Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights
Abstract
1. Introduction
2. Results
2.1. Copromicroscopical Evaluation
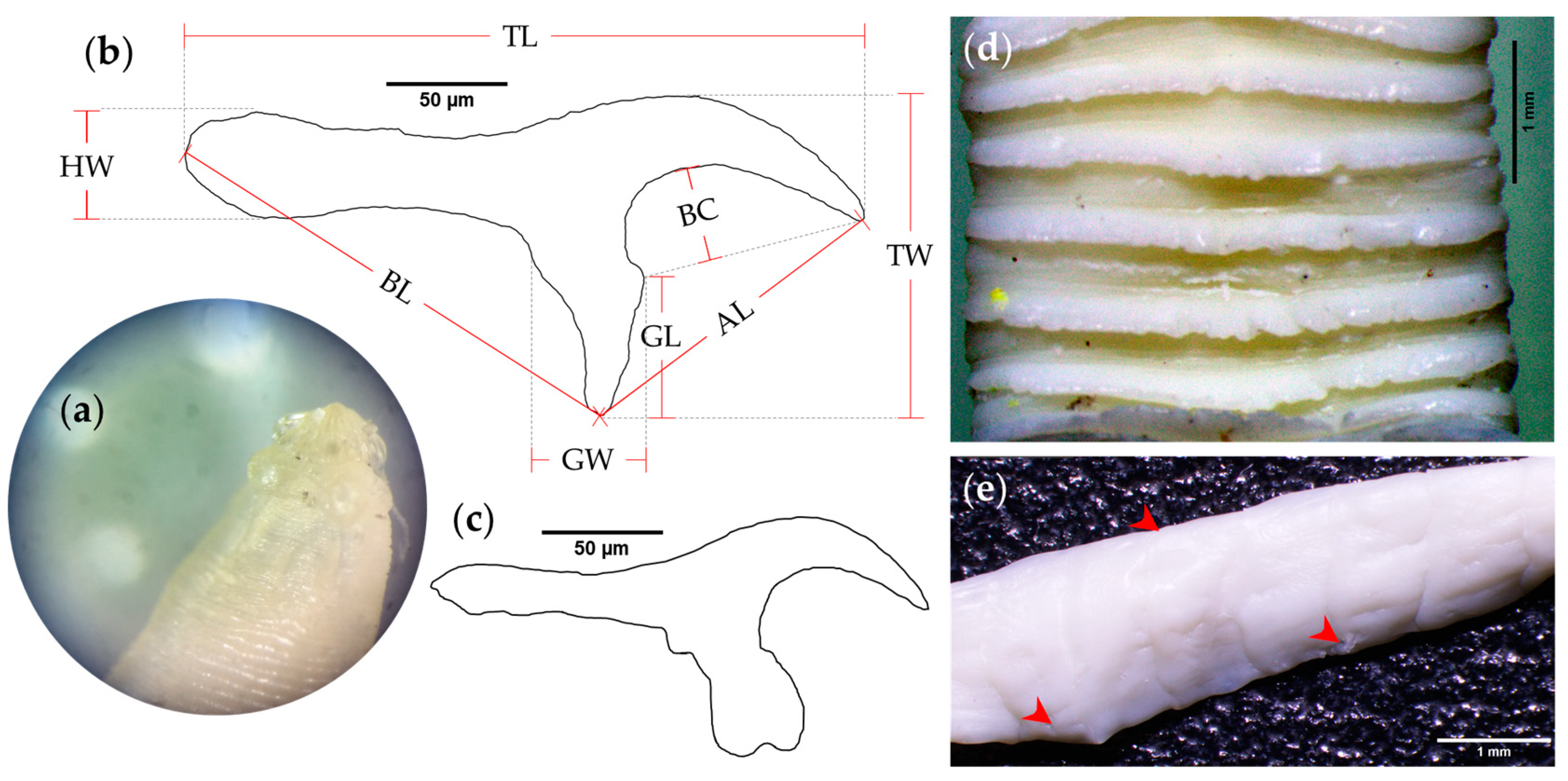
2.2. Cestode Identification and Characterization of Rostellar Hooks
2.3. Toxoplasma gondii PCR
3. Discussion
4. Materials and Methods
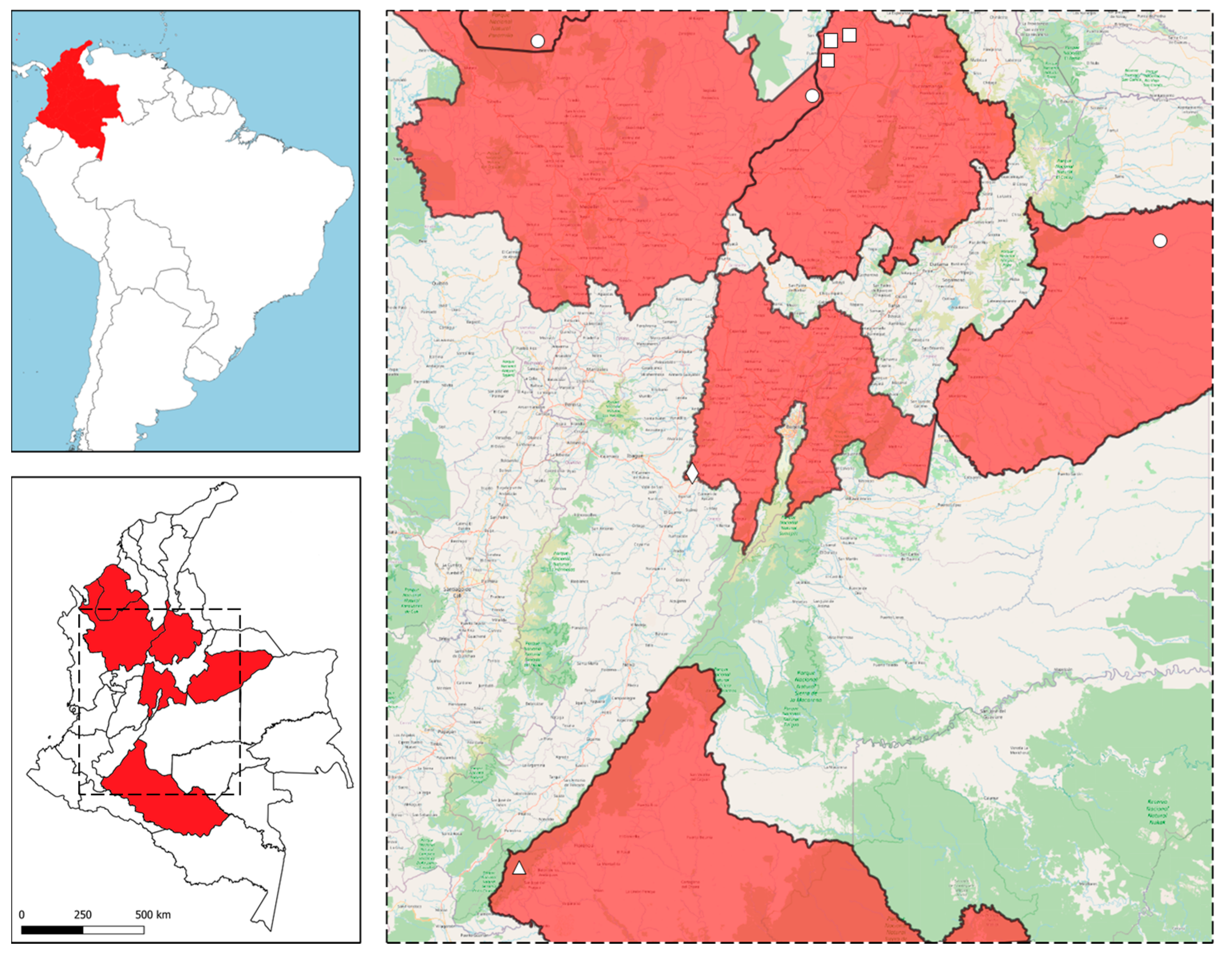
4.1. Study Area
4.2. Sample Collection and Laboratory Procedures
4.2.1. Basic Copromicroscopic Analyses
4.2.2. Molecular Phylogenetics
4.2.3. Faecal DNA Isolation and Toxoplasma gondii Molecular Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitchener, A.C.; Breitenmoser-Würsten, C.; Eizirik, E.; Gentry, A.; Werdelin, L.; Wilting, A.; Yamaguchi, N.; Abramov, A.; Christiansen, P.; Driscoll, C.; et al. A revised taxonomy of the Felidae. The final report of the Cat Classification Task Force of the IUCN/SSC Cat Specialist Group. Cat News 2017. Available online: https://repository.si.edu/handle/10088/32616 (accessed on 29 June 2021).
