Clinical, Radiological and Functional Characteristics of Pulmonary Diseases among HTLV-1 Infected Patients without Prior Active Tuberculosis Infection
Abstract
:1. Introduction
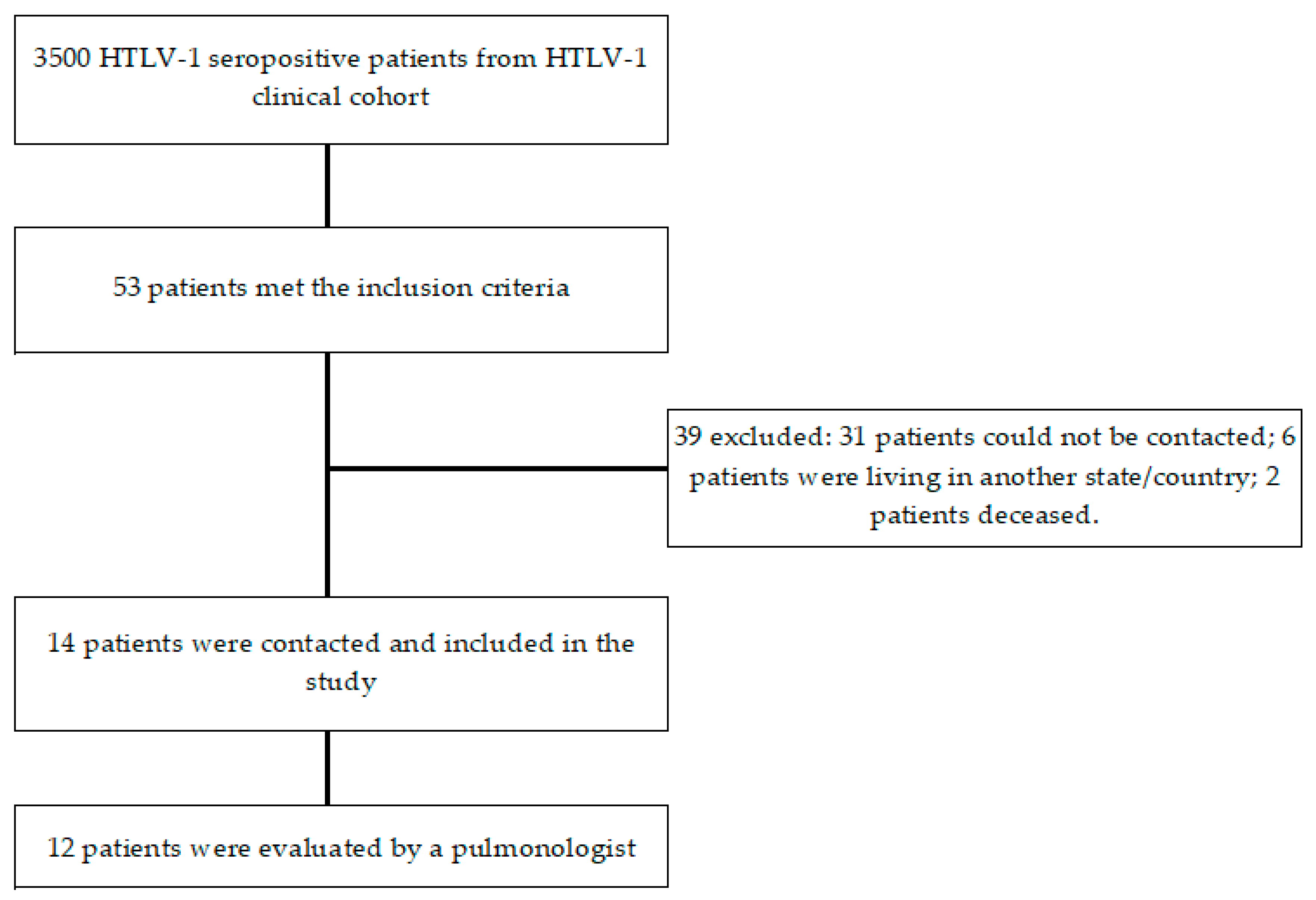
2. Results
3. Discussion
4. Methods
4.1. Study Design and Population
4.2. Procedures
4.3. Statistical Analyses
4.4. Ethical Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
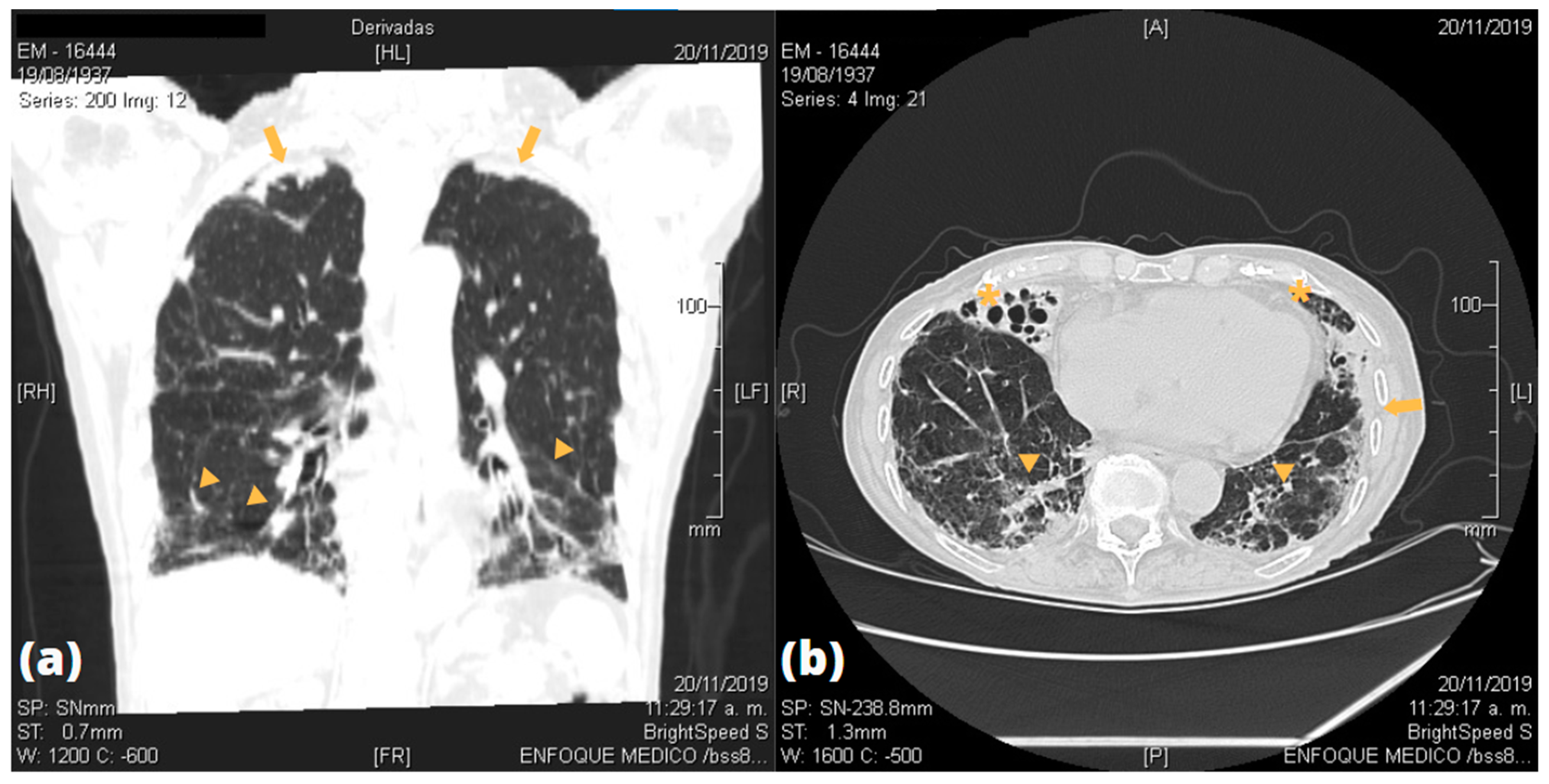
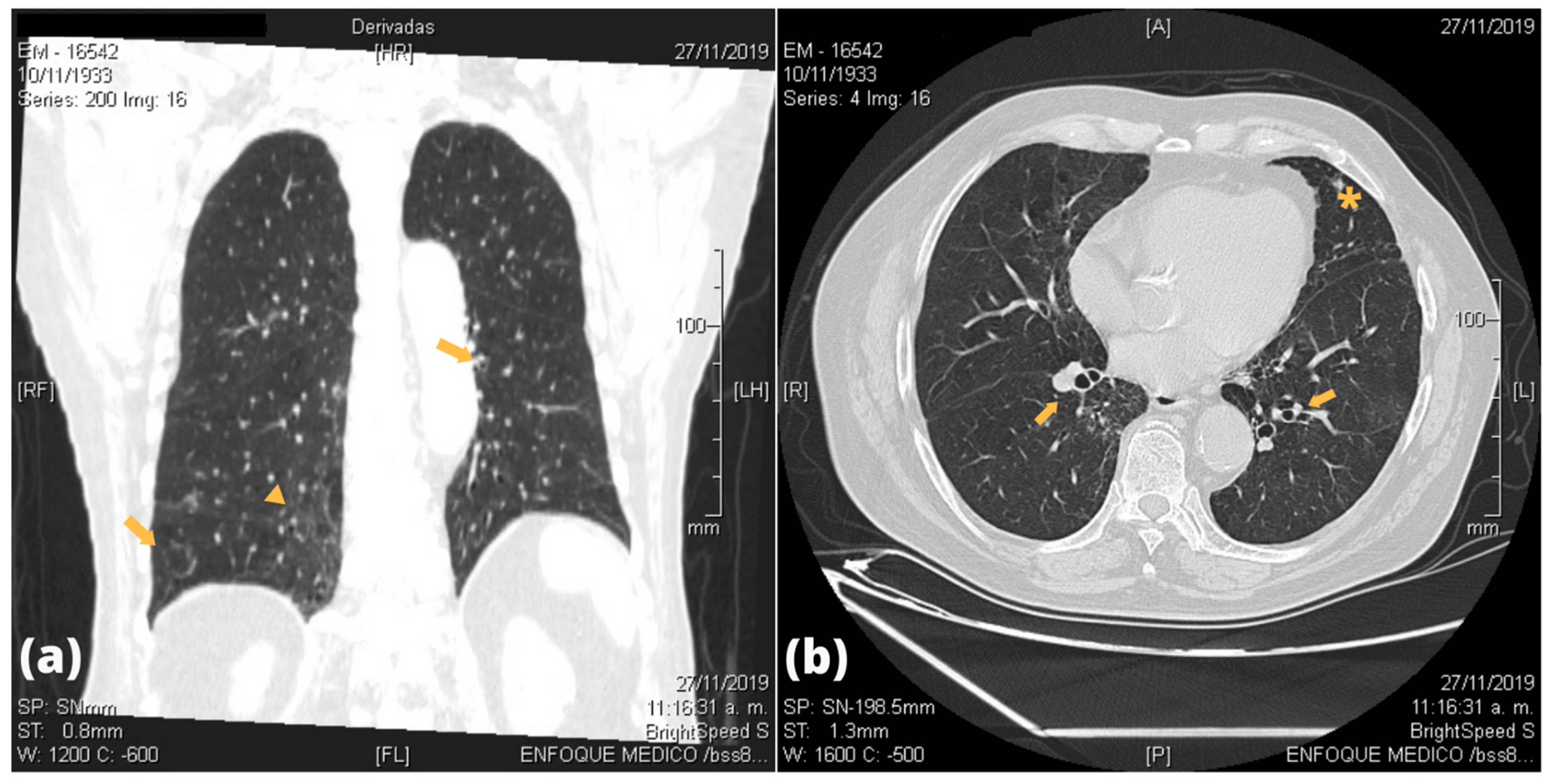
| Patients | Bronchial Lesion | Alveolar Lesion | Parenchymal Lesion | Pleural Lesion |
|---|---|---|---|---|
| P1 | None | None | Scarce right inferior fibrous sequelae tracts | None |
| P2 | Bilateral tubular bronchiectasis | None | Bilateral GGO; scarce right inferior fibrous retractile tracts | None |
| P3 | Bilateral varicose bronchiectasis | None | None | None |
| P4 | None | None | Bilateral apical fibrous retractile tracts | Bilateral apical pleural thickening |
| P5 | Right medial bronchiectasis | None | Bilateral inferior GGO; bilateral apical fibrous retractile tracts; right medial air cyst | Bilateral apical pleural thickening |
| P6 | Right medial and left inferior bronchiectasis | None | Bilateral inferior fibrous retractile tracts; right apical interstitial infiltrate | None |
| P7 | Left atelectasis | None | Bilateral inferior interstitial infiltrate | None |
| P8 | Bilateral cylindrical hilio-perihiliar bronchiectasis; laminar atelectasis | None | Bilateral apical fibrous retractile tracts | Right apical pleural thickening |
| P9 | Bilateral tubular bronchiectasis | None | Bilateral GGO with fibrous tracts | None |
| P11 | Right medial varicose bronchiectasis | None | Bilateral inferior GGO and interlobular septal interstitial thickening; bilateral apical thick fibrous sequelae tracts | Bilateral apical pleural thickening |
