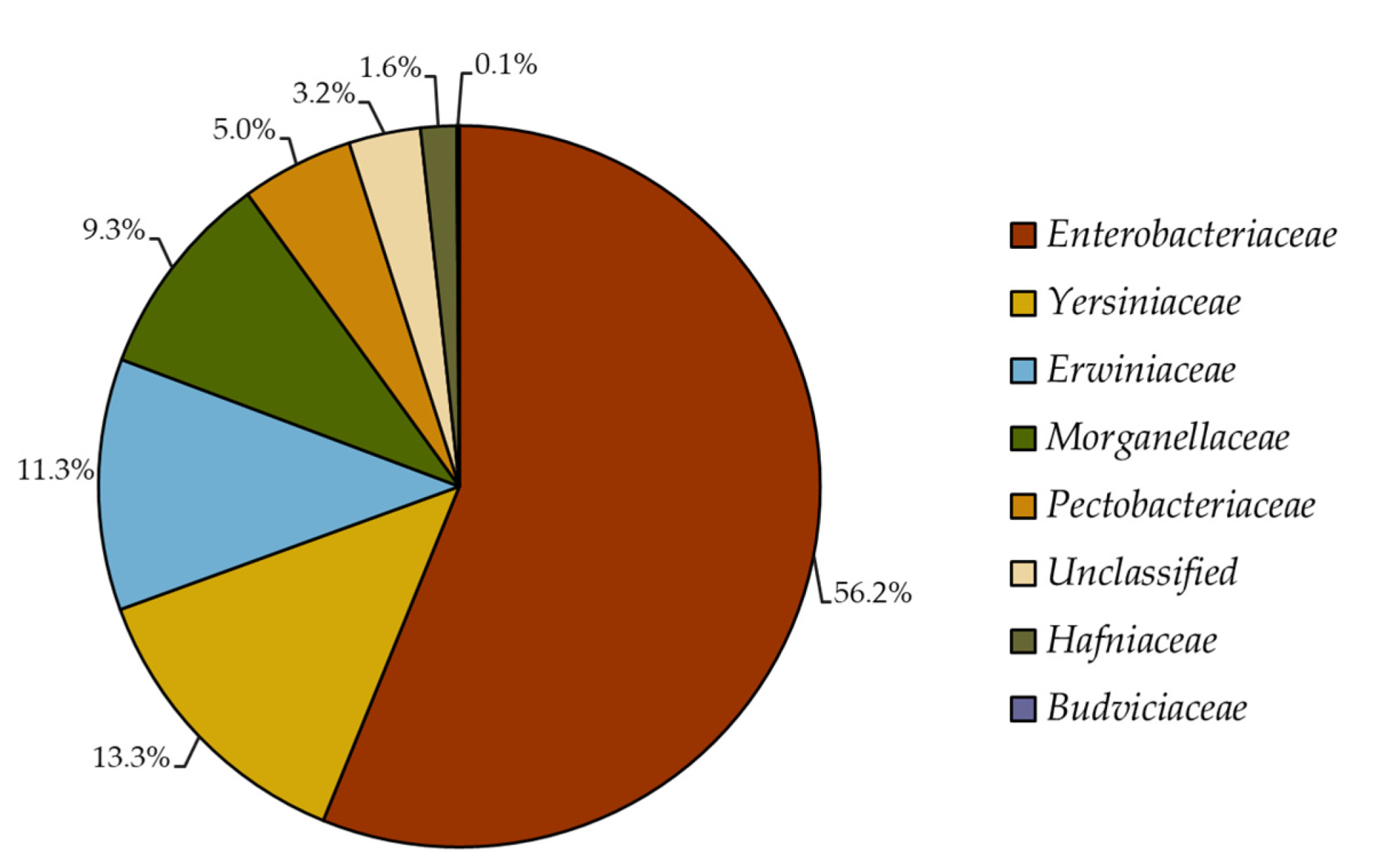
2.1. Reclassification of Formerly Enterobacteriaceae 16S rRNA Gene Sequences
2.2. Identification of Primer Sets
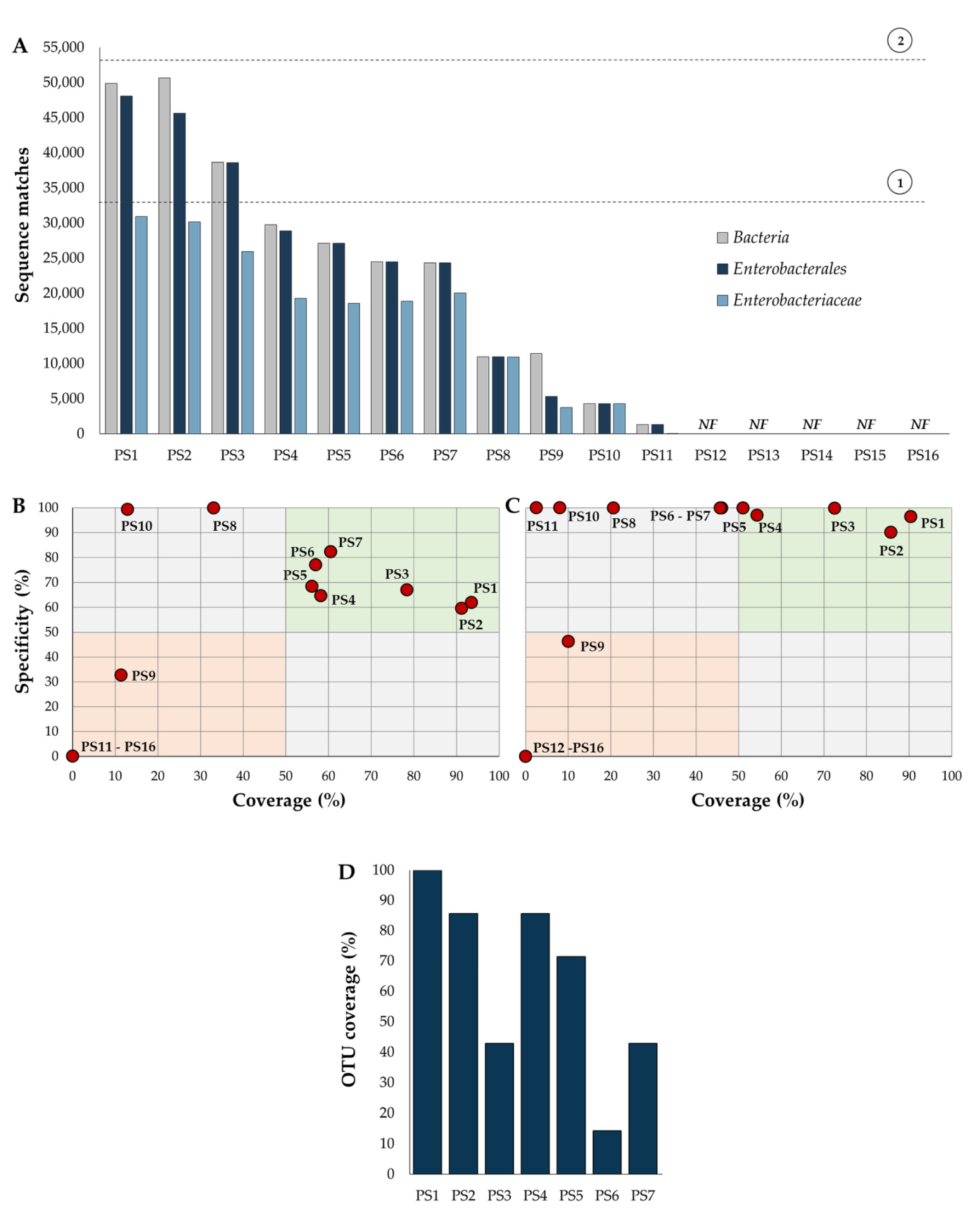
2.3. Primer Specificity and Coverage
3. Discussion
4. Materials and Methods
4.1. Reclassification of Formerly Enterobacteriaceae 16S rRNA Gene Sequences
4.2. Identification of Primer Sets
4.3. Design of Primer Set #3 (PS3)
4.4. Performance of Different Primer Sets Targeting Specific Amplification of Formerly Enterobacteriaceae 16S rRNA Gene
4.5. Identification of Bacterial Groups Targeted by Previously Validated and Published PCR Primer Sets
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Octavia, S.; Lan, R. The Family Enterobacteriaceae. In The Prokaryotes: Gammaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 225–286. [Google Scholar]
- Guanghui, Z.; Jing, L.; Guojun, Z.; Hong, L. Epidemiology and Risk Factors of Neurosurgical Bacterial Meningitis/Encephalitis Induced by Carbapenem Resistant Enterobacteriaceae. J. Infect. Chemother. 2020, 26, 101–106. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Hsia, Y.; Sharland, M.; Heath, P.T. Systematic Review of Carbapenem-Resistant Enterobacteriaceae Causing Neonatal Sepsis in China. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 36. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Wallace, M.J.; Fishbein, S.R.S.; Dantas, G. Antimicrobial Resistance in Enteric Bacteria: Current State and next-Generation Solutions. Gut Microbes 2020, 12, 1799654. [Google Scholar] [CrossRef]
- Xu, N.; Bai, X.; Cao, X.; Yue, W.; Jiang, W.; Yu, Z. Changes in Intestinal Microbiota and Correlation with TLRs in Ulcerative Colitis in the Coastal Area of Northern China. Microb. Pathog. 2021, 150, 104707. [Google Scholar] [CrossRef]
- Wright, E.K.; Kamm, M.A.; Teo, S.M.; Inouye, M.; Wagner, J.; Kirkwood, C.D. Recent Advances in Characterizing the Gastrointestinal Microbiome in Crohn’s Disease: A Systematic Review. Inflamm. Bowel. Dis. 2015, 21, 1219–1228. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut Microbiota in Health, Diverticular Disease, Irritable Bowel Syndrome, and Inflammatory Bowel Diseases: Time for Microbial Marker of Gastrointestinal Disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Ringel, Y. Alterations in Composition and Diversity of the Intestinal Microbiota in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2012, 24, 521-e248. [Google Scholar] [CrossRef]