- Li, G.; Davis, B.W.; Eizirik, E.; Murphy, W.J. Phylogenomic evidence for ancient hybridization in the genomes of living cats (Felidae). Genome Res. 2016, 26, 1–11. [Google Scholar] [CrossRef]
- Thornton, D.; Zeller, K.; Rondinini, C.; Boitani, L.; Crooks, K.; Burdett, C.; Rabinowitz, A.; Quigley, H. Assessing the umbrella value of a range-wide conservation network for jaguars (Panthera onca). Ecol. Appl. 2016, 26, 1112–1124. [Google Scholar] [CrossRef]
- Montazeri, M.; Mikaeili Galeh, T.; Moosazadeh, M.; Sarvi, S.; Dodangeh, S.; Javidnia, J.; Sharif, M.; Daryani, A. The global serological prevalence of Toxoplasma gondii in felids during the last five decades (1967–2017): A systematic review and meta-analysis. Parasit. Vectors 2020, 13, 82. [Google Scholar] [CrossRef]
- Pena, H.F.J.; Marvulo, M.F.V.; Horta, M.C.; Silva, M.A.; Silva, J.C.R.; Siqueira, D.B.; Lima, P.-A.C.P.; Vitaliano, S.N.; Gennari, S.M. Isolation and genetic characterisation of Toxoplasma gondii from a red-handed howler monkey (Alouatta belzebul), a jaguarundi (Puma yagouaroundi), and a black-eared opossum (Didelphis aurita) from Brazil. Vet. Parasitol. 2011, 175, 377–381. [Google Scholar] [CrossRef]
- Zahedi, A.; Paparini, A.; Jian, F.; Robertson, I.; Ryan, U. Public health significance of zoonotic Cryptosporidium species in wildlife: Critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 2016, 5, 88–109. [Google Scholar] [CrossRef]
- Oates, S.C.; Miller, M.A.; Hardin, D.; Conrad, P.A.; Melli, A.; Jessup, D.A.; Dominik, C.; Roug, A.; Tinker, M.T.; Miller, W.A. Prevalence, environmental loading, and molecular characterization of Cryptosporidium and Giardia isolates from domestic and wild animals along the central California coast. Appl. Environ. Microbiol. 2012, 78, 8762–8772. [Google Scholar] [CrossRef]
- Rocha, F.L.; Roque, A.L.R.; de Lima, J.S.; Cheida, C.C.; Lemos, F.G.; de Azevedo, F.C.; Arrais, R.C.; Bilac, D.; Herrera, H.M.; Mourão, G.; et al. Trypanosoma cruzi infection in neotropical wild carnivores (Mammalia: Carnivora): At the top of the T. cruzi transmission chain. PLoS ONE 2013, 8, e67463. [Google Scholar] [CrossRef]
- Alvarado-Rybak, M.; Solano-Gallego, L.; Millán, J. A review of piroplasmid infections in wild carnivores worldwide: Importance for domestic animal health and wildlife conservation. Parasit. Vectors 2016, 9, 538. [Google Scholar] [CrossRef]
- André, M.R. Diversity of Anaplasma and Ehrlichia/Neoehrlichia agents in terrestrial wild carnivores worldwide: Implications for human and domestic animal health and wildlife conservation. Front. Vet. Sci. 2018, 5, 293. [Google Scholar] [CrossRef]
- Diakou, A.; Dimzas, D.; Astaras, C.; Savvas, I.; Di Cesare, A.; Morelli, S.; Neofitos, Κ.; Migli, D.; Traversa, D. Clinical investigations and treatment outcome in a European wildcat (Felis silvestris silvestris) infected by cardio-pulmonary nematodes. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100357. [Google Scholar] [CrossRef]
- Traversa, D.; Morelli, S.; Di Cesare, A.; Diakou, A. Felid cardiopulmonary nematodes: Dilemmas solved and new questions posed. Pathogens 2021, 10, 30. [Google Scholar] [CrossRef]
