Characteristics of Chlamydia suis Ocular Infection in Pigs
Abstract
1. Introduction
2. Results
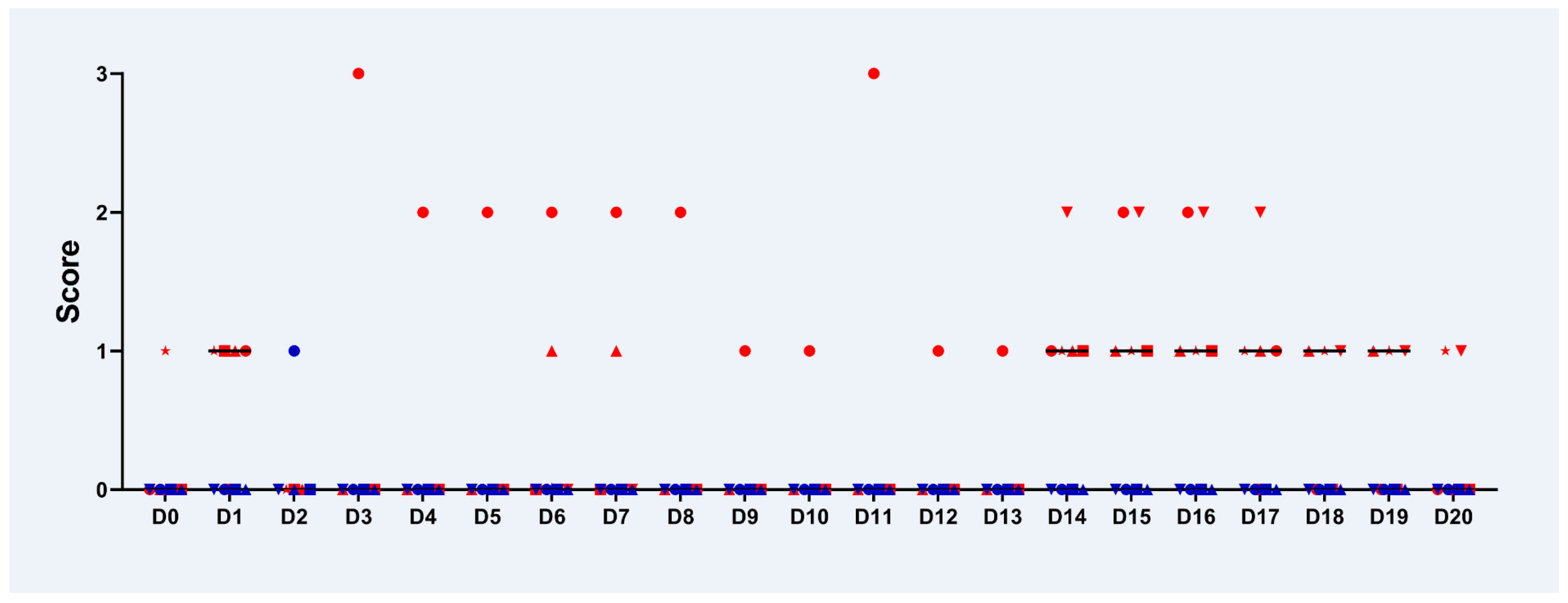
2.1. Clinical Examination
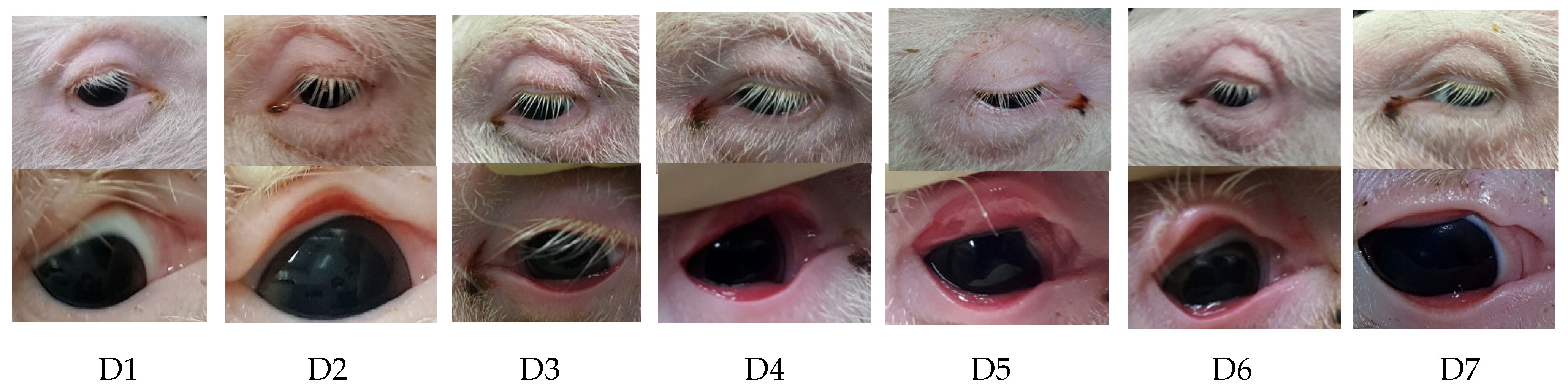
2.2. Examination of the Eyes
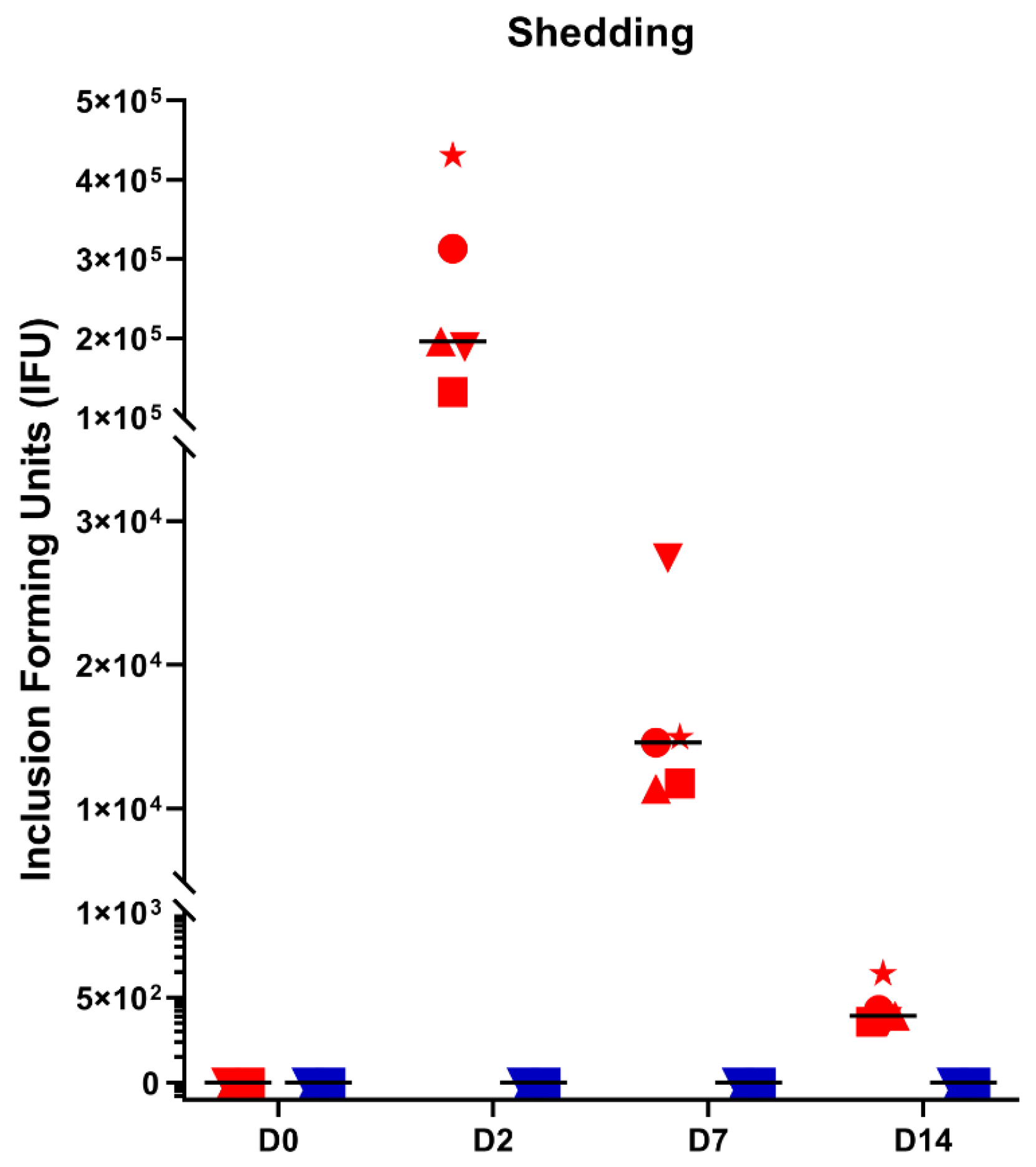
2.3. Ocular Shedding
2.4. Serology
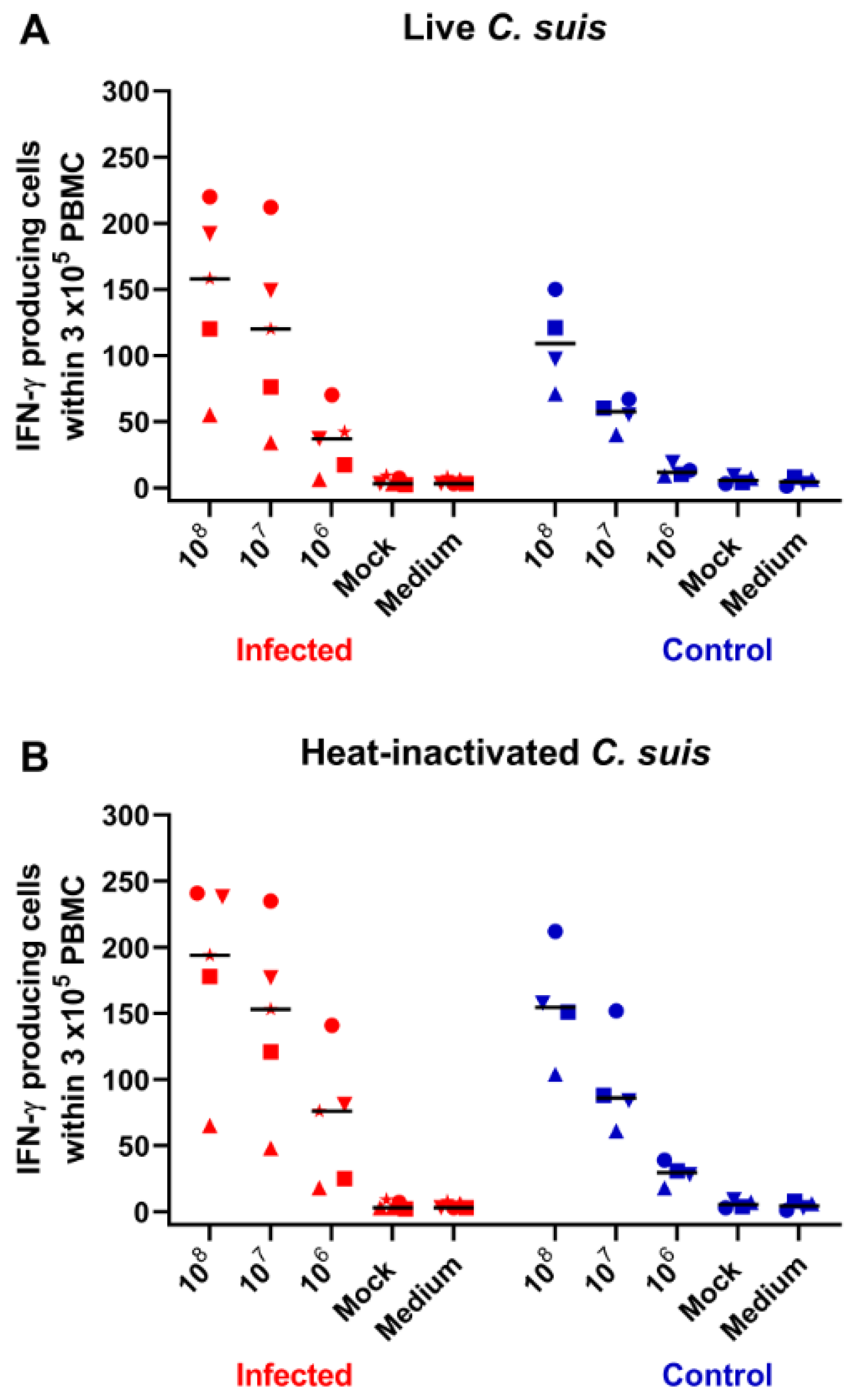
2.5. IFN-γ Production in Blood-Derived Lymphocytes
2.6. Pathomorphological Findings
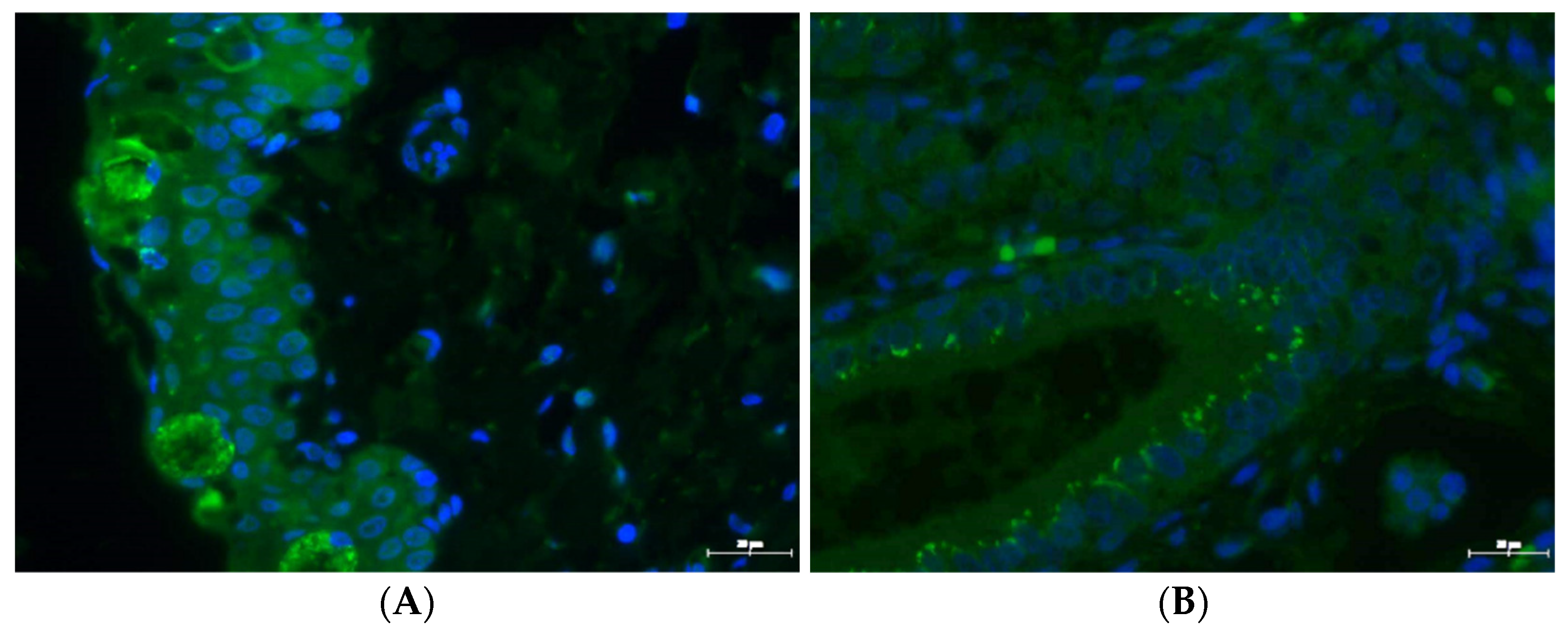
2.7. Immunochemistry
3. Discussion
4. Materials and Methods
4.1. C. suis
4.2. Infection Experiment
4.3. Determination of Chlamydial Inclusion Forming Units (IFUs) from Conjunctival Swabs
4.4. Immunohistochemistry
4.5. Antibody Responses
4.6. IFN-γ ELISpot from PBMC
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schautteet, K.; Vanrompay, D. Chlamydiaceae infections in pig. Vet. Res. 2011, 42, 29. [Google Scholar] [CrossRef]
- Borel, N.; Polkinghorne, A.; Pospischil, A. A Review on Chlamydial Diseases in Animals: Still a Challenge for Pathologists? Vet. Pathol. 2018, 55, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Rothschild, J.; Ruettger, A.; Kandel, R.P.; Sachse, K. Zoonotic Chlamydiaceae species associated with trachoma. Nepal. Emerg. Infect. Dis. 2013, 19, 1948. [Google Scholar] [CrossRef] [PubMed]
- De Puysseleyr, K.; de Puysseleyr, L.; Dhondt, H.; Geens, T.; Braeckman, L.; Morré, S.A.; Cox, E.; Vanrompay, D. Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect. Dis. 2014, 14, 560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Puysseleyr, L.; de Puysseleyr, K.; Braeckman, L.; Morré, S.A.; Cox, E.; Vanrompay, D. Assessment of Chlamydia suis infection in pig