Immunization Coverage and Antibody Retention against Rabies in Domestic Dogs in Lusaka District, Zambia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Cluster Survey Method
2.3. Blood Sample Collection from Owned Dogs
2.4. Measurement of Antibody Titer Against Rabies
2.5. Data and Statistical Analyses
3. Results
3.1. Dog Population Characteristics
3.2. Rabies Vaccination and Immunization Coverage in Dogs
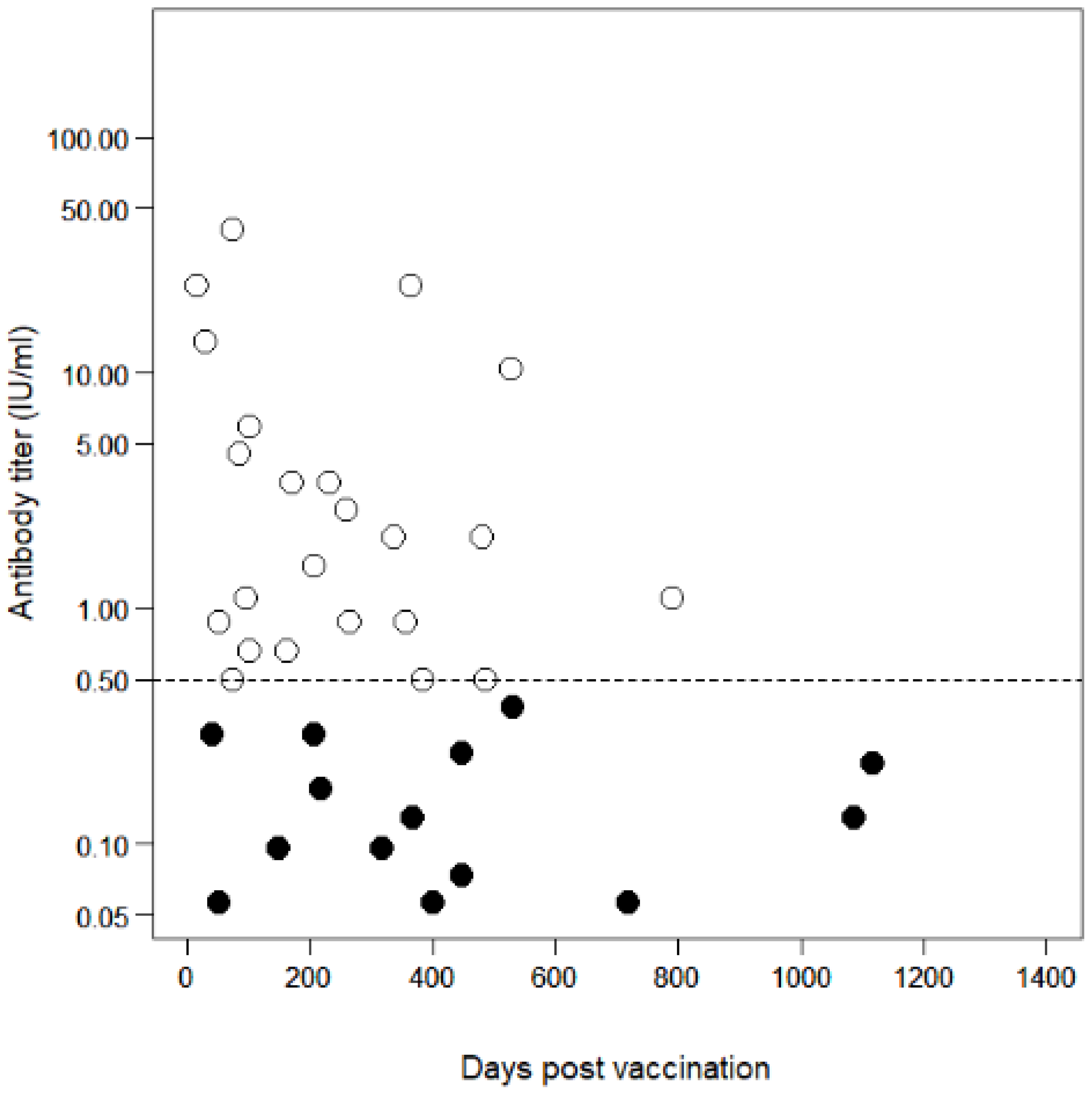
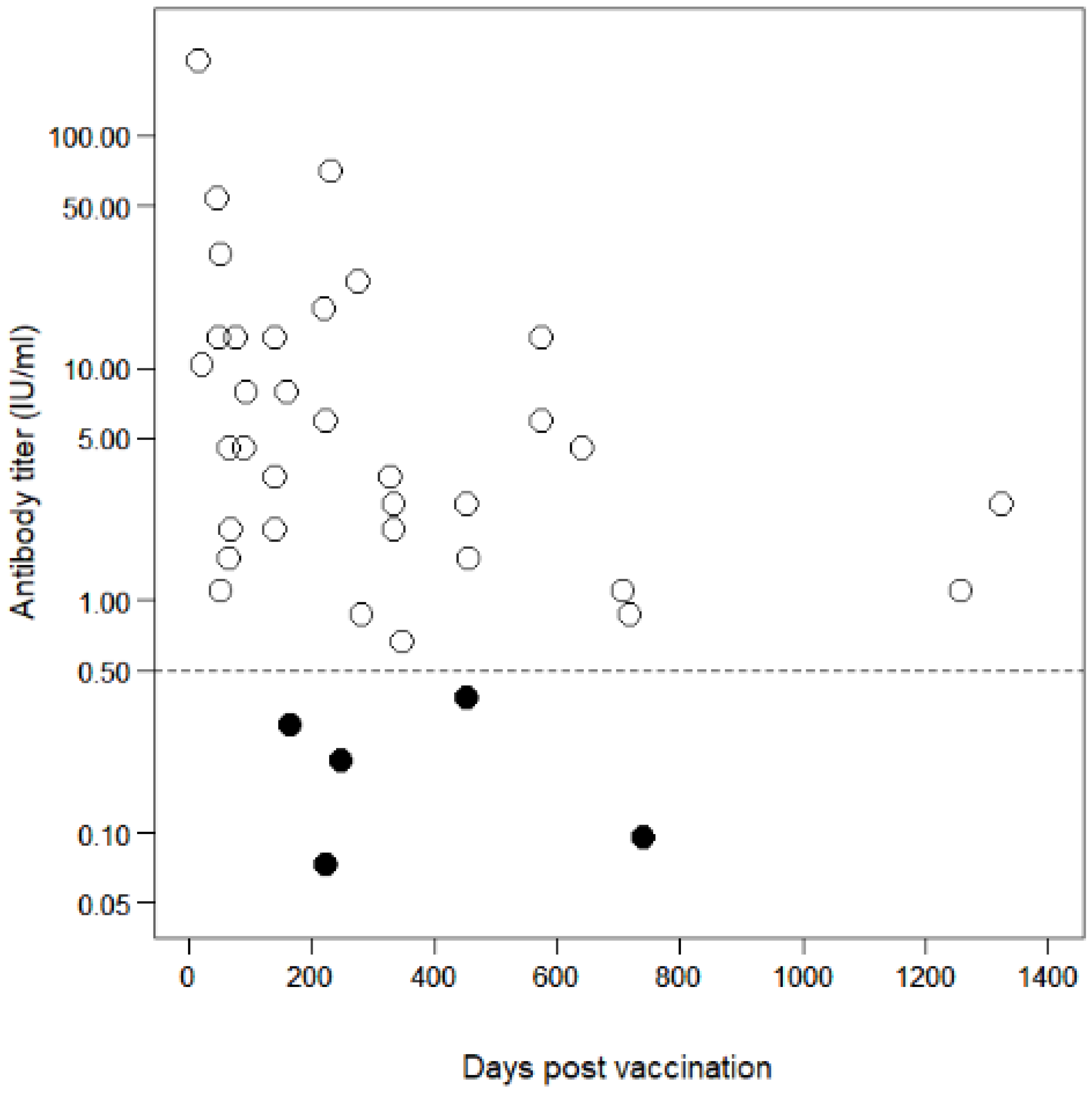
3.3. Antibody Decline in Vaccinated Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; WHO Technical Report Series, No. 1012; World Health Organization: Genova, Switzerland, 2018. [Google Scholar]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Lembo, T.; Hampson, K.; Kaare, M.T.; Ernest, E.; Knobel, D.; Kazwala, R.R.; Haydon, D.T.; Cleaveland, S. The feasibility of canine rabies elimination in Africa: Dispelling doubts with data. PLoS Negl. Trop. Dis. 2010, 4, e626. [Google Scholar] [CrossRef]
- Cleaveland, S.; Hampson, K.; Kaare, M. Living with rabies in Africa. Vet. Rec. 2007, 161, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Kaare, M.; Tiringa, P.; Mlengeya, T.; Barrat, J. A dog rabies vaccination campaign in rural Africa: Impact on the incidence of dog rabies and human dog-bite injuries. Vaccine 2003, 21, 1965–1973. [Google Scholar] [CrossRef]
- Hampson, K.; Dushoff, J.; Cleaveland, S.; Haydon, D.T.; Kaare, M.; Packer, C.; Dobson, A. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009, 7, e53. [Google Scholar] [CrossRef] [PubMed]
- Coleman, P.G.; Dye, C. Immunization coverage required to prevent outbreaks of dog rabies. Vaccine 1996, 14, 185–186. [Google Scholar] [CrossRef]