- Pence, D.B.; Tewes, M.E.; Laack, L.L. Helminths of the ocelot from Southern Texas. J. Wildl. Dis. 2003, 39, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Seguel, M.; Gottdenker, N. The diversity and impact of hookworm infections in wildlife. Int. J. Parasitol. Parasites Wildl. 2017, 6, 177–194. [Google Scholar] [CrossRef]
- González, P.; Carbonell, E.; Urios, V.; Rozhnov, V.V. Coprology of Panthera tigris altaica and Felis bengalensis euptilurus from the Russian far east. J. Parasitol. 2007, 93, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Arrabal, J.P.; Pérez, M.G.; Arce, L.F.; Kamenetzky, L. First identification and molecular phylogeny of Sparganum proliferum from endangered felid (Panthera onca) and other wild definitive hosts in one of the regions with highest worldwide biodiversity. Int. J. Parasitol. Parasites Wildl. 2020, 13, 142–149. [Google Scholar] [CrossRef]
- Ulziijargal, G.; Yeruult, C.; Khulan, J.; Gantsetseg, C.; Wandra, T.; Yamasaki, H.; Narankhajid, M. Molecular identification of Taenia hydatigena and Mesocestoides species based on copro-DNA analysis of wild carnivores in Mongolia. Int. J. Parasitol. Parasites Wildl. 2020, 11, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Lavikainen, A.; Haukisalmi, V.; Deksne, G.; Holmala, K.; Lejeune, M.; Isomursu, M.; Jokelainen, P.; Näreaho, A.; Laakkonen, J.; Hoberg, E.P.; et al. Molecular identification of Taenia spp. in the Eurasian lynx (Lynx lynx) from Finland. Parasitology 2013, 140, 653–662. [Google Scholar] [CrossRef]
- Souza, U.A.; Webster, A.; Dall’Agnol, B.; Peters, F.B.; Favarini, M.O.; Schott, D.; Zitelli, L.C.; Mazim, F.D.; Kasper, C.B.; Ott, R.; et al. Ticks, mites, fleas, and vector-borne pathogens in free-ranging neotropical wild felids from southern Brazil. Ticks Tick. Borne. Dis. 2021, 12, 101706. [Google Scholar] [CrossRef]
- Marcogliese, D.J. Parasites of the superorganism: Are they indicators of ecosystem health? Int. J. Parasitol. 2005, 35, 705–716. [Google Scholar] [CrossRef]
- Hudson, P.J.; Dobson, A.P.; Lafferty, K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006, 21, 381–385. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 1–10. [Google Scholar] [CrossRef]
- Scognamillo, D.; Maxit, I.E.; Sunquist, M.; Polisar, J. Coexistence of jaguar (Panthera onca) and puma (Puma concolor) in a mosaic landscape in the Venezuelan llanos. J. Zool. 2003, 259, 269–279. [Google Scholar] [CrossRef]
- Boron, V.; Xofis, P.; Link, A.; Payan, E.; Tzanopoulos, J. Conserving predators across agricultural landscapes in Colombia: Habitat use and space partitioning by jaguars, pumas, ocelots and jaguarundis. Oryx 2020, 54, 554–563. [Google Scholar] [CrossRef]