- Linninge, C.; Roth, B.; Erlanson-Albertsson, C.; Molin, G.; Toth, E.; Ohlsson, B. Abundance of Enterobacteriaceae in the Colon Mucosa in Diverticular Disease. World J. Gastrointest. Pathophysiol. 2018, 9, 18–27. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Shao, L.; Ling, Z. Alterations of the Predominant Fecal Microbiota and Disruption of the Gut Mucosal Barrier in Patients with Early-Stage Colorectal Cancer. Biomed. Res. Int. 2020, 2020, 2948282. [Google Scholar] [CrossRef]
- Liu, C.-J.; Zhang, Y.-L.; Shang, Y.; Wu, B.; Yang, E.; Luo, Y.-Y.; Li, X.-R. Intestinal Bacteria Detected in Cancer and Adjacent Tissue from Patients with Colorectal Cancer. Oncol. Lett. 2019, 17, 1115–1127. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.-D.; Sun, X.-Q.; Ding, W.-J.; Wang, X.-Y.; Fan, J.-G. Gut Microbiota Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef]
- Diskin, S.; Feygenberg, O.; Maurer, D.; Droby, S.; Prusky, D.; Alkan, N. Microbiome Alterations Are Correlated with Occurrence of Postharvest Stem-End Rot in Mango Fruit. Phytobiome 2017, 1, 117–127. [Google Scholar] [CrossRef]
- Kurina, I.; Popenko, A.; Klimenko, N.; Koshechkin, S.; Chuprikova, L.; Filipenko, M.; Tyakht, A.; Alexeev, D. Development of qPCR Platform with Probes for Quantifying Prevalent and Biomedically Relevant Human Gut Microbial Taxa. Mol. Cell. Probes 2020, 52, 101570. [Google Scholar] [CrossRef]
- Rossmann, B.; Müller, H.; Smalla, K.; Mpiira, S.; Tumuhairwe, J.B.; Staver, C.; Berg, G. Banana-Associated Microbial Communities in Uganda Are Highly Diverse but Dominated by Enterobacteriaceae. Appl. Environ. Microbiol. 2012, 78, 4933–4941. [Google Scholar] [CrossRef]
- Clavel, M.; Barraud, O.; Moucadel, V.; Meynier, F.; Karam, E.; Ploy, M.-C.; François, B. VALIBI study group Molecular Quantification of Bacteria from Respiratory Samples in Patients with Suspected Ventilator-Associated Pneumonia. Clin. Microbiol. Infect. 2016, 22, 812.e1–812.e7. [Google Scholar] [CrossRef]
- Castillo, M.; Martín-Orúe, S.M.; Manzanilla, E.G.; Badiola, I.; Martín, M.; Gasa, J. Quantification of Total Bacteria, Enterobacteria and Lactobacilli Populations in Pig Digesta by Real-Time PCR. Vet. Microbiol. 2006, 114, 165–170. [Google Scholar] [CrossRef]
