Measurement of the IgG Avidity Index in the Diagnosis of Clinical Toxocariasis Patients
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Population Study
4.2. Serology and Avidity Measurements
4.3. Urea’s Effect on Toxocara Western Blot IgG
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magnaval, J.F.; Glickman, L.T.; Dorchies, P.; Morassin, B. Highlights of human toxocariasis. Korean J. Parasitol. 2001, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sowemimo, O.A.; Lee, Y.L.; Asaolu, S.O.; Chuang, T.W.; Akinwale, O.P.; Badejoko, B.O.; Gyang, V.P.; Nwafor, T.; Henry, E.; Fan, C.K. Seroepidemiological study and associated risk factors of Toxocara canis infection among preschool children in Osun State, Nigeria. Acta. Trop. 2017, 173, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.; Beltran, T.; Choi, Y.S. Prevalence of Toxocara species infection in the U.S.: Results from the National Health and Nutrition Examination Survey, 2011–2014. PLoS. Negl. Trop. Dis. 2017, 11, e0005818. [Google Scholar] [CrossRef] [PubMed]
- Kantarakia, C.; Tsoumani, M.E.; Galanos, A.; Mathioudakis, A.G.; Giannoulaki, E.; Beloukas, A.; Voyiatzaki, C. Comparison of the level of awareness about the transmission of echinococcosis and toxocariasis between pet owners and non-pet owners in Greece. Int. J. Environ. Res. Public Health. 2020, 17, 5292. [Google Scholar] [CrossRef] [PubMed]
- Keegan, J.D.; Holland, C.V. A comparison of Toxocara canis embryonation under controlled conditions in soil and hair. J. Helminthol. 2013, 87, 78–84. [Google Scholar] [CrossRef]
- Auer, H.; Walochnik, J. Toxocariasis and the clinical spectrum. Adv. Parasitol. 2020, 109, 111–130. [Google Scholar] [CrossRef]
- Villard, O.; Cimon, B.; L’Ollivier, C.; Fricker-Hidalgo, H.; Godineau, N.; Houze, S.; Paris, L.; Pelloux, H.; Villena, I.; Candolfi, E. Serological diagnosis of Toxoplasma gondii infection: Recommendations from the French National Reference Center for Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2016, 84, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, N.K.; Besselaar, T.G.; Schoub, B.D.; O’Connell, K.F. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 1991, 33, 6–9. [Google Scholar] [CrossRef]
- Gonzaga, H.T.; Ribeiro, V.S.; Feliciano, N.D.; Manhani, M.N.; Silva, D.A.O.; Ueta, M.T.; Costa-Cruz, J.M. IgG avidity in differential serodiagnosis of human strongyloidiasis active infection. Immunol. Lett. 2011, 139, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Luciani, L.; L’Ollivier, C.; Million, M.; Amphoux, B.; Edouard, S.; Raoult, D. Introduction to measurement of avidity of anti-Coxiella burnetii IgG in diagnosis of Q Fever. J. Clin. Microbiol. 2019, 57, e00539-19. [Google Scholar] [CrossRef] [Green Version]
