Aelurostrongylus abstrusus Antibody Seroprevalence Reveals That Cats Are at Risk of Infection throughout Germany
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cat Sera
4.2. ELISA
4.3. Mapping, Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannelli, A.; Capelli, G.; Joachim, A.; Hinney, B.; Losson, B.; Kirkova, Z.; René-Martellet, M.; Papadopoulos, E.; Farkas, R.; Napoli, E.; et al. Lungworms and gastrointestinal parasites of domestic cats: A European perspective. Int. J. Parasitol. 2017, 47, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Crisi, P.E.; Aste, G.; Traversa, D.; Di Cesare, A.; Febo, E.; Vignoli, M.; Santori, D.; Luciani, A.; Boari, A. Single and mixed feline lungworm infections: Clinical, radiographic and therapeutic features of 26 cases (2013–2015). J. Feline Med. Surg. 2016, 19, 1017–1029. [Google Scholar] [CrossRef]
- Genchi, M.; Ferrari, N.; Fonti, P.; De Francesco, I.; Piazza, C.; Viglietti, A. Relation between Aelurostrongylus abstrusus larvae excretion, respiratory and radiographic signs in naturally infected cats. Vet. Parasitol. 2014, 206, 182–187. [Google Scholar] [CrossRef]
- Scott, D.W. Current knowledge of aelurostrongylosis in the cat. Literature review and case reports. Cornell Vet. 1973, 63, 483–500. [Google Scholar]
- Traversa, D.; Lia, R.P.; Iorio, R.; Boari, A.; Paradies, P.; Capelli, G.; Avolio, S.; Otranto, D. Diagnosis and risk factors of Aelurostrongylus abstrusus (Nematoda, Strongylida) infection in cats from Italy. Vet. Parasitol. 2008, 153, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J. Production of immunity in the cat against lungworm disease by administration of third-stage larvae. J. Comp. Pathol. 1969, 79, 161–165. [Google Scholar] [CrossRef]
- Schnyder, M.; Di Cesare, A.; Basso, W.; Guscetti, F.; Riond, B.; Glaus, T.M.; Crisi, P.; Deplazes, P. Clinical, laboratory and pathological findings in cats experimentally infected with Aelurostrongylus abstrusus. Parasitol. Res. 2014, 113, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Dennler, M.; Bass, D.A.; Gutierrez-Crespo, B.; Schnyder, M.; Guscetti, F.; Di Cesare, A.; Deplazes, P.; Kircher, P.; Glaus, T.M. Thoracic computed tomography, angiographic computed tomography, and pathology findings in six cats experimentally infected with Aelurostrongylus abstrusus. Vet. Radiol. Ultrasound 2013, 54, 459–469. [Google Scholar] [CrossRef]
- Hamilton, J. Experimental lungworm disease of the cat. J. Comp. Pathol. 1966, 76, 147–157. [Google Scholar] [CrossRef]
