Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis
Abstract
1. Introduction
2. Results
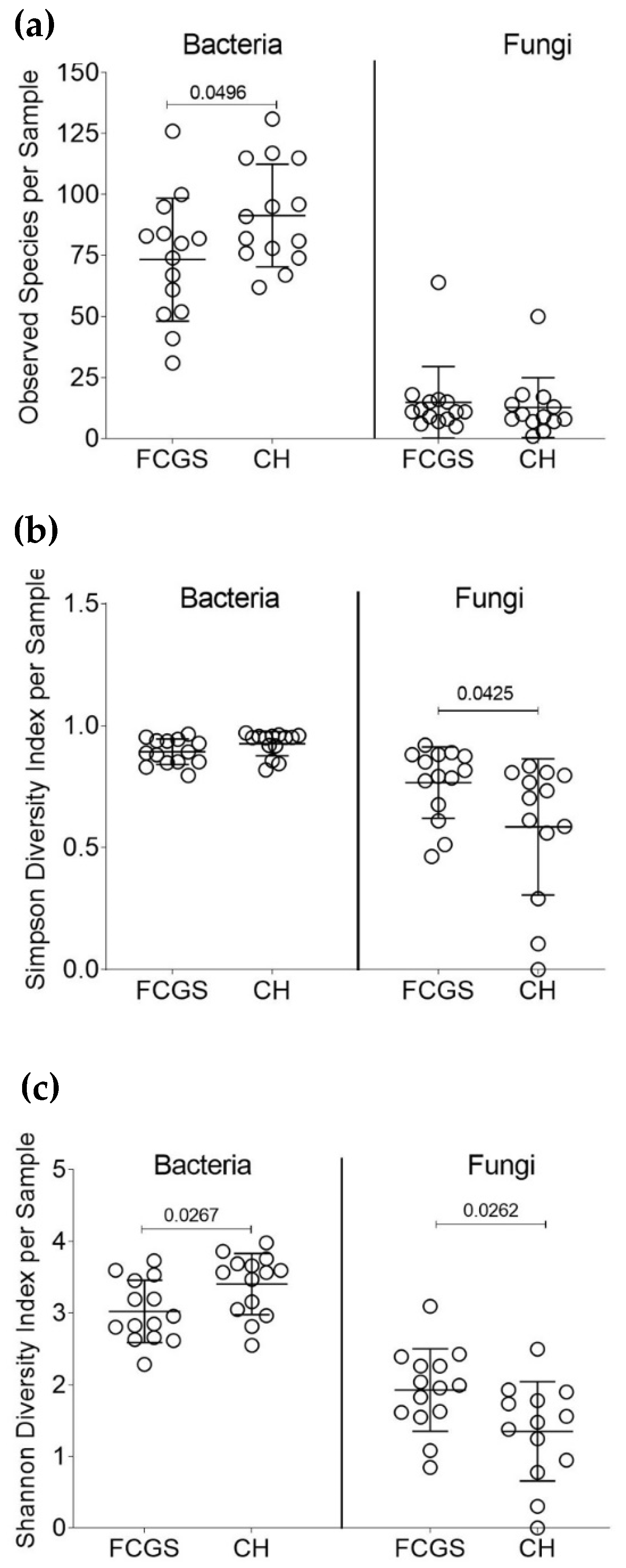
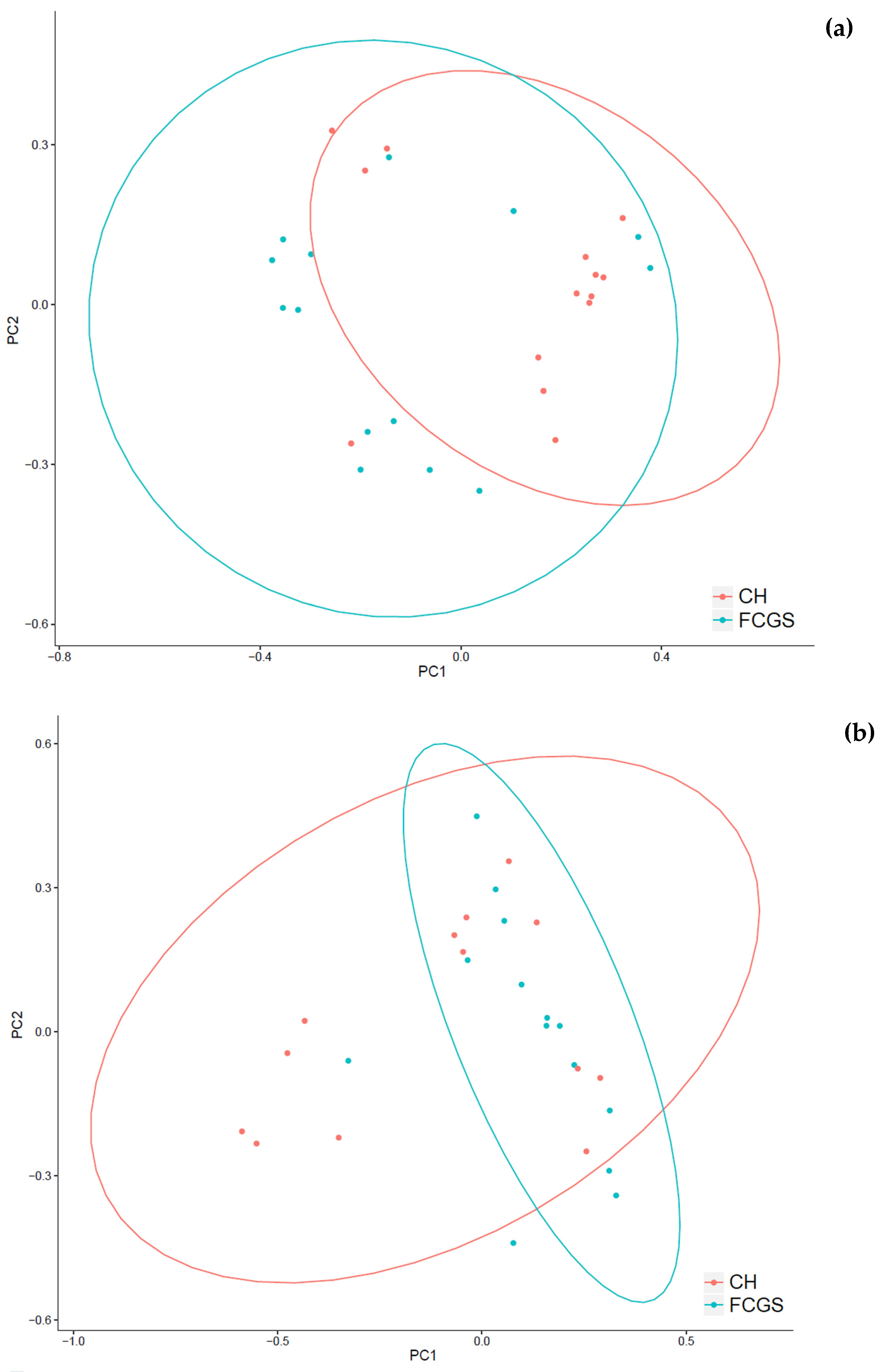
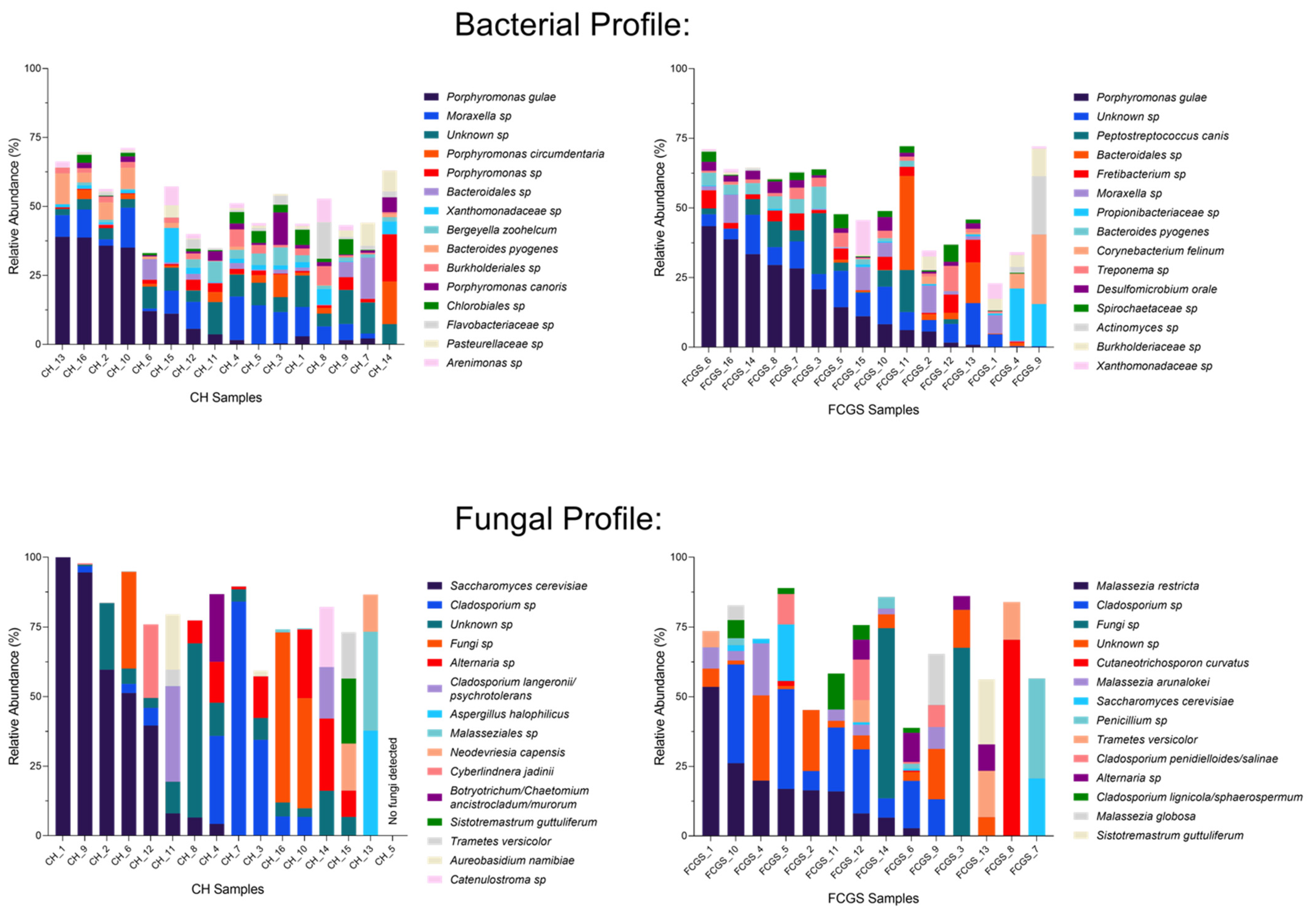
2.1. Analysis of the Bacterial Microbiota in CH and FCGS Cats
2.2. Analysis of the Fungal Microbiota (Mycobiota) in CH and FCGS Cats
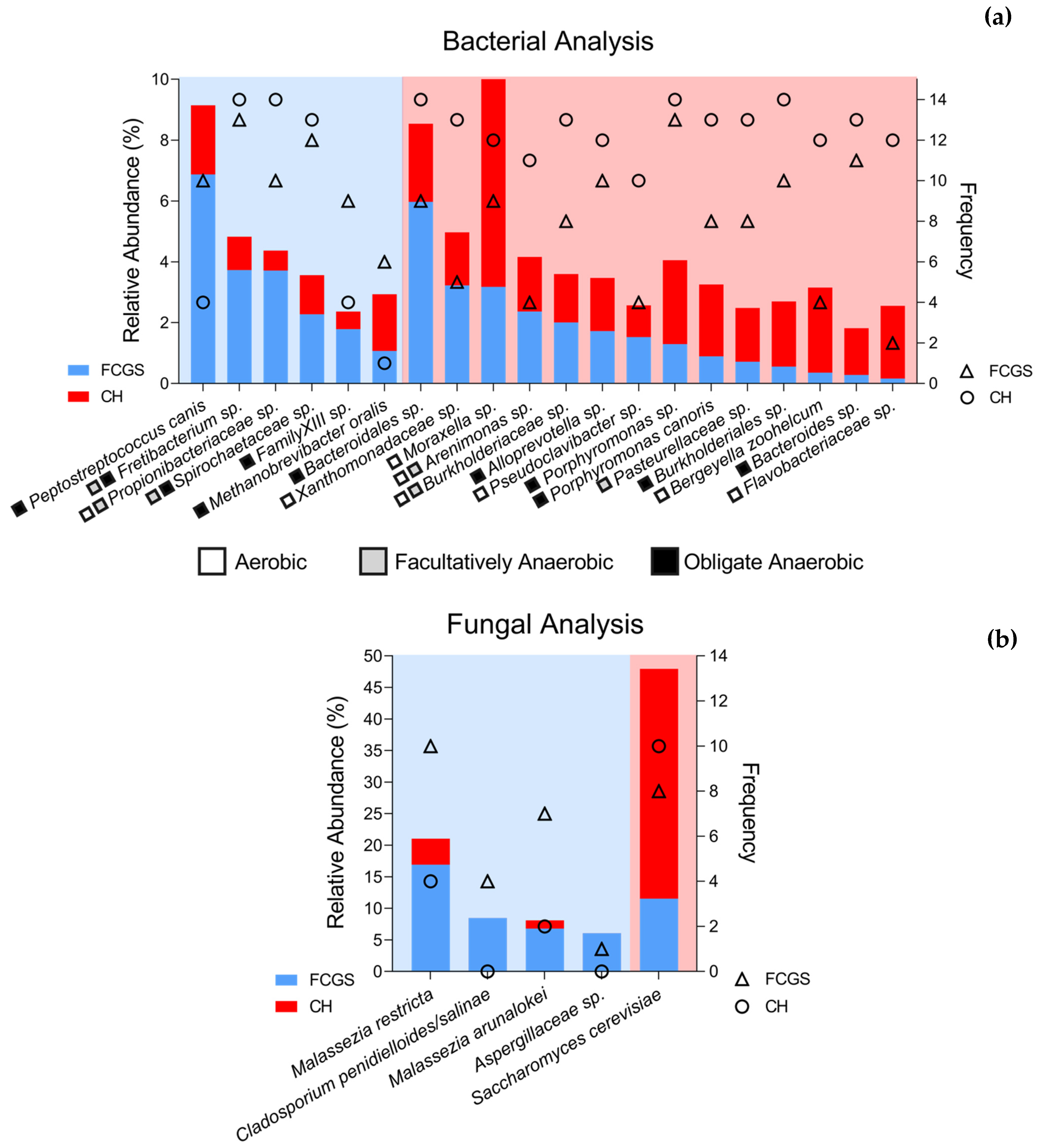
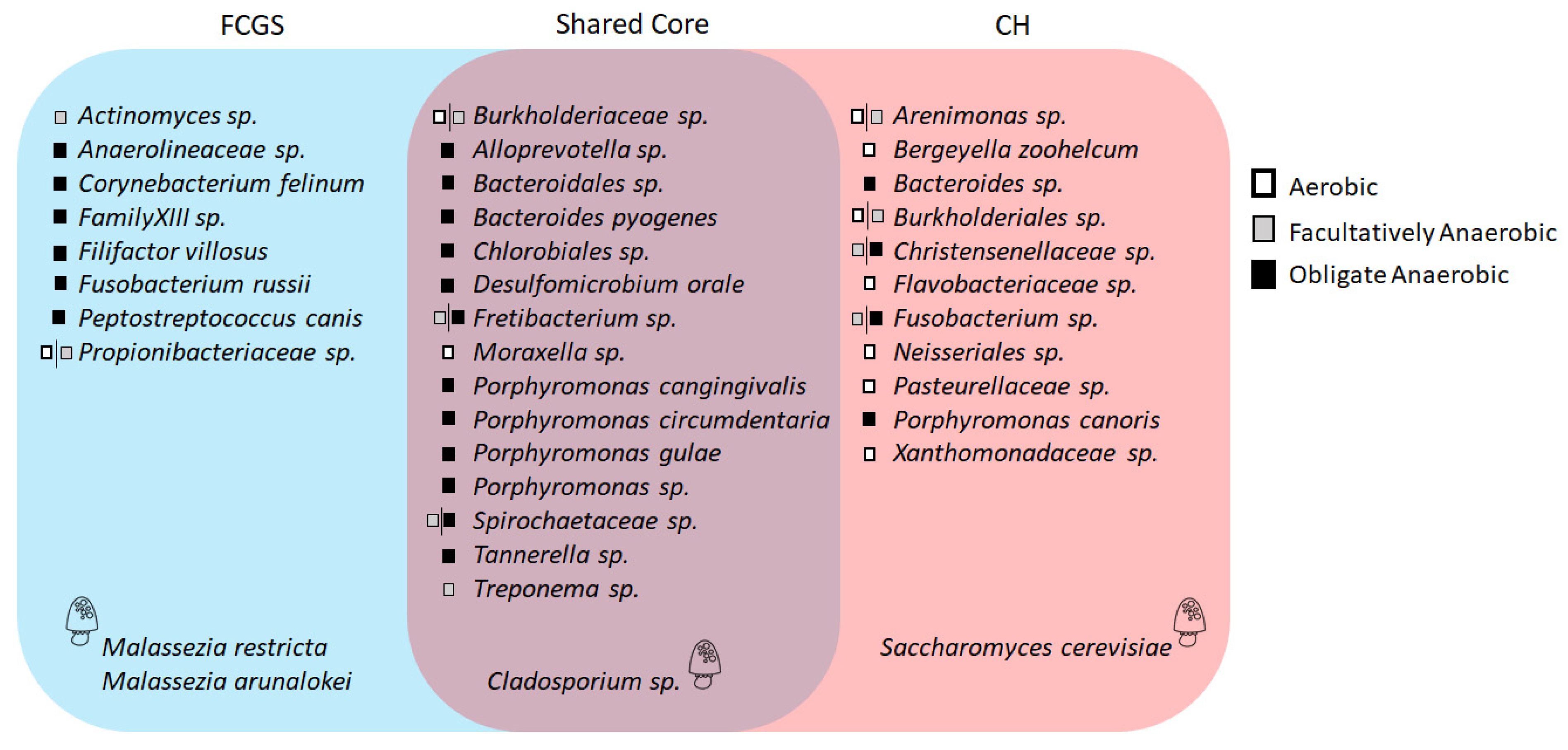
2.3. Microbiota Core Analysis
2.4. Co-occurrence Analysis
3. Discussion
4. Materials and Methods
4.1. Subjects Included and Study Design
4.2. Sample Collection, DNA Extraction, Library Preparation, and Sequencing
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bin Lee, D.; Verstraete, F.J.; Arzi, B. An Update on Feline Chronic Gingivostomatitis. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 973–982. [Google Scholar] [CrossRef]
- Popovici, C.P.; Fit, N.; Bartha, D.; Mircean, M.; Scurtu, I.; Codea, R.; Giurgiu, G.; Neagu, D. Clinical and Microbiological Aspects in Cats With Gingivostomatitis Complex. Agricultura 2017, 103, 133–139. [Google Scholar] [CrossRef]