- Baufine-Ducrocq, H.; Bourillet, B.; Quillet, P.; Couzineau, P. Effect of 2-mercaptoethanol on immunoglobulin M in toxoplasmosis and rubella. Ann. Biol. Clin. 1983, 41, 337–340. [Google Scholar]
- Smith, H.; Holland, C.; Taylor, M.; Magnaval, J.F.; Schantz, P.; Maizels, R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009, 25, 182–188. [Google Scholar] [CrossRef]
- Abu Raya, B.; Bamberger, E.; Almog, M.; Peri, R.; Srugo, I.; Kessel, A. Immunization of pregnant women against pertussis: The effect of timing on antibody avidity. Vaccine. 2015, 15, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Denoël, P.A.; Goldblatt, D.; de Vleeschauwer, I.; Jacquet, J.M.; Pichichero, M.E.; Poolman, J.T. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria-tetanus-acellular pertussis/Hib combination vaccines. Clin. Vacc. Immunol. 2007, 14, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Anttila, M.; Eskola, J.; Ahman, H.; Käyhty, H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999, 9, 1970–1977. [Google Scholar] [CrossRef]
- Collins, C.A.; Gelinas, L.; Yasukawa, L.L.; Audet, S.; Abu-Raya, B.; Turvey, S.E.; Beeler, J.A.; Kollmann, T.R.; Gans, H.A. Measles maternal antibodies with low avidity do not interfere with the establishment of robust quantity and quality antibody responses after the primary dose of Measles, Mumps, and Rubella vaccine administered at 12-Months of age. J. Pediatric Infect. Dis. Soc. 2020, 31, 752–755. [Google Scholar] [CrossRef]
- Rudzińska, M.; Kowalewska, B.; Sikorska, K. Clinical usefulness of Western blotting and ELISA avidity for the diagnosis of human toxocariasis. Parasite Immunol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Meroni, V.; Genco, F.; Tinelli, C.; Lanzarini, P.; Bollani, L.; Stronati, M.; Petersen, E. Spiramycin treatment of Toxoplasma gondii infection in pregnant women impairs the production and the avidity maturation of T. gondii-specific immunoglobulin G antibodies. Clin. Vaccine Immunol. 2009, 16, 1517–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, B.; Bhatti, G.; Thokar, M.A.; Malla, N. Human toxocariasis and ascariasis: Concomitant parasitism in Srinagar, Kashmir, India. Indian J. Pathol. Microbiol. 2002, 45, 315–318. [Google Scholar]
- Mendonça, L.R.; Figueiredo, C.A.; Esquivel, R.; Fiaccone, R.L.; Pontes-de-Carvalho, L.; Cooper, P.; Barreto, M.L.; Alcantara-Neves, N.M. Seroprevalence and risk factors for Toxocara infection in children from an urban large setting in Northeast Brazil. Acta. Trop. 2013, 128, 90–95. [Google Scholar] [CrossRef]