| P12 | Right tubular bronchiectasis | None | Bilateral inferior opacities and fibro retractable tracts | Bilateral pleural thickening |
| P13 | Bilateral tubular and varicose bronchiectasis | None | Right inferior GGO; bilateral inferior intra- and interlobular thickening | None |
| P14 | None | None | None | None |
| P15 | None | None | Subpleural infiltrate | None |
References
- Eusebio-Ponce, E.; Anguita, E.; Paulino-Ramirez, R.; Candel, F.J. HTLV-1 infection: An emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev. Esp. Quim. 2019, 32, 485–496. [Google Scholar]
- Einsiedel, L.; Fernandes, L.; Spelman, T.; Steinfort, D.; Gotuzzo, E. Bronchiectasis Is Associated with Human T-Lymphotropic Virus 1 Infection in an Indigenous Australian Population. Clin. Infect. Dis. 2012, 54, 43–50. [Google Scholar] [CrossRef]
- Honarbakhsh, S.; Taylor, G.P. High prevalence of bronchiectasis is linked to HTLV-1-associated inflammatory disease. BMC Infect. Dis. 2015, 15, 258. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.R.N.; Falcão, L.F.M.; Falcão, A.S.C.; Normando, V.M.F.; Quaresma, J.A.S. Human T Lymphotropic Virus and Pulmonary Diseases. Front. Microbiol. 2018, 9, 1879. [Google Scholar] [CrossRef] [Green Version]
- Einsiedel, L.; Pham, H.; Wilson, K.; Walley, R.; Turpin, J.; Bangham, C.; Gessain, A.; Woodman, R. Human T-Lymphotropic Virus type 1c subtype proviral loads, chronic lung disease and survival in a prospective cohort of Indigenous Australians. PLoS Negl. Trop. Dis. 2018, 12, e0006281. [Google Scholar] [CrossRef]
- Couderc, L.J.; Rain, B.; Desgranges, C. Pulmonary fibrosis in association with human T cell lymphotropic virus type 1 (HTLV-1) infection. Respir. Med. 2000, 94, 1010. [Google Scholar] [CrossRef] [Green Version]
