Source Identification of Klebsiella pneumoniae Causing Six Episodes of Recurrent Sepsis in an Adolescent That Underwent Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
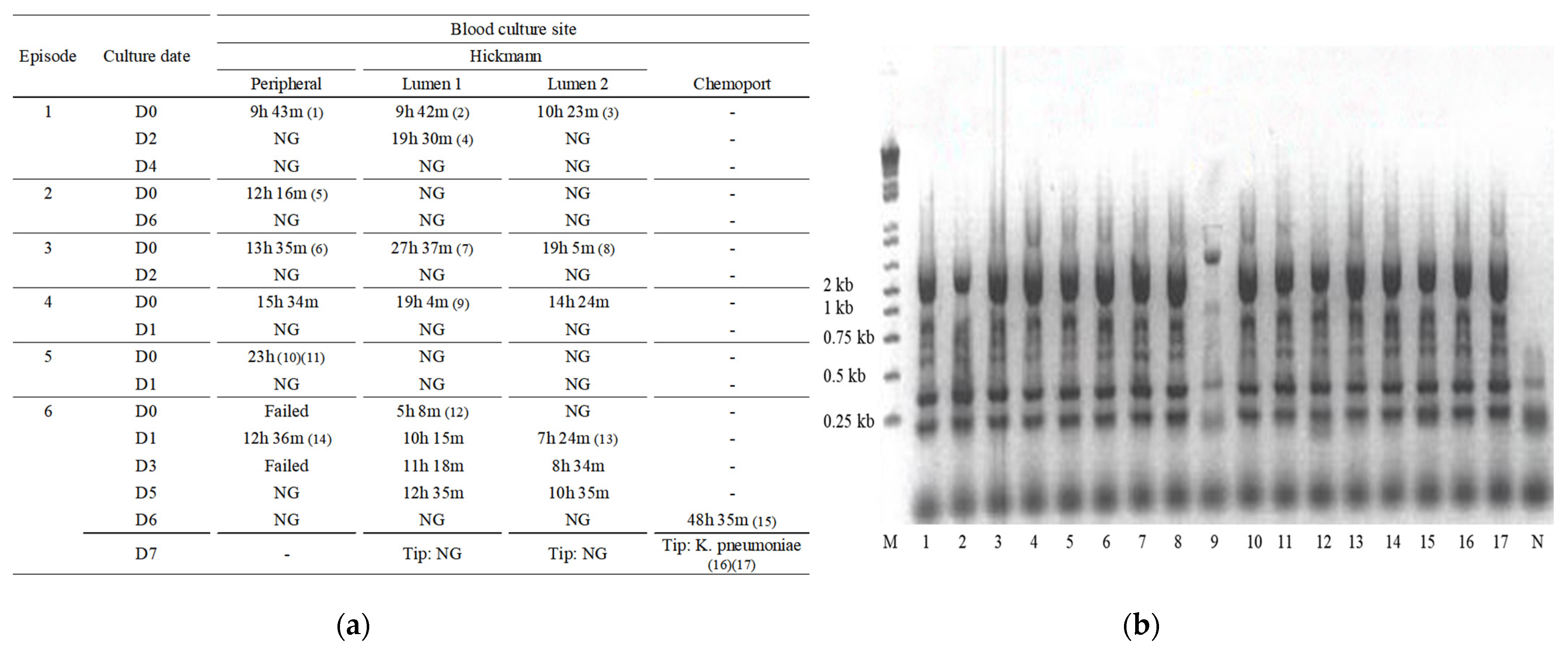
2. Case
3. Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moell, J.; Svenningsson, A.; Af Sandeberg, M.; Larsson, M.; Heyman, M.; Harila-Saari, A.; Nilsson, A. Early central line-associated blood stream infections in children with cancer pose a risk for premature catheter removal. Acta Paediatr. 2019, 108, 361–366. [Google Scholar] [CrossRef]
- Huerta, L.E.; Nelson, G.E.; Stewart, T.G.; Rice, T.W. Factors associated with recurrence and mortality in central line-associated bloodstream infections: A retrospective cohort study. Crit. Care 2018, 22, 266. [Google Scholar] [CrossRef] [PubMed]
- Woudt, S.H.S.; de Greeff, S.C.; Schoffelen, A.F.; Vlek, A.L.M.; Bonten, M.J.M.; Infectious Diseases Surveillance Information System–Antimicrobial Resistance (ISIS-AR) Study Group. Antibiotic Resistance and the Risk of Recurrent Bacteremia. Clin. Infect. Dis. 2017, 66, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.; Kong, V.Y.; Sartorius, B.; Liu, T.Y.; Liu, Y.Y.; Clarke, D.L. Mechanical complications of central venous catheterisation in trauma patients. Ann. R. Coll. Surg. Eng. 2017, 99, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.T.; Geschwind, J.F.; Savader, S.J.; Venbrux, A.C. Entrapment of J-tip guidewires by Venatech and stainless-steel Greenfield vena cava filters during central venous catheter placement: Percutaneous management in four patients. Cardiovasc Intervent. Radiol. 1998, 21, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.; Hanna, H.A.; Alakech, B.; Chatzinikolaou, I.; Johnson, M.M.; Tarrand, J. Differential time to positivity: A useful method for diagnosing catheter-related bloodstream infections. Ann. Intern. Med. 2004, 140, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fifth Informational Supplement [CLSI Document M100-S25]. Available online: https://file.qums.ac.ir/repository/mmrc/CLSI2015.pdf (accessed on 1 January 2015).