- Peralta, S.; Carney, P.C. Feline chronic gingivostomatitis is more prevalent in shared households and its risk correlates with the number of cohabiting cats. J. Feline Med. Surg. 2019, 21, 1165–1171. [Google Scholar] [CrossRef]
- Love, D.N.; Vekselstein, R.; Collings, S. The obligate and facultatively anaerobic bacterial flora of the normal feline gingival margin. Vet. Microbiol. 1990, 22, 267–275. [Google Scholar] [CrossRef]
- Dolieslager, S.M.; Riggio, M.P.; Lennon, A.; Lappin, D.F.; Johnston, N.; Taylor, D.; Bennett, D. Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. Vet. Microbiol. 2011, 148, 93–98. [Google Scholar] [CrossRef]
- Barcina, I.; Ines, A. The viable but nonculturable phenotype: A crossroads in the life-cycle of non-differentiating bacteria? Rev. Environ. Sci. Biotechnol. 2009, 8, 245–255. [Google Scholar] [CrossRef]
- Adler, C.J.; Malik, R.; Browne, G.V.; Norris, J.M. Diet may influence the oral microbiome composition in cats. Microbiome 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Dorn, E.S.; Tress, B.; Suchodolski, J.S.; Nisar, T.; Ravindran, P.; Weber, K.; Hartmann, K.; Schulz, B.S. Bacterial microbiome in the nose of healthy cats and in cats with nasal disease. PLoS ONE 2017, 12, e0180299. [Google Scholar] [CrossRef]