- Normando, V.M.F.; Dias, Á.R.N.; da Silva, A.L.S.E.; da Silva Pinto, D.; de Souza Santos, M.C.; Rodrigues, C.L.; de Oliviera, E.M.; de Souza Filho, L.E.C.; de Brito Vieira, W.; Andriolo, R.B.; et al. HTLV-I induces lesions in the pulmonary system: A systematic review. Life Sci. 2020, 256, 117979. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Izumo, S.; Ijichi, S.; Kubota, H.; Arimura, K.; Kawabata, M.; Osame, M. HTLV-I-associated myelopathy: Analysis of 213 patients based on clinical features and laboratory findings. J. NeuroVirol. 1995, 1, 50–61. [Google Scholar] [CrossRef]
- Okada, F.; Ando, Y.; Yoshitake, S.; Yotsumoto, S.; Matsumoto, S.; Wakisaka, M.; Maeda, T.; Mori, H. Pulmonary CT Findings in 320 Carriers of Human T-Lymphotropic Virus Type 1. Radiology 2006, 240, 559–564. [Google Scholar] [CrossRef]
- Tanaka, T.; Sekioka, T.; Usui, M.; Imashuku, S. Opportunistic Infections in Patients with HTLV-1 Infection. Case Rep. Hematol. 2015, 2015, 943867. [Google Scholar] [CrossRef] [Green Version]
- Bastos, M.d.L.; Santos, S.B.; Souza, A.; Finkmoore, B.; Bispo, O.; Barreto, T.; Cardoso, I.; Bispo, I.; Bastos, F.; Pereira, D.; et al. Influence of HTLV-1 on the clinical, microbiologic and immunologic presentation of tuberculosis. BMC Infect. Dis. 2012, 12, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedral-Sampaio, D.B.; Neto, M.E.; Pedrosa, C.; Brites, C.; Duarte, M.; Harrington, W., Jr. Co-Infection of Tuberculosis and HIV/HTLV Retroviruses: Frequency and Prognosis Among Patients Admitted in a Brazilian Hospital. Braz. J. Infect. Dis. 1997, 1, 31–35. [Google Scholar]
- Verdonck, K.; González, E.; Henostroza, G.; Nabeta, P.; Llanos, F.; Cornejo, H.; Vanham, G.; Seas, C.; Gotuzzo, E. HTLV-1 infection is frequent among out-patients with pulmonary tuberculosis in northern Lima, Peru. Int. J. Tuberc. Lung Dis. 2007, 11, 1066–1072. [Google Scholar]