farmers. Transbound. Emerg. Dis. 2017, 64, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schott, F.; Donati, M.; Di Francesco, A.; Hässig, M.; Wanninger, S.; Sidler, X.; Borel, N. Prevalence of chlamydial infections in fattening pigs and their influencing factors. PLoS ONE 2015, 10, e0143576. [Google Scholar] [CrossRef]
- Rogers, D.G.; Andersen, A.A. Conjunctivitis caused by a swine Chlamydia trachomatis-like organism in gnotobiotic pigs. J. Vet. Diagn. Investig. 1999, 11, 341–344. [Google Scholar] [CrossRef]
- Becker, A.; Lutz-Wohlgroth, L.; Brugnera, E.; Lu, Z.H.; Zimmermann, D.R.; Grimm, F.; Grosse Beilage, E.; Kaps, S.; Spiess, B.; Pospischil, A.; et al. Intensively kept pigs pre-disposed to chlamydial associated conjunctivitis. J. Vet. Med. Ser. A 2007, 54, 307–313. [Google Scholar] [CrossRef]
- Reinhold, P.; Kirschvink, N.; Theegarten, D.; Berndt, A. An experimentally induced Chlamydia suis infection in pigs results in severe lung function disorders and pulmonary inflammation. Vet. Res. 2008, 39, 35. [Google Scholar] [CrossRef] [PubMed]
- Guscetti, F.; Schiller, I.; Sydler, T.; Heinen, E.; Pospischil, A. Experimental enteric infection of gnotobiotic piglets with Chlamydia suis strain S45. Vet. Microbiol. 2009, 135, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.G.; Andersen, A.A. Intestinal lesions caused by a strain of Chlamydia suis in weanling pigs infected at 21 days of age. J. Vet. Diagn. Investig. 2000, 12, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.V.S. A microbiological study of polyarthritis in slaughter pigs. J. S. Afr. Vet. Assoc. 1982, 53, 99–101. [Google Scholar]