- Older, C.E.; Diesel, A.B.; Lawhon, S.D.; Queiroz, C.R.R.; Henker, L.C.; Hoffmann, A.R. The feline cutaneous and oral microbiota are influenced by breed and environment. PLoS ONE 2019, 14, e0220463. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, A.; Pinder, S.L.; Costa, M.C.; Weese, J.S. Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet. J. 2014, 201, 223–229. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Klein, E.A.; Bennett, M.-L.; Croft, J.M.; Harris, S.J.; Marshall-Jones, Z.V. The feline oral microbiome: A provisional 16S rRNA gene based taxonomy with full-length reference sequences. Vet. Microbiol. 2015, 175, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Furuya, M.; Soma, T.; Hayashiuchi, Y.; Yoshiuchi, R.; Matsubayashi, M.; Tani, H.; Sasai, K. Prevalence of microorganisms associated with feline gingivostomatitis. J. Feline Med. Surg. 2018, 21, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Croft, J.; O’Flynn, C.; Deusch, O.; Colyer, A.; Allsopp, J.; Milella, L.; Davis, I.J. A Pyrosequencing Investigation of Differences in the Feline Subgingival Microbiota in Health, Gingivitis and Mild Periodontitis. PLoS ONE 2015, 10, e0136986. [Google Scholar] [CrossRef] [PubMed]