- Hansen, R.; Russell, R.K.; Reiff, C.; Louis, P.; McIntosh, F.; Berry, S.H.; Mukhopadhya, I.; Bisset, W.M.; Barclay, A.R.; Bishop, J.; et al. Microbiota of De-Novo Pediatric IBD: Increased Faecalibacterium Prausnitzii and Reduced Bacterial Diversity in Crohn’s but Not in Ulcerative Colitis. Am. J. Gastroenterol. 2012, 107, 1913–1922. [Google Scholar] [CrossRef]
- Croswell, A.; Amir, E.; Teggatz, P.; Barman, M.; Salzman, N.H. Prolonged Impact of Antibiotics on Intestinal Microbial Ecology and Susceptibility to Enteric Salmonella Infection. Infect. Immun. 2009, 77, 2741–2753. [Google Scholar] [CrossRef]
- Binh, C.T.T.; Heuer, H.; Gomes, N.C.M.; Kaupenjohann, M.; Smalla, K. Similar Bacterial Community Structure and High Abundance of Sulfonamide Resistance Genes in Field-Scale Manures. In Manure: Management, Uses and Environmental Impacts; Dellaguardia, C.S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 141–166. [Google Scholar]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E.T. Characterization of Bacterial Communities in Feces from Healthy Elderly Volunteers and Hospitalized Elderly Patients by Using Real-Time PCR and Effects of Antibiotic Treatment on the Fecal Microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Handschur, M.; Pinar, G.; Gallist, B.; Lubitz, W.; Haslberger, A.G. Culture Free DGGE and Cloning Based Monitoring of Changes in Bacterial Communities of Salad Due to Processing. Food Chem. Toxicol. 2005, 43, 1595–1605. [Google Scholar] [CrossRef]
- Tuomisto, S.; Karhunen, P.J.; Pessi, T. Time-Dependent Post Mortem Changes in the Composition of Intestinal Bacteria Using Real-Time Quantitative PCR. Gut Pathog. 2013, 5, 35. [Google Scholar] [CrossRef]
- Nakano, S.; Kobayashi, T.; Funabiki, K.; Matsumura, A.; Nagao, Y.; Yamada, T. Development of a PCR Assay for Detection of Enterobacteriaceae in Foods. J. Food Prot. 2003, 66, 1798–1804. [Google Scholar] [CrossRef]
- Pélissier, M.-A.; Vasquez, N.; Balamurugan, R.; Pereira, E.; Dossou-Yovo, F.; Suau, A.; Pochart, P.; Magne, F. Metronidazole Effects on Microbiota and Mucus Layer Thickness in the Rat Gut. FEMS Microbiol. Ecol. 2010, 73, 601–610. [Google Scholar] [CrossRef][Green Version]