- Stockdale, P. The pathogenesis of the lesions elicited by Aelurostrongylus abstrusus during its prepatent period. Pathol. Vet. 1970, 7, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, J.A.; Slater, M.R.; Makolinski, K.V.; Looney, A.L.; Appel, L.D.; Martin, N.M.; McDonough, S.P. Post-mortem findings in 54 cases of anesthetic associated death in cats from two spay–neuter programs in New York State. J. Feline Med. Surg. 2011, 13, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.M. Studies on re-infestation of the cat with Aelurostrongylus abstrusus. J. Comp. Pathol. 1968, 78, 69–72. [Google Scholar] [CrossRef]
- Ribeiro, V.M.; Lima Dos Santos, W. Larval production of cats infected and re-infected with Aelurostrongylus abstrusus (Nematoda: Protostrongylidae). Rev. Méd. Vét. 2001, 152, 815–820. [Google Scholar]
- Schnyder, M.; Fahrion, A.; Ossent, P.; Kohler, L.; Webster, P.; Heine, J.; Deplazes, P. Larvicidal effect of imidacloprid/moxidectin spot-on solution in dogs experimentally inoculated with Angiostrongylus vasorum. Vet. Parasitol. 2009, 166, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.; Martin, P.; Braddock, J.; Malik, R. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995–2000). J. Feline Med. Surg. 2004, 6, 189–198. [Google Scholar] [CrossRef]
- Traversa, D.; Iorio, R.; Otranto, D. Diagnostic and Clinical implications of a nested PCR specific for ribosomal DNA of the feline lungworm Aelurostrongylus abstrusus (Nematoda, Strongylida). J. Clin. Microbiol. 2008, 46, 1811–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare, A.; Gueldner, E.K.; Traversa, D.; Veronesi, F.; Morelli, S.; Crisi, P.E.; Pampurini, F.; Strube, C.; Schnyder, M. Seroprevalence of antibodies against the cat lungworm Aelurostrongylus abstrusus in cats from endemic areas of Italy. Vet. Parasitol. 2019, 272, 13–16. [Google Scholar] [CrossRef]
- Gueldner, E.K.; Gilli, U.; Strube, C.; Schnyder, M. Seroprevalence, biogeographic distribution and risk factors for Aelurostrongylus abstrusus infections in Swiss cats. Vet. Parasitol. 2019, 266, 27–33. [Google Scholar] [CrossRef]
- Zottler, E.-M.; Strube, C.; Schnyder, M. Detection of specific antibodies in cats infected with the lung nematode Aelurostrongylus abstrusus. Vet. Parasitol. 2017, 235, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019, 193, 227–235. [Google Scholar] [CrossRef]
- Cavalera, M.A.; Schnyder, M.; Gueldner, E.K.; Furlanello, T.; Iatta, R.; Brianti, E.; Strube, C.; Colella, V.; Otranto, D. Serological survey and risk factors of Aelurostrongylus abstrusus infection among owned cats in Italy. Parasitol. Res. 2019, 118, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Schnyder, M.; Colombo, M.; Strube, C.; Dimzas, D.; Latino, R.; Traversa, D. Feline lungworms in Greece: Copromicroscopic, molecular and serological study. Parasitol. Res. 2020, 119, 2877–2883. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Endoparasites in dogs and cats in Germany 1999–2002. Parasitol. Res. 2003, 90, S148–S150. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Natural infections of Angiostrongylus vasorum and Crenosoma vulpis in dogs in Germany (2007–2009). Parasitol. Res. 2009, 105, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Pantchev, N.; Vrhovec, M.G.; Bauer, C.; Hermosilla, C. Lungworm infections (Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrongylus abstrusus) in dogs and cats in Germany and Denmark in 2003–2007. Vet. Parasitol. 2009, 159, 175–180. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Results of parasitological examinations of faecal samples from cats and dogs in Germany between 2003 and 2010. Parasitol. Res. 2011, 109, 45–60. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.-C.; Rohen, M.; Epe, C.; Schnieder, T. Prevalence of endoparasites in stray and fostered dogs and cats in Northern Germany. Parasitol. Res. 2012, 111, 849–857. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Occurrence and regional distribution of Aelurostrongylus abstrusus in cats in Germany. Parasitol. Res. 2012, 112, 855–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schug, K.; Kramer, F.; Schaper, R.; Hirzmann, J.; Failing, K.; Hermosilla, C.; Taubert, A. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in central Germany. Parasites Vectors 2018, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Steeb, S.; Hirzmann, J.; Eskens, U.; Volmer, K.; Bauer, C. Lungenwurm-Infektionen bei der europäischen Wildkatze. Kompakt Vet. 2014, 3, 9. [Google Scholar]
- Zottler, E.-M.; Bieri, M.; Basso, W.; Schnyder, M. Intestinal parasites and lungworms in stray, shelter and privately owned cats of Switzerland. Parasitol. Int. 2018, 69, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Raue, K.; Raue, J.; Hauck, D.; Söbbeler, F.; Morelli, S.; Traversa, D.; Schnyder, M.; Volk, H.; Strube, C. Do all roads lead to Rome? The potential of different approaches to diagnose Aelurostrongylus abstrusus infection in cats. Pathogens 2021, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.M.; McCaw, A.W. The output of first stage larvae by cats infested with Aelurostrongylus abstrusus. J. Helminthol. 1968, 42, 295–298. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Taubert, A.; Staubach, C.; Sauter-Louis, C.; Conraths, F.J.; Vrhovec, M.G.; Pantchev, N. GIS-supported epidemiological analysis on canine Angiostrongylus vasorum and Crenosoma vulpis infections in Germany. Parasites Vectors 2017, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Lange, M.; Penagos-Tabares, F.; Hirzmann, J.; Failing, K.; Schaper, R.; Van Bourgonie, Y.; Backeljau, T.; Hermosilla, C.; Taubert, A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis larvae in native slug populations in Germany. Vet. Parasitol. 2018, 254, 120–130. [Google Scholar] [CrossRef]