- Segovia, B.M.; Torras, M.Á.C. Communication of the Results of the Treatment with Probiotics in Two Cats with Chronic Gingivostomatitis. Open J. Vet. Med. 2018, 8, 9–14. [Google Scholar] [CrossRef][Green Version]
- Kouki, M.I.; Papadimitriou, S.A.; Psalla, D.; Kolokotronis, A.; Rallis, T.S. Chronic Gingivostomatitis with Esophagitis in Cats. J. Vet. Intern. Med. 2017, 31, 1673–1679. [Google Scholar] [CrossRef]
- Fausto, A.; Rodrigues, M.L.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Weese, S.J.; Nichols, J.; Jalali, M.; Litster, A. The oral and conjunctival microbiotas in cats with and without feline immunodeficiency virus infection. Vet. Res. 2015, 46, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, M.X.; Bicalho, R.C.; Fiani, N.; Lima, S.F.; Peralta, S. The subgingival microbial community of feline periodontitis and gingivostomatitis: Characterization and comparison between diseased and healthy cats. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Li, K.; Bihan, M.; Yooseph, S.; Methe, B.A. Analyses of the Microbial Diversity across the Human Microbiome. PLoS ONE 2012, 7, e32118. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Bosco, S.M.G.; Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56, S165–S187. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Johnson, T.J.; Mansell, J.M.; Suchodolski, J.S.; Hoffmann, A.R. Characterization of the cutaneous mycobiota in healthy and allergic cats using next generation sequencing. Vet. Dermatol. 2017, 28, 71-e17. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.A.; Mochel, J.P.; Du, Y.; Priestnall, S.L.; Moore, F.; Slayter, M.; Rodrigues, A.; Ackermann, M.; Krockenberger, M.; Mansell, J.; et al. Correlating Gastrointestinal Histopathologic Changes to Clinical Disease Activity in Dogs With Idiopathic Inflammatory Bowel Disease. Vet. Pathol. 2019, 56, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Older, C.E.; Gomes, M.O.S.; Hoffmann, A.R.; Policano, M.D.; Reis, C.A.C.D.; Carregaro, A.B.; Ambrósio, C.E.; Carregaro, V.M.L. Influence of the FIV status and chronic gingivitis on feline oral microbiota. Pathogens 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Lof, M.; Janus, M.M.; Krom, B.P. Metabolic interactions between bacteria and fungi in commensal oral biofilms. J. Fungi 2017, 3, 40. [Google Scholar] [CrossRef]