- Thoma, R.; Guscetti, F.; Schiller, I.; Schmeer, N.; Corboz, L.; Pospischil, A. Chlamydiae in porcine abortion. Vet. Pathol. 1997, 34, 467–469. [Google Scholar] [CrossRef]
- Woollen, N.; Daniels, E.K.; Yeary, T.; Leipold, H.W.; Phillips, R.M. Chlamydial infection and perinatal mortality in a swine herd. J. Am. Vet. Med. Assoc. 1990, 197, 600–601. [Google Scholar] [PubMed]
- Kauffold, J.; Melzer, F.; Berndt, A.; Hoffmann, G.; Hotzel, H.; Sachse, K. Chlamydiae in oviducts and uteri of repeat breeder pigs. Theriogenology 2006, 66, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Camenisch, U.; Lu, Z.H.; Vaughan, L.; Corboz, L.; Zimmermann, D.R.; Wittenbrink, M.M.; Pospischil, A.; Sydler, T. Diagnostic investigation into the role of Chlamydiae in cases of increased rates of return to oestrus in pigs. Vet. Rec. 2004, 155, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Eggemann, G.; Wendt, M.; Hoelzle, L.E.; Jäger, C.; Weiss, R.; Failing, K. Zum Vorkommen von Chlamydien-Infektionen in Zuchtsauenbeständen und deren Bedeutung für das Fruchtbarkeitsgeschehen. DTW Dtsch. Tierarztl. Wochenschr. 2000, 107, 3–10. [Google Scholar] [PubMed]
- Szeredi, L.; Schiller, I.; Sydler, T.; Guscetti, F.; Heinen, E.; Corboz, L.; Eggenberger, E.; Jones, G.E.; Pospischil, A. Intestinal Chlamydia in finishing pigs. Vet. Pathol. 1996, 33, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Vanrompay, D.; Geens, T.; Desplanques, A.; Hoang, T.Q.T.; Vos, L.D.; van Loock, M.; Huyck, E.; Mirry, C.; Cox, E. Immunoblotting, ELISA and culture evidence for Chlamydiaceae in sows on 258 Belgian farms. Vet. Microbiol. 2004, 99, 59–66. [Google Scholar] [CrossRef]