- Kim, J.; Lee, J.Y.; Kim, S.I.; Song, W.; Kim, J.S.; Jung, S.; Yu, J.K.; Park, K.G.; Park, Y.J. Rates of fecal transmission of extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacteriaceae among patients in intensive care units in Korea. Ann. Lab. Med. 2014, 34, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Maier, R.V.; Jimenez, M.; Dellinger, E.P. Source control in the management of severe sepsis and septic shock: An evidence-based review. Crit. Care Med. 2004, 32, S513–S526. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, W.J.F.M.; Herbers, A.H.E.; Netea, M.G.; Blijlevens, N.M.A. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: Introducing the paradigm febrile mucositis. Br. J. Haematol. 2014, 167, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Gustinetti, G.; Mikulska, M. Bloodstream infections in neutropenic cancer patients: A practical update. Virulence 2016, 7, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. C. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, E.A.H.; Knops, R.R.G.; Boerhof, J.; Feijen, E.; Merks, J.H.M.; Reedijk, A.M.J.; Lieverst, J.A.; Pieters, R.; Boezen, H.M.; Kremer, L.C.M.; et al. Treatment-related mortality in children with cancer: Prevalence and risk factors. Eur. J. Cancer 2019, 121, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Böll, B.; Schalk, E.; Buchheidt, D.; Hasenkamp, J.; Kiehl, M.; Kiderlen, T.R.; Kochanek, M.; Koldehoff, M.; Kostrewa, P.; Claßen, A.Y.; et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 239–259. [Google Scholar] [CrossRef] [PubMed]
| Episode No./Culture Site | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic Susceptibility | 1 | 2 | 3 | 4 | 5 | 6 | |||||||||
| P | H1 | H2 | P | P | H1 | H2 | P | H1 | H2 | P | P | H1 | H2 | Chemoport | |
| Amikacin | S | S | S | I | S | R | R | S | S | S | S | S | S | S | S |
| Amoxicilin-clavulanic acid | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Ampicillin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Aztreonam | R | R | R | R | R | S | S | R | R | R | R | I | S | S | R |
| Carbapenemase | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Cefazolin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cefepime | S | S | S | R | S | S | S | S | S | S | S | S | S | S | S |
| Cefotaxime | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cefoxitin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Ceftazidime | R | R | R | R | R | I | I | R | R | R | R | R | R | R | R |
| Ciprofloxacin | R | R | R | I | R | R | R | R | R | R | R | R | R | R | R |
| ESBL | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Ertapenem | R | R | R | R | R | S | S | I | S | S | I | S | S | S | S |
| Gentamicin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Imipenem | S | S | S | R | I | S | S | S | S | S | S | S | S | S | S |
| Meropenem | S | S | S | I | I | S | S | S | S | S | S | S | S | S | S |
| Piperacillin/tazobactam | I | I | I | R | R | S | S | R | I | I | R | I | I | I | R |
| Tigecycline | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Trimethoprim/sulfamethoxazole | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Episode No. | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| WBC Count (106/L) | 80 | 40 | 30 | 1720 | 1840 | 9150 |
| Hemoglobin (g/dL) | 7.9 | 9.2 | 8.2 | 9.2 | 8.7 | 12 |
| Platelet count (109/L) | 35 | 17 | 26 | 27 | 55 | 45 |
| ANC (106/L) | 40 | 0 | 0 | 880 | 1180 | 8690 |
| hs-CRP (mg/dL) | 4.97 | 4.86 | 15.15 | 4.22 | 0.12 | 6.25 |
| AST (mg/dL) | 130 | 32 | 21 | 53 | 53 | 60 |
| ALT (mg/dL) | 352 | 139 | 65 | 145 | 49 | 178 |
| Total Bilirubin (mg/dL) | 1.21 | 2.18 | 3.07 | 1.85 | 0.85 | 1.64 |
| Urea Nitrogen (mg/dL) | 33.6 | 18.7 | 11.4 | 3.2 | 7.1 | 15.9 |
| Creatinine (mg/dL) | 0.57 | 0.47 | 0.32 | 0.33 | 0.37 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Kang, H.M.; Kim, S.K.; Lee, J.W.; Chung, N.-G.; Cho, B.; Jeong, D.C.; Park, Y.-J. Source Identification of Klebsiella pneumoniae Causing Six Episodes of Recurrent Sepsis in an Adolescent That Underwent Hematopoietic Stem Cell Transplantation. Pathogens 2021, 10, 1123. https://doi.org/10.3390/pathogens10091123
Jo S, Kang HM, Kim SK, Lee JW, Chung N-G, Cho B, Jeong DC, Park Y-J. Source Identification of Klebsiella pneumoniae Causing Six Episodes of Recurrent Sepsis in an Adolescent That Underwent Hematopoietic Stem Cell Transplantation. Pathogens. 2021; 10(9):1123. https://doi.org/10.3390/pathogens10091123
Chicago/Turabian StyleJo, Suejung, Hyun Mi Kang, Seong Koo Kim, Jae Wook Lee, Nack-Gyun Chung, Bin Cho, Dae Chul Jeong, and Yeon-Joon Park. 2021. "Source Identification of Klebsiella pneumoniae Causing Six Episodes of Recurrent Sepsis in an Adolescent That Underwent Hematopoietic Stem Cell Transplantation" Pathogens 10, no. 9: 1123. https://doi.org/10.3390/pathogens10091123
APA StyleJo, S., Kang, H. M., Kim, S. K., Lee, J. W., Chung, N.-G., Cho, B., Jeong, D. C., & Park, Y.-J. (2021). Source Identification of Klebsiella pneumoniae Causing Six Episodes of Recurrent Sepsis in an Adolescent That Underwent Hematopoietic Stem Cell Transplantation. Pathogens, 10(9), 1123. https://doi.org/10.3390/pathogens10091123