- Nagy-Reis, M.; Oshima, J.E.d.F.; Kanda, C.Z.; Palmeira, F.B.L.; Melo, F.R.; Morato, R.G.; Bonjorne, L.; Magioli, M.; Leuchtenberger, C.; Rohe, F.; et al. Neotropical Carnivores: A data set on carnivore distribution in the Neotropics. Ecology 2020, 101. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Robinson, H.S.; Abarca, M.; Zeller, K.A.; Velasquez, G.; Paemelaere, E.A.D.; Goldberg, J.F.; Payan, E.; Hoogesteijn, R.; Boede, E.O.; et al. Estimating large carnivore populations at global scale based on spatial predictions of density and distribution—Application to the jaguar (Panthera onca). PLoS ONE 2018, 13, e0194719. [Google Scholar] [CrossRef] [PubMed]
- Boron, V.; Tzanopoulos, J.; Gallo, J.; Barragan, J.; Jaimes-Rodriguez, L.; Schaller, G.; Payán, E. Jaguar densities across human-dominated landscapes in Colombia: The contribution of unprotected areas to long term conservation. PLoS ONE 2016, 11, e0153973. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Lymbery, A.J.; Smith, A. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 2010, 40, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. Int. J. Parasitol. Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Payán, E.; Boron, V. The future of wild mammals in oil palm landscapes in the Neotropics. Front. For. Glob. Chang. 2019, 2, 61. [Google Scholar] [CrossRef]
- Boron, V.; Deere, N.J.; Xofis, P.; Link, A.; Quiñones-Guerrero, A.; Payan, E.; Tzanopoulos, J. Richness, diversity, and factors influencing occupancy of mammal communities across human-modified landscapes in Colombia. Biol. Conserv. 2019, 232, 108–116. [Google Scholar] [CrossRef]
- Reperant, L.A.; Cornaglia, G.; Osterhaus, A.D.M.E. The importance of understanding the human–animal interface. In One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases; Mackenzie, J.S., Jeggo, M., Daszak, P., Richt, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 365, pp. 49–81. [Google Scholar]
- Kuchta, R.; Kołodziej-Sobocińska, M.; Brabec, J.; Młocicki, D.; Sałamatin, R.; Scholz, T. Sparganosis (Spirometra) in Europe in the molecular era. Clin. Infect. Dis. 2021, 72, 882–890. [Google Scholar] [CrossRef]
- Mueller, J.F.; Miranda Froes, O.; Fernandez, T. On the occurrence of Spirometra mansonoides in South America. J. Parasitol. 1975, 61, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.D.; Martin, R.L. Tapeworms of the Chaco Boreal, Paraguay, by two new species. J. Helminthol. 1978, 52, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Olea, F.; Sacristán, I.; Aguilar, E.; García, S.; López, M.J.; Oyarzún-Ruiz, P.; Brito, J.L.; Fredes, F.; Napolitano, C. Gastrointestinal and cardiorespiratory endoparasites in the wild felid guigna (Leopardus guigna) in Chile: Richness increases with latitude and first records for the host species. Int. J. Parasitol. Parasites Wildl. 2020, 13, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.G.; Coscarelli, D.; Melo, M.N.; Melo, A.L.; Pinto, H.A. Molecular identification of Spirometra spp. (Cestoda: Diphyllobothriidae) in some wild animals from Brazil. Parasitol. Int. 2016, 65, 428–431. [Google Scholar] [CrossRef]
- Tantaleán, M.; Michaud, C. Huéspedes definitivos de Spirometra mansonoides (Cestoda: Diphyllobothriidae) en el Perú. Rev. Peru. Biol. 2005, 12, 153–157. [Google Scholar] [CrossRef][Green Version]