- Riggio, M.P.; Lennon, A.; Taylor, D.J.; Bennett, D. Molecular identification of bacteria associated with canine periodontal disease. Vet. Microbiol. 2011, 150, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.; Marshall, M.; Colyer, A.; O’Flynn, C.; Deusch, O.; Harris, S. A longitudinal assessment of changes in bacterial community composition associated with the development of periodontal disease in dogs. Vet. Microbiol. 2015, 181, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Özavci, V.; Erbas, G.; Parin, U.; Yüksel, H.T.; Kirkan, Ş. Molecular detection of feline and canine periodontal pathogens. Vet. Anim. Sci. 2019, 8, 100069. [Google Scholar] [CrossRef] [PubMed]
- Cunha, E.; Rebelo, S.; Carneiro, C.; Tavares, L.; Carreira, L.M.; Oliveira, M. A polymicrobial biofilm model for testing the antimicrobial potential of a nisin-biogel for canine periodontal disease control. BMC Vet. Res. 2020, 16, 469. [Google Scholar] [CrossRef]
- Bringuier, A.; Khelaifia, S.; Richet, H.; Aboudharam, G.; Drancourt, M. Real-time PCR quantification of methanobrevibacter oralis in periodontitis. J. Clin. Microbiol. 2013, 51, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Prete, S.D.; Vullo, D.; Ghobril, C.; Hitce, J.; Clavaud, C.; Marat, X.; Clemente, C.; Supuran, C.T. Cloning, purification, and characterization of a β-carbonic anhydrase from Malassezia restricta, an opportunistic pathogen involved in dandruff and seborrheic dermatitis. Int. J. Mol. Sci. 2019, 20, 2447. [Google Scholar] [CrossRef]
- Honnavar, P.; Prasad, G.S.; Ghosh, A.; Dogra, S.; Handa, S.; Rudramurthy, S.M. Malassezia arunalokei sp. nov., a novel yeast species isolated from seborrheic dermatitis patients and healthy individuals from India. J. Clin. Microbiol. 2016, 54, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.; Strausbaugh, L.D. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: Discovery of Malassezia as a prominent commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.M.; Hasnain, S.; Al-Frayh, A. Allergy and asthma: Prevalence and frequency of inhalant allergens in the middle-east. J. Dis. Glob. Health 2016, 27, 1–13. Available online: https://ikprress.org/index.php/JODAGH/article/view/2077 (accessed on 23 May 2021).