- Di Francesco, A.; Baldelli, R.; Cevenini, R.; Magnino, S.; Pignanelli, S.; Salvatore, D.; Galuppi, R.; Donati, M. Seroprevalence to Chlamydiae in Pigs in Italy. Vet. Rec. 2006, 159, 849–850. [Google Scholar] [PubMed]
- Di Francesco, A.; Donati, M.; Morandi, F.; Renzi, M.; Masia, M.A.; Ostanello, F.; Salvatore, D.; Cevenini, R.; Baldelli, R. Seroepidemiologic survey for Chlamydia suis in wild boar (Sus scrofa) populations in Italy. J. Wildl. Dis. 2011, 47, 709–712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pospischil, A.; Wood, R.L. Intestinal Chlamydia in pigs. Vet. Pathol. 1987, 24, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jelocnik, M.; Yang, F.; Gong, J.; Kaltenboeck, B.; Polkinghorne, A.; Feng, Z.; Pannekoek, Y.; Borel, N.; Song, C.; et al. Asymptomatic infections with highly polymorphic Chlamydia suis are ubiquitous in pigs. BMC Vet. Res. 2017, 13, 370. [Google Scholar] [CrossRef]
- Hoque, M.M.; Adekanmbi, F.; Barua, S.; Rahman, K.S.; Aida, V.; Anderson, B.; Poudel, A.; Kalalah, A.; Bolds, S.; Madere, S.; et al. Peptide ELISA and FRET-qPCR Identified a Significantly Higher Prevalence of Chlamydia suis in Domestic Pigs Than in Feral Swine from the State of Alabama, USA. Pathogens 2020, 10, 11. [Google Scholar] [CrossRef]
- Reinhold, P.; Liebler-Tenorio, E.; Sattler, S.; Sachse, K. Recurrence of Chlamydia suis infection in pigs after short-term antimicrobial treatment. Vet. J. 2011, 187, 405–407. [Google Scholar] [CrossRef]
- Chahota, R.; Ogawa, H.; Ohya, K.; Yamaguchi, T.; Everett, K.D.E.; Fukushi, H. Involvement of multiple Chlamydia suis genotypes in porcine conjunctivitis. Transbound. Emerg. Dis. 2018, 65, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Englund, S.; af