- Conan, A.; Akerele, O.; Simpson, G.; Reininghaus, B.; van Rooyen, J.; Knobel, D. Population Dynamics of Owned, Free-Roaming Dogs: Implications for Rabies Control. PLoS Negl. Trop. Dis. 2015, 9, e0004177. [Google Scholar] [CrossRef] [PubMed]
- Morters, M.K.; McKinley, T.J.; Restif, O.; Conlan, A.J.; Cleaveland, S.; Hampson, K.; Whay, H.R.; Damriyasa, I.M.; Wood, J.L. The demography of free-roaming dog populations and applications to disease and population control. J. Appl. Ecol. 2014, 51, 1096–1106. [Google Scholar] [CrossRef]
- Knobel, D.L.; Hampson, K.; Lembo, T.; Cleaveland, S.; Davis, A. Dog rabies and its control. In Rabies, 4th ed.; Fooks, A.R., Jackson, A.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 567–603. [Google Scholar]
- Ferguson, E.A.; Hampson, K.; Cleaveland, S.; Consunji, R.; Deray, R.; Friar, J.; Haydon, D.T.; Jimenez, J.; Pancipane, M.; Townsend, S.E. Heterogeneity in the spread and control of infectious disease: Consequences for the elimination of canine rabies. Sci. Rep. 2015, 5, 18232. [Google Scholar] [CrossRef]
- Townsend, S.E.; Lembo, T.; Cleaveland, S.; Meslin, F.X.; Miranda, M.E.; Putra, A.A.G.; Haydon, D.T.; Hampson, K. Surveillance guidelines for disease elimination: A case study of canine rabies. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 249–261. [Google Scholar] [CrossRef]
- Morters, M.K.; Restif, O.; Hampson, K.; Cleaveland, S.; Wood, J.L.; Conlan, A.J. Evidence-based control of canine rabies: A critical review of population density reduction. J. Anim. Ecol. 2013, 82, 6–14. [Google Scholar] [CrossRef]