- Verdonck, K.; González, E.; Schrooten, W.; Vanham, G.; Gotuzzo, E.; Verdonck, K.; González, E.; Schrooten, W.; Vanham, G.; Gotuzzo, E. HTLV-1 infection is associated with a history of active tuberculosis among family members of HTLV-1-infected patients in Peru. Epidemiol. Infect. 2008, 136, 1076–1083. [Google Scholar] [CrossRef]
- Grassi, M.F.R.; Dos Santos, N.P.; Lírio, M.; Kritski, A.L.; Almeida, M.d.C.C.; Santana, L.P.; Lazaro, N.; Dias, J.; Neto, E.M.; Galvao-Castro, B. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect. Dis. 2016, 16, 491. [Google Scholar] [CrossRef] [Green Version]
- Pasipanodya, J.G.; Miller, T.L.; Vecino, M.; Munguia, G.; Garmon, R.; Bae, S.; Drewyer, G.; Weis, S.E. Pulmonary Impairment After Tuberculosis. Chest 2007, 131, 1817–1824. [Google Scholar] [CrossRef]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Tuberculosis Report 2020. 2020. Available online: https://www.who.int/teams/global-tuberculosis-programme/data (accessed on 1 January 2021).
- Ita, F.; Mayer, E.F.; Verdonck, K.; Gonzalez, E.; Clark, D.; Gotuzzo, E. Human T-lymphotropic virus type 1 infection is frequent in rural communities of the southern Andes of Peru. Int. J. Infect. Dis. 2014, 19, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Cachay, R.; Schwalb, A.; Mora, R.; Caceres, T.; Gotuzzo, E. Infección por virus linfotrópico de células T humanas tipo 1 en pacientes con diagnóstico de tuberculosis multidrogorresistente. Rev. Peru. Med. Exp. Salud Pública 2019, 36, 150–151. [Google Scholar] [CrossRef] [Green Version]
- Zihlmann, K.F.; De Alvarenga, A.T.; Casseb, J. Living Invisible: HTLV-1-Infected Persons and the Lack of Care in Public Health. PLoS Negl. Trop. Dis. 2012, 6, e1705. [Google Scholar] [CrossRef]
- Yamazato, Y.; Miyazato, A.; Kawakami, K.; Yara, S.; Kaneshima, H.; Saito, A. High Expression of p40 tax and Pro-inflammatory Cytokines and Chemokines in the Lungs of Human T-Lymphotropic Virus Type 1-Related Bronchopulmonary Disorders. Chest 2003, 124, 2283–2292. [Google Scholar] [CrossRef]