- Dziemian, E.; Zarnowska, H.; Kołodziej-Sobocińska, M.; Machnicka, B. Determination of the relative avidity of the specific IgG antibodies in human toxocariasis. Parasite Immunol. 2008, 30, 187–190. [Google Scholar] [CrossRef]
- Boldiš, V.; Ondriska, F.; Špitalská, E.; Reiterová, K. Immunodiagnostic approaches for the detection of human toxocarosis. Exp. Parasitol. 2015, 159, 252–258. [Google Scholar] [CrossRef]
- Mauracher, C.A.; Mitchell, L.A.; Tingle, A.J. Differential IgG avidity to rubella virus structural proteins. J. Med. Virol. 1992, 36, 202–208. [Google Scholar] [CrossRef] [PubMed]
- de Gouvêa Viana, L.; Rabello, A.; Katz, N. Antibody subclass profile and avidity during acute and chronic human Schistosoma mansoni infection. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 550–556. [Google Scholar] [CrossRef]
- Sterla, S.; Sato, H.; Nieto, A. Echinococcus granulosus human infection stimulates low avidity anticarbohydrate IgG2 and high avidity antipeptide IgG4 antibodies. Parasite Immunol. 1999, 21, 27–34. [Google Scholar] [CrossRef]
- Fricker-Hidalgo, H.; L’Ollivier, C.; Bosson, C.; Imbert, S.; Bailly, S.; Dard, C.; Piarroux, R.; Paris, L.; Pelloux, H. Interpretation of the Elecsys Toxo IgG avidity results for very low and very high index: Study on 741 sera with a determined date of toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Grangeot-Keros, L. Mesure de l’avidité des immunoglobulines G: Techniques, intérêt et limites. Biol. Méd. 2011, 90, 55-0066. [Google Scholar] [CrossRef]


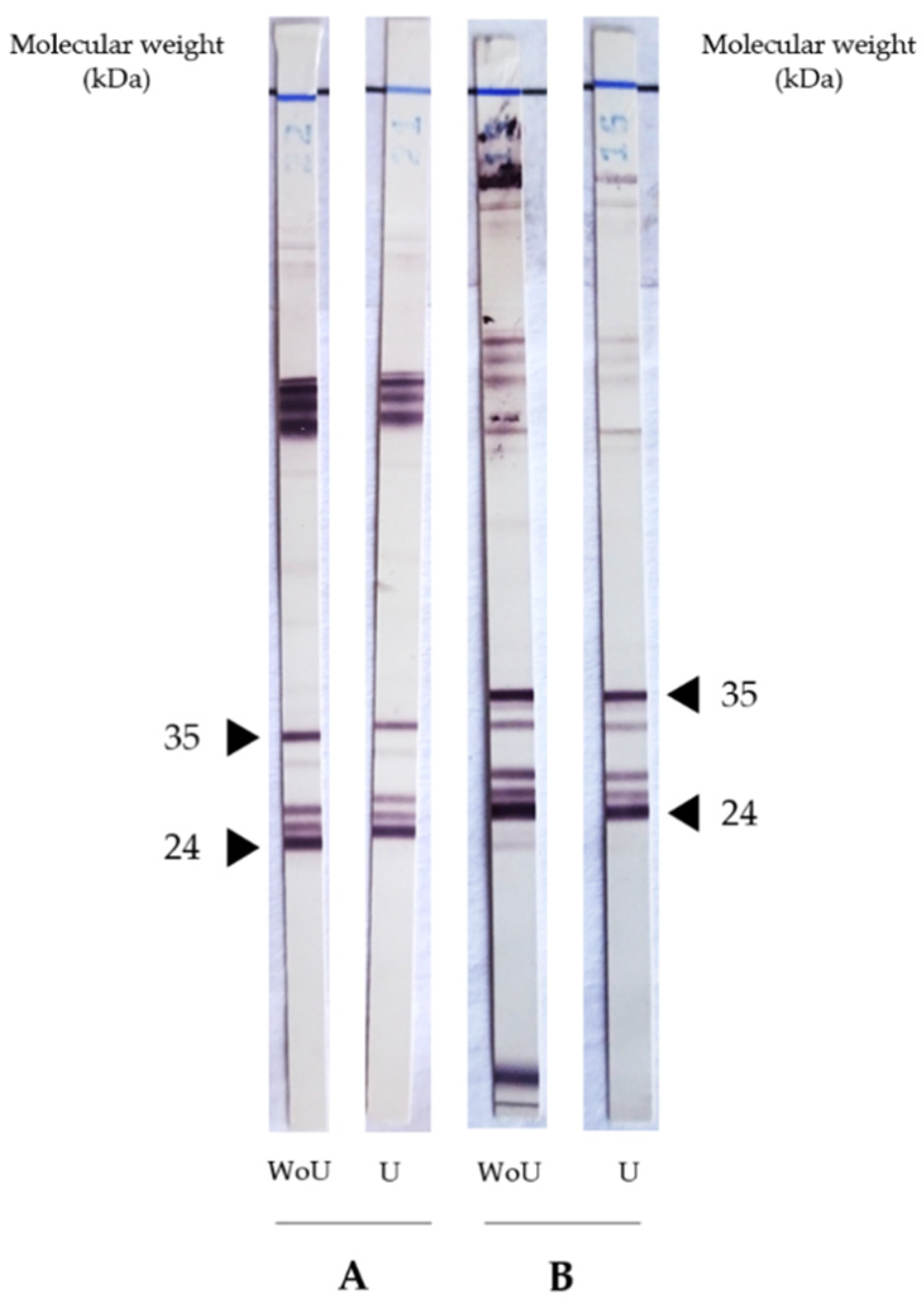
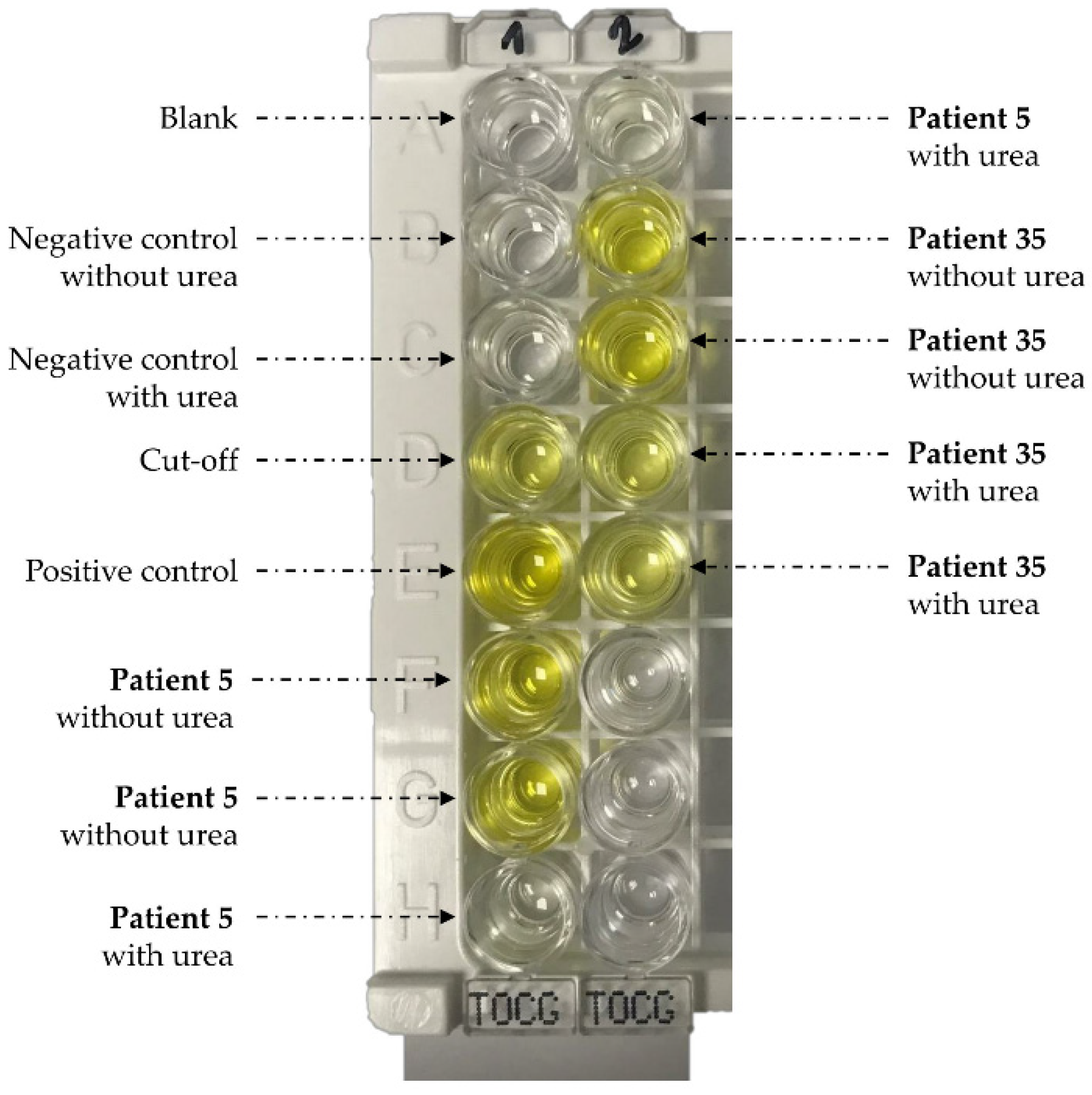
| Patient | Gender | Age | NovaLisa (NTU) 1 | Avidity Index (%) | Clinical Group | Eosinophilia | IgE (kUI/L) 2 | Clinical Presentation | Complementary Information | Treatment | Evolution of Symptoms | Final Diagnosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABZ | IVE | ||||||||||||
| 1 | M | 61 | 22.05 | 18.58 * | Active | N | - | Diffuse myalgia associated with chronic pruritus and abdominal pain | Y | Y | Favourable (except pruritus = pruritus sine materia) | Toxocariasis | |
| 2 | F | 19 | 19.57 | 20.97 * | Active | Y | - | Long-term abdominal pain associated with a major hyper eosinophilia | Y | Y | Favourable for eosinophilia | Toxocariasis | |
| 3 | M | 77 | 36.43 | 21.45 * | Active | Y | 918 | Itchy rash and pruritus | Previous serum negative (2 years before) | Y | Y | Favourable | Toxocariasis |
| 4 | M | 53 | 29.74 | 22.78 * | Active | N | - | Acute confusional and cerebellar syndrome evolving for 5 days | Presence of anti-Toxocara antibodies in the CSF | ND | N | Favourable | Toxocariasis |