- Martinon, A.; Cronin, U.P.; Wilkinson, M.G. Comparison of In-House and Commercial Real-Time PCR Systems for the Detection of Enterobacteriaceae and Their Evaluation Within an Interlaboratory Study Using Infant Formula Samples. Food Anal. Methods 2011, 4, 485–496. [Google Scholar] [CrossRef]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-Based Phylogeny and Taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales Ord. Nov. Divided into the Families Enterobacteriaceae, Erwiniaceae Fam. Nov., Pectobacteriaceae Fam. Nov., Yersiniaceae Fam. Nov., Hafniaceae Fam. Nov., Morganellaceae Fam. Nov., and Budviciaceae Fam. Nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Chen, S.; Zhang, Z.; Song, J.; Long, Z.; Yu, Y.; Fang, H. Enterobacteriaceae Predominate in the Endophytic Microbiome and Contribute to the Resistome of Strawberry. Sci. Total Environ. 2020, 727, 138708. [Google Scholar] [CrossRef]
- Saenz-García, C.E.; Castañeda-Serrano, P.; Mercado Silva, E.M.; Alvarado, C.Z.; Nava, G.M. Insights into the Identification of the Specific Spoilage Organisms in Chicken Meat. Foods 2020, 9, 225. [Google Scholar] [CrossRef]
- Parte, A.C. LPSN—List of Prokaryotic Names with Standing in Nomenclature. Nucleic Acids Res. 2014, 42, D613–D616. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and Tools for High Throughput RRNA Analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Qian, P.-Y. Sensitivity and Correlation of Hypervariable Regions in 16S RRNA Genes in Phylogenetic Analysis. BMC Bioinform. 2016, 17, 135. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Marquina, D.; Andersson, A.F.; Ronquist, F. New Mitochondrial Primers for Metabarcoding of Insects, Designed and Evaluated Using in Silico Methods. Mol. Ecol. Resour. 2019, 19, 90–104. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Bahlinger, E.; Dorn-In, S.; Beindorf, P.-M.; Mang, S.; Kaltner, F.; Gottschalk, C.; Gareis, M.; Schwaiger, K. Development of Two Specific Multiplex QPCRs to Determine Amounts of Pseudomonas, Enterobacteriaceae, Brochothrix Thermosphacta and Staphylococcus in Meat and Heat-Treated Meat Products. Int. J. Food Microbiol. 2021, 337, 108932. [Google Scholar] [CrossRef] [PubMed]
- Moura, I.B.; Normington, C.; Ewin, D.; Clark, E.; Wilcox, M.H.; Buckley, A.M.; Chilton, C.H. Method Comparison for the Direct Enumeration of Bacterial Species Using a Chemostat Model of the Human Colon. BMC Microbiol. 2020, 20, 2. [Google Scholar] [CrossRef]
- Štšepetova, J.; Baranova, J.; Simm, J.; Parm, Ü.; Rööp, T.; Sokmann, S.; Korrovits, P.; Jaagura, M.; Rosenstein, K.; Salumets, A.; et al. The Complex Microbiome from Native Semen to Embryo Culture Environment in Human in Vitro Fertilization Procedure. Reprod. Biol. Endocrinol. 2020, 18, 3. [Google Scholar] [CrossRef]