- Moulinier, R.; Martinez, E.; Torres, J.; Noya, O.; de NOya, B.A.; Reyes, O. Human proliferative sparganosis in Venezuela: Report of a case. Am. J. Trop. Med. Hyg. 1982, 31, 358–363. [Google Scholar] [CrossRef]
- Beaver, P.C.; Rolon, F.A. Proliferating larval cestode in a man in Paraguay. Am. J. Trop. Med. Hyg. 1981, 30, 625–637. [Google Scholar] [CrossRef]
- Kikuchi, T.; Maruyama, H. Human proliferative sparganosis update. Parasitol. Int. 2020, 75, 102036. [Google Scholar] [CrossRef]
- Gomez, J.J.; Botero, D. The first case of sparganosis in Colombia. Am. J. Trop. Med. Hyg. 1958, 7, 597–599. [Google Scholar] [CrossRef]
- Peng, Z.-W.; Ning, Y.; Liu, D.; Sun, Y.; Wang, L.-X.; Zhai, Q.-A.; Hou, Z.-J.; Chai, H.-L.; Jiang, G.-S. Ascarid infection in wild Amur tigers (Panthera tigris altaica) in China. BMC Vet. Res. 2020, 16, 86. [Google Scholar] [CrossRef]
- Bolivar-Mejia, A.; Alarcón-Olave, C.; Calvo-Betancourt, L.S.; Paniz-Mondolfi, A.; Delgado, O.; Rodriguez-Morales, A.J. Toxocariasis in the Americas: Burden and disease control. Curr. Trop. Med. Rep. 2014, 1, 62–68. [Google Scholar] [CrossRef]
- López-Osorio, S.; Penagos-Tabares, F.; Chaparro-Gutiérrez, J.J. Prevalence of Toxocara spp. in dogs and cats in South America (excluding Brazil). Adv. Parasitol. 2020, 109, 743–778. [Google Scholar] [CrossRef]
- Duszynski, D.W.; Kvičerová, J.; Seville, R.S. The Biology and Identification of the Coccidia (Apicomplexa) of Carnivores of the World; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Dubey, J.P. A review of Cystoisospora felis and C. rivolta-induced coccidiosis in cats. Vet. Parasitol. 2018, 263, 34–48. [Google Scholar] [CrossRef]
- Lühe, M.F.L. Cystotänien südamerikanischer Feliden. Zool Jahrb 1910, 12, 687–710. [Google Scholar]
- Gomez-Puerta, L.A.; Yucra, D.; Lopez-Urbina, M.T.; Gonzalez, A.E. The alpaca (Vicugna pacos) as a natural intermediate host of Taenia omissa (Cestoda: Taeniidae). Vet. Parasitol. 2017, 246, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puerta, L.A.; Alarcon, V.; Pacheco, J.; Franco, F.; Lopez-Urbina, M.T.; Gonzalez, A.E. Molecular and morphological evidence of Taenia omissa in pumas (Puma concolor) in the Peruvian Highlands. Rev. Bras. Parasitol. Veterinária 2016, 25, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.M.; de Carvalho, L.M.; González, M.J.P.; Torres, R.T.; Pla, S.; Núñez-Arjona, J.C.; Rueda, C.; Vallverdú-Coll, N.; Silvestre, F.; Peña, J.; et al. Parasites of the reintroduced Iberian lynx (Lynx pardinus) and sympatric mesocarnivores in Extremadura, Spain. Pathogens 2021, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Amin, O.M. Classification of the Acanthocephala. Folia Parasitol. 2013, 60, 273–305. [Google Scholar] [CrossRef]