| 5 | M | 56 | 35.37 | 22.79 * | Active | Y | 25.1 | Bipulmonary transplant patient—respiratory degradation | Previous serum negative (2 months before) | N | N | Stable | Toxocariasis |
| 6 | M | 75 | 31.42 | 26.75 * | Active | Y | 33,974 | Transient eosinophilia associated with persistent neuropathy | Y | N | Favourable for eosinophilia | Toxocariasis | |
| 7 | F | 76 | 29.47 | 28.59 * | Active | N | - | Itchy rash and pruritus | N | Y | Favourable | Toxocariasis | |
| 8 | F | 50 | 19.43 | 30.81 * | Active | N | - | Granulomatous anterior uveitis manifested by decreased visual acuity in the left eye for 3 weeks | ND | ND | ND | Toxocariasis | |
| 9 | M | 40 | 53.00 | 31.47 * | Active | Y | 10.9 | Eczematiform dermatosis with eosinophilia | N | N | Favourable | Toxocariasis | |
| 10 | F | 60 | 30.90 | 37.44 * | Active | Y | 591 | Long-term abdominal pain | Y | Y | Favourable | Toxocariasis | |
| 11 | M | 81 | 35.93 | 67.93 * | Active | N | - | Bilateral granulomatous panuveitis and meningitis manifested by sudden deafness and decreased visual acuity | Presence of anti-Toxocara antibodies in both CSF and AH | Y | N | Favourable | Neuromeningeal toxocariasis |
| 12 | M | 46 | 17.59 | 34.25 | Active | Y | - | Fever and episodes of urticaria and chills in a patient with psoriasis | Y | N | Persistence | Toxocariasis | |
| 13 | M | 65 | 39.18 | 41.68 | Active | Y | 14.7 | Eosinophilic pneumonia | Y | N | Favourable | Toxocariasis | |
| 14 | F | 28 | 34.69 | 33.73 | Active | Y | >1000 | Etiological assessment of eosinophilia—asthma exacerbation and scan images suggestive of larva migrans syndrome | Y | Y | Favourable | Toxocariasis | |
| 15 | M | 48 | 27.92 | 32.73 * | Chronic | N | - | Bilateral granulomatous panuveitis | N | N | Favourable | Neurosyphilis with ocular involvement | |
| 16 | F | 33 | 25.16 | 33.16* | Chronic | N | - | Persistent headache with recent onset of left hemiparesis | Absence of anti-Toxocara antibodies in the CSF | N | N | Persistence | Inflammatory encephalitis of undetermined etiology |
| 17 | F | 62 | 23.24 | 34.76 * | Chronic | N | - | Significant leptomeningitis associated with frontal ischemic stroke | N | N | Recurrent | Probable dysimmune meningoencephalitis | |
| 18 | F | 68 | 12.84 | 36.21 * | Chronic | N | - | Bilateral uveitis | N | N | Recurrent | Sarcoidosis | |
| 19 | M | 38 | 58.00 | 37.91 | Chronic | N | - | Chronic pruritus evolving over 3 years | N | N | Persistence | Trichophyton mentagrophytes dermatophytosis | |
| 20 | M | 77 | 25.17 | 39.78 | Chronic | N | - | Sub-diaphragmatic lymph nodes | N | N | Follow-up in haematology oncology | Low-grade B lymphoma | |