- Hamilton, J.M.; McCaw, A.W. An investigation into the longevity of first stage larvae of Aelurostrongylus abstrusus. J. Helminthol. 1967, 41, 313–320. [Google Scholar] [CrossRef]
- Di Cesare, A.; Crisi, P.E.; Bartolini, R.; Iorio, R.; Talone, T.; Filippi, L.; Traversa, D. Larval development of Angiostrongylus vasorum in the land snail Helix aspersa. Parasitol. Res. 2015, 114, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Jeżewski, W.; Buńkowska-Gawlik, K.; Hildebrand, J.; Perec-Matysiak, A.; Laskowski, Z. Intermediate and paratenic hosts in the life cycle of Aelurostrongylus abstrusus in natural environment. Vet. Parasitol. 2013, 198, 401–405. [Google Scholar] [CrossRef]
- Hobmaier, M.; Hobmaier, A. Intermediate hosts of Aelurostrongylus abstrusus of the cat. Exp. Biol. Med. 1935, 32, 1641–1647. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Schnyder, M.; Traversa, D.; Di Cesare, A.; Wright, I.; Lacher, D.W. Updates on feline aelurostrongylosis and research priorities for the next decade. Parasites Vectors 2016, 9, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, S.; Diakou, A.; Colombo, M.; Di Cesare, A.; Barlaam, A.; Dimzas, D.; Traversa, D. Cat respiratory nematodes: Current knowledge, novel data and warranted Studies on clinical features, treatment and control. Pathogens 2021, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Martin, P.; Allan, G.; Barrs, V.; Malik, R. Lower respiratory tract infections in cats: 21 cases (1995–2000). J. Feline Med. Surg. 2004, 6, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Bourdeau, P.; Chalvet-Monfray, K.; Cozma, V.; Farkas, R.; Guillot, J.; Halos, L.; Joachim, A.; Losson, B.; Miró, G.; et al. Parasites of domestic owned cats in Europe: Co-infestations and risk factors. Parasites Vectors 2014, 7, 291. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.P.; Skarbye, L.K.; Vinther, L.M.; Willesen, J.; Pipper, C.B.; Olsen, C.S.; Mejer, H. Occurrence and clinical significance of Aelurostrongylus abstrusus and other endoparasites in Danish cats. Vet. Parasitol. 2017, 234, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gueldner, E.K.; Schuppisser, C.; Borel, N.; Hilbe, M.; Schnyder, M. First case of a natural infection in a domestic cat (Felis catus) with the canid heart worm Angiostrongylus vasorum. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100342. [Google Scholar] [CrossRef] [PubMed]
- Varcasia, A.; Tamponi, C.; Brianti, E.; Cabras, P.A.; Boi, R.; Pipia, A.P.; Giannelli, A.; Otranto, D.; Scala, A. Angiostrongylus chabaudi Biocca, 1957: A new parasite for domestic cats? Parasites Vectors 2014, 7, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare, A.; Morelli, S.; Colombo, M.; Simonato, G.; Veronesi, F.; Marcer, F.; Diakou, A.; D’Angelosante, R.; Pantchev, N.; Psaralexi, E.; et al. Is Angiostrongylosis a Realistic Threat for Domestic Cats? Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, M.; Tanner, I.; Webster, P.; Barutzki, D.; Deplazes, P. An ELISA for sensitive and specific detection of circulating antigen of Angiostrongylus vasorum in serum samples of naturally and experimentally infected dogs. Vet. Parasitol. 2011, 179, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Schnyder, M.; Schaper, R.; Bilbrough, G.; Morgan, E.; Deplazes, P. Seroepidemiological survey for canine angiostrongylosis in dogs from Germany and the UK using combined detection of Angiostrongylus vasorum antigen and specific antibodies. Parasitology 2013, 140, 1442–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
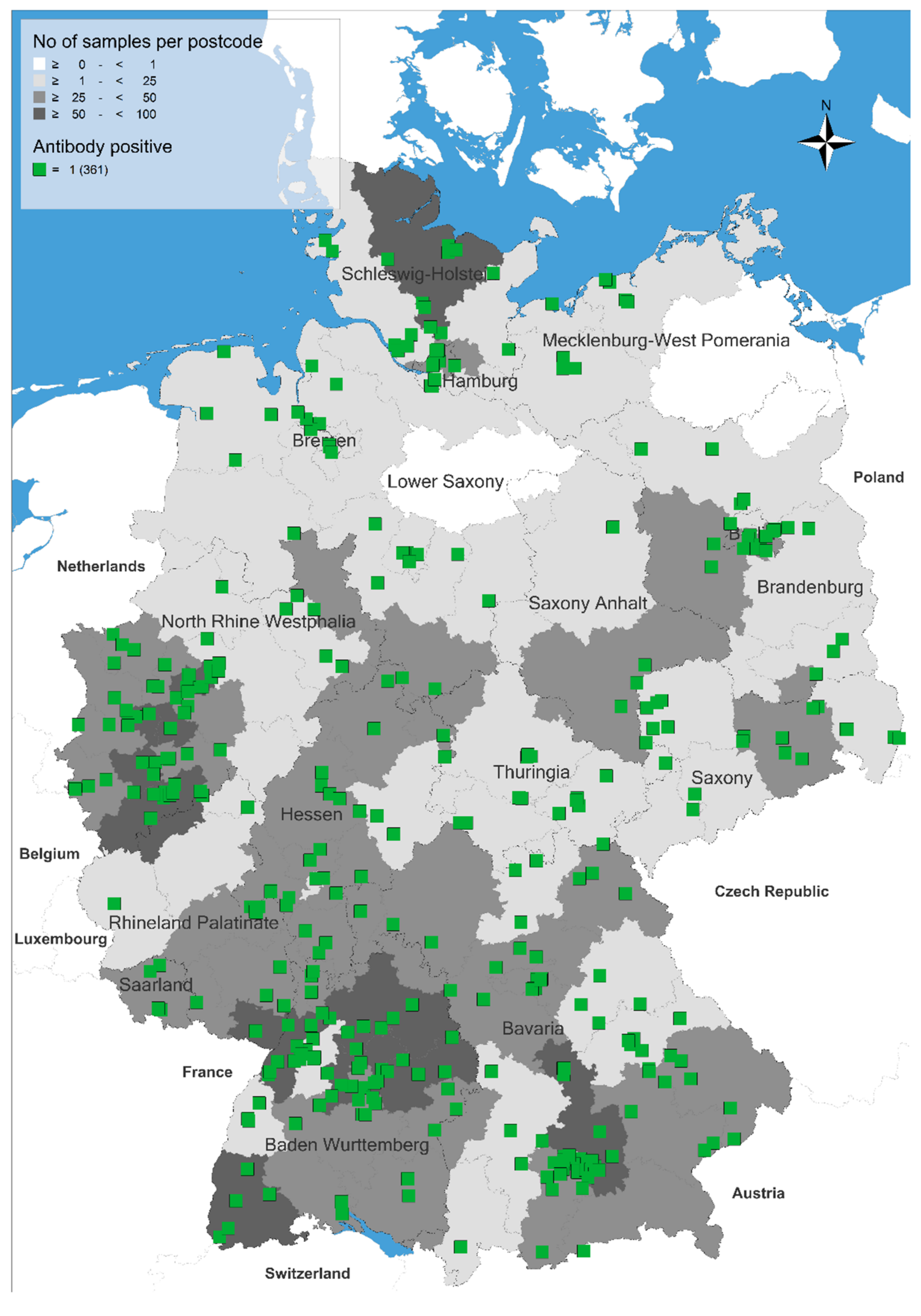
| Federal State of Germany | Number of Examined Sera (n) | Number of Positive Sera (n) | Seroprevalence and 95% Confidence Intervals (%, Range) |
|---|---|---|---|
| Baden-Württemberg | 546 | 64 | 11.7 (9.1–14.7) |
| Bavaria | 548 | 77 | 14.1 (11.3–17.2) |
| Berlin | 105 | 9 | 8.3 (4.0–15.6) |
| Brandenburg | 110 | 14 | 12.7 (7.1–20.4) |
| Bremen | 17 | 4 | 23.5 (6.8–49.9) |
| Hamburg | 78 | 8 | 10.3 (4.5–19.2) |
| Hesse | 252 | 23 | 9.1 (5.9–13.4) |
| Mecklenburg-West Pomerania | 25 | 8 | 32.0 (14.9–53.5) |
| Lower Saxony | 186 | 20 | 10.8 (6.7–16.1) |
| North-Rhine Westphalia | 587 | 61 | 10.4 (8.0–13.1) |
| Rhineland Palatinate | 133 | 12 | 9.0 (4.7–15.2) |
| Saarland | 37 | 5 | 13.5 (4.5–28.8) |
| Saxony | 138 | 19 | 13.8 (8.5–20.7) |
| Saxony-Anhalt | 62 | 4 | 6.5 (1.8–15.7) |
| Schleswig-Holstein | 122 | 19 | 15.6 (9.6–23.2) |
| Thuringia | 52 | 14 | 26.9 (15.6–41.0) |
| Total | 2998 | 361 | 12.0 (10.9–13.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnyder, M.; Schaper, R.; Gori, F.; Hafner, C.; Strube, C. Aelurostrongylus abstrusus Antibody Seroprevalence Reveals That Cats Are at Risk of Infection throughout Germany. Pathogens 2021, 10, 1011. https://doi.org/10.3390/pathogens10081011
Schnyder M, Schaper R, Gori F, Hafner C, Strube C. Aelurostrongylus abstrusus Antibody Seroprevalence Reveals That Cats Are at Risk of Infection throughout Germany. Pathogens. 2021; 10(8):1011. https://doi.org/10.3390/pathogens10081011
Chicago/Turabian StyleSchnyder, Manuela, Roland Schaper, Francesca Gori, Carola Hafner, and Christina Strube. 2021. "Aelurostrongylus abstrusus Antibody Seroprevalence Reveals That Cats Are at Risk of Infection throughout Germany" Pathogens 10, no. 8: 1011. https://doi.org/10.3390/pathogens10081011
APA StyleSchnyder, M., Schaper, R., Gori, F., Hafner, C., & Strube, C. (2021). Aelurostrongylus abstrusus Antibody Seroprevalence Reveals That Cats Are at Risk of Infection throughout Germany. Pathogens, 10(8), 1011. https://doi.org/10.3390/pathogens10081011