Segerstad, C.H.; Arnlund, F.; Westergren, E.; Jacobson, M. The occurrence of Chlamydia spp. in pigs with and without clinical disease. BMC Vet. Res. 2012, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Belij-Rammerstorfer, S.; Inic-Kanada, A.; Stojanovic, M.; Marinkovic, E.; Lukic, I.; Stein, E.; Montanaro, J.; Bintner, N.; Schürer, N.; Ghasemian, E. Infectious dose and repeated infections are key factors influencing immune response characteristics in guinea pig ocular chlamydial infection. Microbes Infect. 2016, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Seth-Smith, H.M.B.; Wanninger, S.; Bachmann, N.; Marti, H.; Qi, W.; Donati, M.; Di Francesco, A.; Polkinghorne, A.; Borel, N. The Chlamydia suis Genome Exhibits High Levels of Diversity, Plasticity, and Mobile Antibiotic Resistance: Comparative Genomics of a Recent Livestock Cohort Shows Influence of Treatment Regimes. Genome Biol. Evol. 2017, 9, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Kieckens, E.; van den Broeck, L.; van Gils, M.; Morré, S.; Vanrompay, D. Co-Occurrence of Chlamydia suis DNA and Chlamydia suis-Specific Antibodies in the Human Eye. Vector Borne Zoonotic Dis. 2018, 18, 677–682. [Google Scholar] [CrossRef]
- De Puysseleyr, K.; Kieckens, E.; De Puysseleyr, L.; Van den Wyngaert, H.; Ahmed, B.; Van Lent, S.; Creasy, H.H.; Myers, G.S.A.; Vanrompay, D. Development of a Chlamydia suis-specific antibody enzyme-linked immunosorbent assay based on the use of a B-cell epitope of the polymorphic membrane protein C. Transbound. Emerg. Dis. 2018, 65, e457–e469. [Google Scholar] [CrossRef] [PubMed]