| 21 | F | 69 | 16.53 | 39.87 | Chronic | N | 89.3 | Chronic urticaria evolving over 4 years | Y | N | Persistence | Primary hyperparathyroidism | |
| 22 | M | 67 | 14.86 | 41.05 | Chronic | N | 151 | Pruritic papular eruptions evolving over 10 years | N | N | Persistence | Cutaneous mastocytosis | |
| 23 | M | 62 | 28.32 | 41.53 | Chronic | Y | - | Pruritic and erythematous rash of the face, skull and neck | Previous serum positive (2 years before) | N | N | Persistence | ND |
| 24 | F | 71 | 13.46 | 44.42 | Chronic | N | - | Chronic urticaria evolving over 1 year | Y | N | Persistence | Hypothesis of a drug-related cause | |
| 25 | F | 74 | 39.44 | 44.56 | Chronic | N | - | Chronic pruritus | N | Y | ND | Bullous pre-pemphigoid | |
| 26 | M | 70 | 14.14 | 46.23 | Chronic | N | - | Granulomatous uveitis of the left eye | N | N | Persistence | Neurolyme with ocular involvement (Borrelia burgdorferi) | |
| 27 | M | 73 | 12.68 | 47.94 | Chronic | N | - | Investigation of a pachymeningitis manifested by chronic headaches for more than 10 years and recent episode of visual haze | N | N | Favourable | Hypotension of the CSF in a patient with an implanted spinal cord neurostimulation system | |
| 28 | F | 66 | 36.97 | 50.68 | Chronic | N | 2577 | Assessment of exudative ascites associated with cirrhosis | N | N | Persistence | Refractory ascites in the context of child cirrhosis B9 | |
| 29 | F | 38 | 17.96 | 55.43 | Chronic | N | - | Pruritus sine materia evolving over 5 months | Y | N | ND | ND | |
| 30 | M | 61 | 13.10 | 57.67 | Chronic | Y | 7666 | Erythrodermic psoriasis with chronic eosinophilia | Y | N | Persistence | Eczema in a patient treated for psoriasis | |
| 31 | M | 76 | 21.26 | 58.65 | Chronic | N | - | Urticaria evolving over several months | Previous positive serum (1 year before) | N | N | Persistence | ND |
| 32 | F | 57 | 16.56 | 58.65 | Chronic | N | - | Epigastric pain revealing intra-abdominal adenomegaly | N | N | Favourable | Mesenteric panniculitis | |
| 33 | M | 85 | 77.59 | 59.45 | Chronic | Y | Erythematous skin lesions in bullous pemphigoid associated with eczema and dermatitis evolving for 2 years | Previous positive (serum 2 years before) | Y | N | Favourable | Diffuse eczematiform dermatosis | |
| 34 | F | 74 | 11.10 | 59.84 | Chronic | N | 28.9 | Etiological assessment of pruritus sine materia for 3 months (limbs) | N | N | Favourable | Dolichocolon | |
| 35 | M | 53 | 20.52 | 60.53 | Chronic | NR | - | Repetitive uveitis | Previous positive serum (24 months before) | Y | N | Persistence | Axial and peripheral spondyloarthropathy |
| 36 | M | 75 | 12.86 | 61.73 | Chronic | Y | - | Chronic dermatitis evolving over one month | N | N | Persistence | Vascularitis | |