- Sugimoto, M.; Mita, S.; Tokunaga, M.; Yamaguchi, K.; Cho, I.; Matsumoto, M.; Mochizuki, M.; Araki, S.; Takatsuki, K.; Ando, M. Pulmonary involvement in human T-cell lymphotropic virus type-I uveitis: T-lymphocytosis and high proviral DNA load in bronchoalveolar lavage fluid. Eur. Respir. J. 1993, 6, 938–943. [Google Scholar]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- von Reyn, C.F.; Arbeit, R.D.; Tosteson, A.N.; Ristola, M.A.; Barber, T.W.; Waddell, R.; Sox, C.H.; Brindle, R.J.; Gilks, C.F.; Ranki, A.; et al. The international epidemiology of disseminated Mycobacterium avium complex infection in AIDS. International MAC Study Group. AIDS 1996, 10, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Cordero, F.M.; Miranda, J.G.; Mercado, J.H.; Leiva, P.L.; Ramos, C.S. Compromiso intestinal por Mycobacterium avium en un paciente con VIH/ SIDA. Rev. Gastroenterol. Peru 2014, 34, 59–61. [Google Scholar]
- Carrasco, J.; Soto, L.; Samalvides, F.; Asencios, L.; Quispe, N.; Valencia, E. Infección pulmonar por Mycobacterium avium en paciente VIH/SIDA: Primer reporte en Perú. Rev. Peru. Med. Exp. Salud Pública 2014, 31, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Ticona-Huaroto, C.; Astocondor-Salazar, L.; Montenegro-Idrogo, J.; Valencia-Mesias, G.; Soria, J. Infección por el complejo Mycobacterium avium—Intracellulare en pacientes con VIH/SIDA en un hospital peruano: Una serie de casos. Rev. Peru. Med. Exp. Salud Pública 2017, 34, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Falcão, L.F.M.; Falcão, A.S.C.; Sousa, R.C.M.; Vieira, W.d.B.; de Oliveira, R.T.M.; Normando, V.M.F.; da Silva Dias, G.A.; De Souza Santos, M.C.; Rocha, R.S.B.; Yoshikawa, G.T.; et al. CT Chest and pulmonary functional changes in patients with HTLV-associated myelopathy in the Eastern Brazilian Amazon. PLoS ONE 2017, 12, e0186055. [Google Scholar]