- Sianto, L.; de Souza, M.V.; Chame, M.; da Luz, M.d.F.; Guidon, N.; Pessis, A.-M.; Araújo, A. Helminths in feline coprolites up to 9000 years in the Brazilian Northeast. Parasitol. Int. 2014, 63, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.; Rabinowitz, A.; Randolph, S.; Johnson, S.S. A coprological survey of parasites of wild Neotropical Felidae. J. Parasitol. 1986, 72, 517. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.G.N.; Chame, M.; Chagas-Moutinho, V.A.; Santos, C.P. Morphology and molecular analysis of Oncicola venezuelensis (Acanthocephala: Oligacanthorhynchidae) from the ocelot Leopardus pardalis in Brazil. J. Helminthol. 2017, 91, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Marteau, M. Oncicola venezuelensis n. sp. (Archiacanthocephala: Oligacanthorhynchida), parasite de l’Ocelot (Felis pardalis L.). Ann. Parasitol. Hum. Comparée 1977, 52, 25–33. [Google Scholar] [CrossRef]
- Thatcher, V.E.; Nickol, B.B. Some acanthocephalans from Panama and Colombia. Proc. Helminthol. Soc. Wash. 1972, 39, 245–248. [Google Scholar]
- Benatti, D.; De Santi, M.; Werther, K.; Tebaldi, J.H.; Hoppe, E.G.L. Helminthfauna of road-killed cougars (Puma concolor) from the Northeastern Region of São Paulo State, Brazil. Rev. Bras. Parasitol. Veterinária 2021, 30, e024120. [Google Scholar] [CrossRef]
- Palmer, J.P.S.; Dib, L.V.; Lobão, L.F.; Pinheiro, J.L.; Ramos, R.C.F.; Uchoa, C.M.A.; Bastos, O.M.P.; Silva, M.E.M.; Nascimento, J.L.D.; Pissinatti, A.; et al. Oncicola venezuelensis (Marteau, 1977) (Acanthocephala: Oligacanthorhynchidae) in puma concolor in Rio de Janeiro, Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, 1–13. [Google Scholar] [CrossRef]
- Orrell, T. NMNH Extant Specimen Records; National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2017. [Google Scholar]
- Vieira, F.M.; Muniz-Pereira, L.C.; de Souza Lima, S.; Neto, A.H.A.M.; Guimarães, E.V.; Luque, J.L. A new metastrongyloidean species (Nematoda) parasitizing pulmonary arteries of Puma (Herpailurus) yagouaroundi (É. Geoffroy, 1803) (Carnivora: Felidae) from Brazil. J. Parasitol. 2013, 99, 327–331. [Google Scholar] [CrossRef]
- Di Cesare, A.; Morelli, S.; Colombo, M.; Simonato, G.; Veronesi, F.; Marcer, F.; Diakou, A.; D’Angelosante, R.; Pantchev, N.; Psaralexi, E.; et al. Is angiostrongylosis a realistic threat for domestic cats? Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, A.; Zeller, K.A. A range-wide model of landscape connectivity and conservation for the jaguar, Panthera onca. Biol. Conserv. 2010, 143, 939–945. [Google Scholar] [CrossRef]
- Chame, M. Terrestrial mammal feces: A morphometric summary and description. Mem. Inst. Oswaldo Cruz 2003, 98, 71–94. [Google Scholar] [CrossRef]
- Zuercher, G.L.; Gipson, P.S.; Stewart, G.C. Identification of carnivore feces by local peoples and molecular analyses. Wildl. Soc. Bull. 2003, 31, 961–970. [Google Scholar]
- Wultsch, C.; Waits, L.P.; Hallerman, E.M.; Kelly, M.J. Optimizing collection methods for noninvasive genetic sampling of neotropical felids. Wildl. Soc. Bull. 2015, 39, 403–412. [Google Scholar] [CrossRef]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Sikes, R.S. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Scholten, T. A Fixative for intestinal parasites permitting the use of concentration and permanent staining procedures. Am. J. Clin. Pathol. 1977, 67, 300–304. [Google Scholar] [CrossRef]
- Dennis, W.R.; Stone, W.M.; Swanson, L.E. A new laboratory and field diagnostic test for fluke ova in feces. J. Am. Vet. Med. Assoc. 1954, 124, 47–50. [Google Scholar]