- Nel Van Zyl, K.; Whitelaw, A.C.; Newton-Foot, M. The Effect of Storage Conditions on Microbial Communities in Stool. PLoS ONE 2020, 15, e0227486. [Google Scholar] [CrossRef]
- Poeker, S.A.; Lacroix, C.; de Wouters, T.; Spalinger, M.R.; Scharl, M.; Geirnaert, A. Stepwise Development of an in Vitro Continuous Fermentation Model for the Murine Caecal Microbiota. Front. Microbiol. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A Web Server for Clustering and Comparing Biological Sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Kalendar, R.; Lee, D.; Schulman, A.H. Java Web Tools for PCR, in Silico PCR, and Oligonucleotide Assembly and Analysis. Genomics 2011, 98, 137–144. [Google Scholar] [CrossRef] [PubMed]

| Primer Set | Original Name | Sequence (5′-3′) | Amplicon Size (bp) b | Position c | Reference |
|---|---|---|---|---|---|
| PS1 a | Forward | GGGGATAACYACTGGAAACGGTRGC | 236 | 144–379 | [15] |
| Reverse | GCATGGCTGCATCAGGSTTKC | ||||
| PS2 | Forward | CGTTACYCGCAGAAGAAGCA | 259 | 482–740 | [17] |
| Reverse | CTGAGCGTCAGTCTTYGTCC | ||||
| PS3 | Entero353-F | GCAGTGGGGAATATTGCA | 474 | 353–826 | This study |
| Entero809-R | AAGGGCACAACCTCCAA | ||||
| PS4 | F-ent | ATGGCTGTCGTCAGCTCGT | 363 | 1054–1416 | [18] |
| R-ent | CCTACTTCTTTTGCAACCCACTC | ||||
| PS5 | Entero-F234 | GATGWRCCCRKATGGGA | 1198 | 226–1423 | [19] |
| Entero-R1423 | AKCTAMCTRCTTCTTTTGCAA | ||||
| PS6 | EnterobactDmod2F | GACCTCGCGAGAGCA | 161 | 1259–1419 | [20] |
| Enter1432mod | CCTACTTCTTTTGCAACCCA | ||||
| PS7 | 515F | GTGCCAGCMGCCGCGGTAA | 312 | 514–825 | [21] |
| 826R | GCCTCAAGGGCACAACCTCCAAG | ||||
| PS8 | Eco1457F | CATTGACGTTACCCGCAGAAGAAGC | 170 | 476–645 | [22] |
| Eco1652R | CTCTACGAGACTCAAGCTTGC | ||||
| PS9 | fd2 | AGAGTTTGATCATGGCTCAG | 1485 | 7–1491 | [23] |
| rp1 | ACGGTTACCTTGTTACGACTT | ||||
| PS10 | Forward | GCGGTAGCACAGAGAGCTT | 49 | 65–113 | [24] |
| Reverse | GGCAGTTTCCCAGACATTACTCA | ||||
| PS11 | Forward 2 | CGTTACCGACAGAAGAAGCA | 259 | 482–740 | [17] |
| Reverse | CTGAGCGTCAGTCTTYGTCC | ||||
| PS12 | ENT-F | GTTGTAAAGCACTTTCAGTGGTGAGGAAGG | NF d | NF | [25] |
| ENT-R | GCCTCAAGGGCACAACCTCCAAG | ||||
| PS13 | DG74f | AGGAGGTGATCCAACCGCA | NF | NF | [23] |
| RW01r | AACTGGAGGAGGGTGGGGAT | ||||
| PS14 | Ent 1113 | TGGCAACAAAGGATAAGG | NF | NF | [26] |
| Ent 1418 | CTTTTGCAACCCACT | ||||