- Coroneo, M.T. The eye as the discrete but defensible portal of coronavirus infection. Ocul. Surf. 2021, 19, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior eye segment drug delivery systems: Current treatments and future challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Hegemann, J.; Suetterlin, C. Chlamydia Biology: From Genome to Disease; Caister Academic Press: Norfolk, UK, 2020; ISBN 978-1-912530-28-1. [Google Scholar] [CrossRef]
- De Clercq, E.; Devriendt, B.; Yin, L.; Chiers, K.; Cox, E.; Vanrompay, D. The immune response against Chlamydia suis genital tract infection partially protects against re-infection. Vet. Res. 2014, 45, 95. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, H.; McSorley, S.J. Salmonella as a model for non-cognate Th1 cell stimulation. Front. Immunol. 2014, 5, 621. [Google Scholar] [CrossRef]
- Barral, R.; Desai, R.; Zheng, X.; Frazer, L.C.; Sucato, G.S.; Haggerty, C.L.; O’Connell, C.M.; Zurenski, M.A.; Darville, T. Frequency of Chlamydia trachomatis-specific T cell interferon-γ and interleukin-17 responses in CD4-enriched peripheral blood mononuclear cells of sexually active adolescent females. J. Reprod. Immunol. 2014, 103, 29–37. [Google Scholar] [CrossRef]
- Weygaerde, Y.V.; Versteele, C.; Thijs, E.; de Spiegeleer, A.; Boelens, J.; Vanrompay, D.; van Braeckel, E.; Vermaelen, K. An unusual presentation of a case of human psittacosis. Respir. Med. Case Rep. 2018, 23, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Koelbl, O. Untersuchungen über das Vorkommen von Miyagawanellen beim Schwein. Wien. Tierarztl. Mon. 1969, 56, 355–361. [Google Scholar]
- Käser, T.; Pasternak, J.A.; Delgado-Ortega, M.; Hamonic, G.; Lai, K.; Erickson, J.; Walker, S.; Dillon, J.R.; Gerdts, V.; Meurens, F. Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 T cells in pigs. Vaccine 2017, 35, 91–100. [Google Scholar] [CrossRef]