- Yamashiro, T.; Kamiya, H.; Miyara, T.; Gibo, S.; Ogawa, K.; Akamine, T.; Moromizato, H.; Yara, S.; Murayama, S. CT Scans of the Chest in Carriers of Human T-cell Lymphotropic Virus Type 1: Presence of Interstitial Pneumonia. Acad. Radiol. 2012, 19, 952–957. [Google Scholar] [CrossRef]
- Jacobson, S.; Massoud, R. Human T-Cell Lymphotropic Virus Type 1 Infection. In Viral Infections of the Human Nervous System; Jackson, A.C., Ed.; Springer: Basel, Switzerland, 2013; pp. 183–207. [Google Scholar]
- Diette, G.B.; Accinelli, R.A.; Balmes, J.R.; Buist, A.S.; Checkley, W.; Garbe, P.; Hansel, N.N.; Kapil, V.; Gordon, S.; Lagat, D.K.; et al. Obstructive lung disease and exposure to burning biomass fuel in the indoor environment. Glob. Heart 2012, 7, 265–270. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Dennis, R.J.; Maldonado, D.; Norman, S.; Baena, E.; Martinez, G. Woodsmoke Exposure and Risk for Obstructive Airways Disease Among Women. Chest 1996, 109, 115–119. [Google Scholar] [CrossRef]
- Sandoval, J.; Salas, J.; Martinez-Guerra, M.L.; Gómez, A.; Martinez, C.; Portales, A.; Palomar, A.; Villegas, M.; Barrios, R. Pulmonary Arterial Hypertension and Cor Pulmonale Associated with Chronic Domestic Woodsmoke Inhalation. Chest 1993, 103, 12–20. [Google Scholar] [CrossRef]
- Hu, G.; Zhou, Y.; Tian, J.; Yao, W.; Li, J.; Li, B.; Ran, P. Risk of COPD from exposure to biomass smoke: A metaanalysis. Chest 2010, 138, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Estarriol, M.H.; Goday, M.R.; Turmo, L.B. Lesiones Pulmonares Inducidas por Fármacos; Elsevier: Amsterdam, The Netherlands, 2002; Available online: http://www.elsevier.es/es-revista-medicina-integral-63-articulo-lesiones-pulmonares-inducidas-por-farmacos-13034633 (accessed on 1 January 2021).