| PS15 | LUX—F | CGGTGTACCCGCAGAAGAAGCACG | NF | NF | [27] |
| LUX—R | GCTTGCACCCTCCGTATTACC | ||||
| PS16 | LUX—F | CGGTGTACCCGCAGAAGAAGCACG | NF | NF | [27] |
| ENT—R | GCCTCAAGGGCACAACCTCCAAG |
| Primer Set | Targeted Taxa (Coverage) a |
|---|---|
| PS1 | Enterobacterales (90%) |
| PS2 | Budviciaceae (67%) + Enterobacteriaceae (89%) + Erwiniaceae (67%) + Hafniaceae (50%) + Pectobacteriaceae (80%) + Yersiniaceae (71%) |
| PS3 | Enterobacteriaceae (58%) + Hafniaceae (100%) + Yersiniaceae (71%) |
| PS4 | Budviciaceae (67%) + Enterobacteriaceae (65%) + Hafniaceae (100%) + Morganellaceae (63%) + Pectobacteriaceae (100%) + Yersiniaceae (71%) |
| PS5 | Budviciaceae (67%) + Enterobacteriaceae (65%) + Hafniaceae (100%) + Pectobacteriaceae (80%) + Yersiniaceae (71%) |
| PS6 | Enterobacteriaceae (62%) |
| PS7 | Enterobacteriaceae (50%) + Hafniaceae (50%) + Yersiniaceae (57%) |
| PS8 | Escherichia/Shigella (89%) + Pseudescherichia (78%) |
| PS9 | Pragia (87%) + Salmonella (65%) + Chania (100%) + Ewingella (50%) |
| PS10 | Klebsiella (53%) + Lelliottia (71%) + Raoultella (55%) |
| PS11 | Morganella (89%) + Providencia (87%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resendiz-Nava, C.N.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Mercado-Silva, E.M.; Nava, G.M. A Comprehensive Evaluation of Enterobacteriaceae Primer Sets for Analysis of Host-Associated Microbiota. Pathogens 2022, 11, 17. https://doi.org/10.3390/pathogens11010017
Resendiz-Nava CN, Silva-Rojas HV, Rebollar-Alviter A, Rivera-Pastrana DM, Mercado-Silva EM, Nava GM. A Comprehensive Evaluation of Enterobacteriaceae Primer Sets for Analysis of Host-Associated Microbiota. Pathogens. 2022; 11(1):17. https://doi.org/10.3390/pathogens11010017
Chicago/Turabian StyleResendiz-Nava, Carolina N., Hilda V. Silva-Rojas, Angel Rebollar-Alviter, Dulce M. Rivera-Pastrana, Edmundo M. Mercado-Silva, and Gerardo M. Nava. 2022. "A Comprehensive Evaluation of Enterobacteriaceae Primer Sets for Analysis of Host-Associated Microbiota" Pathogens 11, no. 1: 17. https://doi.org/10.3390/pathogens11010017
APA StyleResendiz-Nava, C. N., Silva-Rojas, H. V., Rebollar-Alviter, A., Rivera-Pastrana, D. M., Mercado-Silva, E. M., & Nava, G. M. (2022). A Comprehensive Evaluation of Enterobacteriaceae Primer Sets for Analysis of Host-Associated Microbiota. Pathogens, 11(1), 17. https://doi.org/10.3390/pathogens11010017