- Amaral, A.F.; Rahman, K.S.; Kick, A.R.; Cortes, L.M.; Robertson, J.; Kaltenboeck, B.; Gerdts, V.; O’Connell, C.M.; Poston, T.B.; Zheng, X.; et al. Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-γ+ Effector Memory Cells. Vaccines 2020, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Sachse, K.; Vretou, E.; Livingstone, M.; Borel, N.; Pospischil, A.; Longbottom, D. Recent developments in the laboratory diagnosis of chlamydial infections. Vet. Microbiol. 2009, 135, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Vanrompay, D.; Cox, E.; Mast, J.; Goddeeris, B.; Volckaert, G. High-level expression of Chlamydia psittaci major outer membrane protein in COS cells and in skeletal muscles of turkeys. Infect. Immun. 1998, 66, 5494–5500. [Google Scholar] [CrossRef] [PubMed]

| Animal Number | Conjunctiva | Glandula Lacrimalis | Lungs | Duodenum | Kidney | Testes |
|---|---|---|---|---|---|---|
| control 1 | − | − | − | − | − | − |
| control 2 | − | − | − | + | − | − |
| control 3 | (+) | (+) | − | + | − | − |
| control 4 | − | − | − | + | − | − |
| C. suis 1 | + | + | − | + | − | − |
| C. suis 2 | + | + | − | + | − | − |
| C. suis 3 | + | + | − | + | − | − |
| C. suis 4 | + | + | + | + | − | − |
| C. suis 5 | + | + | − | + | − | − |
| Animal Number | D-7 | D0 Infection | D2 | D7 | D14 | D21 Necropsy |
|---|---|---|---|---|---|---|
| control 1 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| control 2 | W, CS | |||||