- Dutta, N.K.; Karakousis, C. Latent Tuberculosis Infection: Myths, Models, and Molecular Mechanisms. Microbiol. Mol. Biol. Rev. 2014, 78, 343–371. [Google Scholar] [CrossRef] [Green Version]
- Murray, M.P.; Pentland, J.L.; Turnbull, K.; MacQuarrie, S.; Hill, A.T. Sputum colour: A useful clinical tool in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2009, 34, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Williams, N. The MRC breathlessness scale. Occup. Med. 2017, 67, 496–497. [Google Scholar] [CrossRef] [Green Version]
| Characteristics (n = 14) | Frequency |
|---|---|
| Female sex, (%) | 8 (57) |
| Age, years, median (IQR) | 60.6 (52–69) |
| BMI, kg/m2 | |
| Normal (18.5–24.9) | 6/11 |
| Overweight (25–29.9) | 1/11 |
| Obese (>30) | 4/11 |
| Place of origin | |
| Highlands | 5/14 |
| Coast region | 9/14 |
| Place of residence | |
| Lima | 12/14 |
| Outside Lima | 2/14 |
| Pneumoconiosis-risk occupation | 3/12 |
| Comorbidities | 4/12 |
| Cardiovascular: high blood pressure, heart failure | 4/12 |
| Endocrine: diabetes mellitus type II, hypothyroidism | 3/12 |
| Cancer | 1/12 |
| Lung damage-associated drugs: losartan and sertraline | 3/12 |
| Previous HTLV-1 associated disease | 5/12 |
| Infective dermatitis | 1/12 |
| Tropical spastic paraparesis | 4/12 |
| Strongyloidiasis | 1/12 |
| Uveitis | 1/12 |
| Sjögren’s syndrome | 1/12 |
| Patient ID | HTLV-1 Associated Disorder |
|---|---|
| P2 | HAM/TSP |
| P4 | Strongyloidiasis |
| P6 | Strongyloidiasis, HAM/TSP, uveitis |
| P11 | Infective dermatitis, HAM/TSP, Sjögren’s syndrome |
| P15 | HAM/TSP |
| Radiological Lung Lesion | Frequency |
|---|---|
| Lobe compromise | |
| Lower lobe: RL, LF | 10/14, 9/14 |
| Medial lobe: RL | 7/14 |
| Upper lobe: RL, LF | 7/14, 5/14 |
| Bronchial lesions | |
| Bronchiectasis | 9/14 |
| Bilateral | 5/9 |
| Atelectasis | 2/14 |
| Alveolar damage | 0/14 |
| Parenchymal damage | |
| GGO/infiltrates | 7/14 |
| Fibro-retractable tracts | 10/14 |
| Bilateral pleural thickness | 5/14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cachay, R.; Gil-Zacarias, M.; Watanabe-Tejada, T.; Schwalb, A.; Mejía, F.; Gayoso, O.; Gotuzzo, E. Clinical, Radiological and Functional Characteristics of Pulmonary Diseases among HTLV-1 Infected Patients without Prior Active Tuberculosis Infection. Pathogens 2021, 10, 895. https://doi.org/10.3390/pathogens10070895
Cachay R, Gil-Zacarias M, Watanabe-Tejada T, Schwalb A, Mejía F, Gayoso O, Gotuzzo E. Clinical, Radiological and Functional Characteristics of Pulmonary Diseases among HTLV-1 Infected Patients without Prior Active Tuberculosis Infection. Pathogens. 2021; 10(7):895. https://doi.org/10.3390/pathogens10070895
Chicago/Turabian StyleCachay, Rodrigo, Marcela Gil-Zacarias, Takashi Watanabe-Tejada, Alvaro Schwalb, Fernando Mejía, Oscar Gayoso, and Eduardo Gotuzzo. 2021. "Clinical, Radiological and Functional Characteristics of Pulmonary Diseases among HTLV-1 Infected Patients without Prior Active Tuberculosis Infection" Pathogens 10, no. 7: 895. https://doi.org/10.3390/pathogens10070895
APA StyleCachay, R., Gil-Zacarias, M., Watanabe-Tejada, T., Schwalb, A., Mejía, F., Gayoso, O., & Gotuzzo, E. (2021). Clinical, Radiological and Functional Characteristics of Pulmonary Diseases among HTLV-1 Infected Patients without Prior Active Tuberculosis Infection. Pathogens, 10(7), 895. https://doi.org/10.3390/pathogens10070895