| 37 | M | 56 | 12.24 | 62.70 | Chronic | N | - | Acute anterior uveitis | N | N | Favourable | Probable HSV uveitis | |
| 38 | M | 54 | 11.32 | 64.18 | Chronic | N | - | Exploration of neurological and psychiatric disorders | Absence of anti-Toxocara antibodies in the CSF | Y | N | Worsening | Dysimmune encephalitis |
| 39 | M | 64 | 39.33 | 64.64 | Chronic | N | - | Unilateral granulomatous uveitis | N | N | Favourable | No etiology found | |
| 40 | M | 81 | 136.36 | 67.39 | Chronic | N | - | Bulbar form of amyotrophic lateral sclerosis | N | N | Worsening | Degenerative motor neuron disease | |
| 41 | M | 80 | 19.63 | 70.13 | Chronic | N | - | Bilateral endophthalmitis after cataract surgery | N | N | Favourable | Improvement under antibiotics | |
| 42 | M | 63 | 78.33 | 71.38 | Chronic | Y | - | Asthenia and chronic eosinophilia | Y | Y | ND | ND | |
| 43 | M | 72 | 12.97 | 74.07 | Chronic | Y | 26.8 | Discovery of eosinophilia during a follow-up of Minkowski–Chauffard disease | Y | N | Follow-up in haematology–oncology | ND | |
| 44 | F | 74 | 35.70 | 87.80 | Chronic | Y | - | Chronic cough evolving over more than 40 years associated with eosinophilia | N | N | Follow-up in pneumology | Bronchial hyperreactivity with bronchial dilatation | |
| 45 | F | 71 | 20.30 | 23.71 | Chronic | N | - | Suspicion of encephalitis in a context of atypical fibromyalgia | Absence of anti-Toxocara antibodies in the CSF | N | N | Favourable | Unexplained encephalitis |
| 46 | M | 40 | 15.33 | 33.26 | Chronic | Y | 1765 | Etiological assessment of eosinophilia in an HIV patient | Y | N | Persistence | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menu, E.; Kopec, L.; Luciani, L.; Legrand, S.; L’Ollivier, C. Measurement of the IgG Avidity Index in the Diagnosis of Clinical Toxocariasis Patients. Pathogens 2021, 10, 1086. https://doi.org/10.3390/pathogens10091086
Menu E, Kopec L, Luciani L, Legrand S, L’Ollivier C. Measurement of the IgG Avidity Index in the Diagnosis of Clinical Toxocariasis Patients. Pathogens. 2021; 10(9):1086. https://doi.org/10.3390/pathogens10091086
Chicago/Turabian StyleMenu, Estelle, Lora Kopec, Léa Luciani, Sophie Legrand, and Coralie L’Ollivier. 2021. "Measurement of the IgG Avidity Index in the Diagnosis of Clinical Toxocariasis Patients" Pathogens 10, no. 9: 1086. https://doi.org/10.3390/pathogens10091086
APA StyleMenu, E., Kopec, L., Luciani, L., Legrand, S., & L’Ollivier, C. (2021). Measurement of the IgG Avidity Index in the Diagnosis of Clinical Toxocariasis Patients. Pathogens, 10(9), 1086. https://doi.org/10.3390/pathogens10091086