| control 3 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| control 4 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| control 5 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| C. suis 1 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| C. suis 2 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| C. suis 3 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| C. suis 4 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
| C. suis 5 | W, CS | W, CS, S | W, CS, S | W, CS, S | W, CS, S, PBMC | W, CS, S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unterweger, C.; Inic-Kanada, A.; Setudeh, S.; Knecht, C.; Duerlinger, S.; Stas, M.; Vanrompay, D.; Kiekens, C.; Steinparzer, R.; Gerner, W.; et al. Characteristics of Chlamydia suis Ocular Infection in Pigs. Pathogens 2021, 10, 1103. https://doi.org/10.3390/pathogens10091103
Unterweger C, Inic-Kanada A, Setudeh S, Knecht C, Duerlinger S, Stas M, Vanrompay D, Kiekens C, Steinparzer R, Gerner W, et al. Characteristics of Chlamydia suis Ocular Infection in Pigs. Pathogens. 2021; 10(9):1103. https://doi.org/10.3390/pathogens10091103
Chicago/Turabian StyleUnterweger, Christine, Aleksandra Inic-Kanada, Sara Setudeh, Christian Knecht, Sophie Duerlinger, Melissa Stas, Daisy Vanrompay, Celien Kiekens, Romana Steinparzer, Wilhelm Gerner, and et al. 2021. "Characteristics of Chlamydia suis Ocular Infection in Pigs" Pathogens 10, no. 9: 1103. https://doi.org/10.3390/pathogens10091103
APA StyleUnterweger, C., Inic-Kanada, A., Setudeh, S., Knecht, C., Duerlinger, S., Stas, M., Vanrompay, D., Kiekens, C., Steinparzer, R., Gerner, W., Ladinig, A., & Barisani-Asenbauer, T. (2021). Characteristics of Chlamydia suis Ocular Infection in Pigs. Pathogens, 10(9), 1103. https://doi.org/10.3390/pathogens10091103