- Kano, R.; Itamoto, K.; Okuda, M.; Inokuma, H.; Hasegawa, A.; Balajee, S. Isolation of Aspergillus udagawae from a fatal case of feline orbital aspergillosis. Mycoses 2008, 51, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Takahashi, T.; Hayakawa, T.; Yamaya, Y.; Hasegawa, A.; Kamata, H. The first case of feline sinonasal aspergillosis due to Aspergillus fischeri in Japan. J. Vet. Med. Sci. 2015, 77, 1183–1185. [Google Scholar] [CrossRef]
- Costa, F.; Spanamberg, A.; Araujo, R.; Werner, J.; Ferreiro, L. Feline sino-orbital fungal infection caused by Aspergillus and Scopulariopsis. Acta Sci. Vet. Porto Alegre 2019, 47, 383. Available online: https://seer.ufrgs.br/ActaScientiaeVeterinariae/article/view/91581/52589 (accessed on 5 May 2021).
- Palma, M.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A., Jr.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: Is there room for improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef] [PubMed]
- Shareef, A.M.; Al-Dabbagh, A.S.A. Effect of probiotic (Saccharomyces cerevisiae) on performance of broiler chicks. Iraqi J. Vet. Sci. 2009, 23, 23–29. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.596.3152&rep=rep1&type=pdf (accessed on 10 May 2021).
- Ramasamy, S.; Barnard, E.; Dawson, T.L., Jr.; Li, H. The role of the skin microbiota in acne pathophysiology. Br. J. Dermatol. 2019, 181, 691–699. [Google Scholar] [CrossRef]
- Deepa, A.; Nair, B.; Sivakumar, T.; Joseph, A. Uncommon opportunistic fungal infections of oral cavity: A review. J. Oral Maxillofac. Pathol. 2014, 18, 235–243. [Google Scholar] [CrossRef]
- Kim, D.; Liu, Y.; Benhamou, R.I.; Sanchez, H.; Simón-Soro, Á.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.R.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Strausbaugh, L.D.; Dongari-Bagtzoglou, A. Fungal-bacterial interactions and their relevance to oral health: Linking the clinic and the bench. Front. Cell Infect. Microbiol. 2014, 4, 101. [Google Scholar] [CrossRef]
- Rolim, V.M.; Pavarini, S.P.; Campos, F.S.; Pignone, V.; Faraco, C.; Muccillo, M.D.; Roehe, P.M.; Costa, F.V.A.; Driemeier, D. Clinical, pathological, immunohistochemical and molecular characterization of feline chronic gingivostomatitis. J. Feline Med. Surg. 2017, 19, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Prem, A.; Tjokrosurjo, J.; Sary, M.; Bel, M.A.V.; Rodrigues-Hoffmann, A.; Kavanagh, M.; Wu, G.; Eden, M.E.; Krumbeck, J.A. The Canine Skin and Ear Microbiome: A Comprehensive Survey of Pathogens Implicated in Canine Skin and Ear Infections Using a Novel Next-Generation-Sequencing-Based Assay. Vet. Microbiol. 2020, 247, 108764. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumbeck, J.A.; Reiter, A.M.; Pohl, J.C.; Tang, S.; Kim, Y.J.; Linde, A.; Prem, A.; Melgarejo, T. Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens 2021, 10, 904. https://doi.org/10.3390/pathogens10070904
Krumbeck JA, Reiter AM, Pohl JC, Tang S, Kim YJ, Linde A, Prem A, Melgarejo T. Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens. 2021; 10(7):904. https://doi.org/10.3390/pathogens10070904
Chicago/Turabian StyleKrumbeck, Janina A., Alexander M. Reiter, James C. Pohl, Shuiquan Tang, Young J. Kim, Annika Linde, Aishani Prem, and Tonatiuh Melgarejo. 2021. "Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis" Pathogens 10, no. 7: 904. https://doi.org/10.3390/pathogens10070904
APA StyleKrumbeck, J. A., Reiter, A. M., Pohl, J. C., Tang, S., Kim, Y. J., Linde, A., Prem, A., & Melgarejo, T. (2021). Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens, 10(7), 904. https://doi.org/10.3390/pathogens10070904