- Heine, J. A simple technic for the demonstration of cryptosporidia in feces. Zentralbl. Veterinarmed. B 1982, 29, 324–327. [Google Scholar] [CrossRef]
- Deplazes, P.; Eckert, J.; Mathis, A.; von Samson-Himmelstjerna, G.; Zahner, H. Parasitology in Veterinary Medicine; Wageningen Academic Publishers: Wagenigen, The Netherlands, 2016; ISBN 978-90-8686-274-0. [Google Scholar]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef]
- Wicht, B.; Yanagida, T.; Scholz, T.; Ito, A.; Jiménez, J.A.; Brabec, J. Multiplex PCR for differential identification of broad tapeworms (Cestoda : Diphyllobothrium) infecting humans. J. Clin. Microbiol. 2010, 48, 3111–3116. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating Maximum-Likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Homan, W.L.; Vercammen, M.; De Braekeleer, J.; Verschueren, H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000, 30, 69–75. [Google Scholar] [CrossRef]



| Risk Classification | |||||
|---|---|---|---|---|---|
| Genus | Species | Common Name | CITES a | UICN b | National b |
| Herpailurus | yagouaroundi * | Jaguarundi, Eyra cat | Appx II | LC | NE |
| Leopardus | pardalis * | Ocelot | Appx I | LC | NE |
| Leopardus | wiedii | Margay, Tree ocelot | Appx I | NT | NE |
| Leopardus | tigrinus | Northern tiger cat | Appx I | VU | NE |
| Panthera | onca * | Jaguar | Appx I | NT | VU |
| Puma | concolor * | Puma, Cougar | Appx I | LC | NE |
| Department | Municipality | Sampling Location | Climate a | Sample Type |
|---|---|---|---|---|
| Antioquia | Yondó | Ciénaga de Barbacoas | Am Af | Feces o |
| Caquetá | San José del Fragüa | Puerto bello | Af | Metazoan Δ |
| Casanare | Hato Corozal | La Chapa | Am | Feces o |
| Córdoba | Puerto Libertador | La Esmeralda | Af | Feces o |
| Cundinamarca | - | - | Aw | Metazoan ◊ |
| Santander | El Hato | Las Pampas | Af | Feces □ |
| Santander | Puerto Wilches | Las Palmas | Am | Feces □ |
| Santander | Puerto Wilches | Caño Limón | Am | Feces □ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, M.; Payán, E.; Brabec, J.; Vélez, J.; Taubert, A.; Chaparro-Gutiérrez, J.J.; Hermosilla, C. Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights. Pathogens 2021, 10, 822. https://doi.org/10.3390/pathogens10070822
Uribe M, Payán E, Brabec J, Vélez J, Taubert A, Chaparro-Gutiérrez JJ, Hermosilla C. Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights. Pathogens. 2021; 10(7):822. https://doi.org/10.3390/pathogens10070822
Chicago/Turabian StyleUribe, Manuel, Esteban Payán, Jan Brabec, Juan Vélez, Anja Taubert, Jenny J. Chaparro-Gutiérrez, and Carlos Hermosilla. 2021. "Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights" Pathogens 10, no. 7: 822. https://doi.org/10.3390/pathogens10070822
APA StyleUribe, M., Payán, E., Brabec, J., Vélez, J., Taubert, A., Chaparro-Gutiérrez, J. J., & Hermosilla, C. (2021). Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights. Pathogens, 10(7), 822. https://doi.org/10.3390/pathogens10070822