- Sambo, M.; Cleaveland, S.; Ferguson, H.; Lembo, T.; Simon, C.; Urassa, H.; Hampson, K. The burden of rabies in Tanzania and its impact on local communities. PLoS Negl. Trop. Dis. 2013, 7, e2510. [Google Scholar] [CrossRef]
- Wallace, R.M.; Mehal, J.; Nakazawa, Y.; Recuenco, S.; Bakamutumaho, B.; Osinubi, M.; Tugumizemu, V.; Blanton, J.D.; Gilbert, A.; Wamala, J. The impact of poverty on dog ownership and access to canine rabies vaccination: Results from a knowledge, attitudes and practices survey, Uganda 2013. Infect. Dis. Poverty 2017, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- De Balogh, K.K.; Wandeler, A.I.; Meslin, F.X. A dog ecology study in an urban and a semi-rural area of Zambia. Onderstepoort J. Vet. Res. 1993, 60, 437–443. [Google Scholar]
- Olugasa, B.O.; Aiyedun, J.O.; Emikpe, B.O. Prevalence of antibody against rabies among confined, free-roaming and stray dogs in a transit city of Nigeria. Vet. Ital. 2011, 47, 453–460. [Google Scholar]
- Muleya, W.; Chambaro, H.M.; Sasaki, M.; Gwenhure, L.F.; Mwenechanya, R.; Kajihara, M.; Saasa, N.; Mupila, Z.; Mori-Kajihara, A.; Qiu, Y.; et al. Genetic diversity of rabies virus in different host species and geographic regions of Zambia and Zimbabwe. Virus Genes 2019, 55, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Muleya, W.; Namangala, B.; Mweene, A.; Zulu, L.; Fandamu, P.; Banda, D.; Kimura, T.; Sawa, H.; Ishii, A. Molecular epidemiology and a loop-mediated isothermal amplification method for diagnosis of infection with rabies virus in Zambia. Virus Res. 2012, 163, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Mweene, A.S.; Siamudaala, V.; Muma, J.B.; Matandiko, W. Rabies status in Zambia for the period 1985-2004. Zoonoses Public Health 2011, 58, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Babaniyi, O.; Songolo, P.; Matapo, B.; Masaninga, F.; Mulenga, F.; Michelo, C.; Mubanga, J.; Kazembe, L.N. Epidemiological characteristics of rabies in Zambia: A retrospective study (2004–2013). Clin. Epidemiol. Glob. Health 2016, 4, 83–88. [Google Scholar] [CrossRef]
- Hamoonga, R. Ending Dog-mediated Human Rabies by 2030: A Zambian Perspective. Health Press Zambia Bull. 2018, 2, 2–4. [Google Scholar]
- Banda, R.; Sandøy, I.F.; Fylkesnes, K.; Janssen, F. Impact of Pregnancy-Related Deaths on Female Life Expectancy in Zambia: Application of Life Table Techniques to Census Data. PLoS ONE 2015, 10, e0141689. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Immunization Coverage Cluster Survey: Reference Manual [WHO/IVB/04.23]; World Health Organization: Genova, Switzerland, 2005. [Google Scholar]
- World Health Organization. Training for Mid-Level Managers (MLM): The EPI Coverage Survey [WHO/IVB/08.07]; World Health Organization: Genova, Switzerland, 2008. [Google Scholar]
- Hoshaw-Woodard, S.; World Health Organization. Description and Comparison of the Methods of Cluster Sampling and Lot Quality Assurance Sampling to Assess Immunization Coverage [WHO/V&B/01.26]; World Health Organization: Genova, Switzerland, 2001. [Google Scholar]
- Cliquet, F.; Aubert, M.; Sagné, L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods 1998, 212, 79–87. [Google Scholar] [CrossRef]
- Aubert, M.F.A. Methods for the calculation of titers. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.-X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Genova, Switzerland, 1996; pp. 445–459. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies: Second Report; WHO Technical Report Series, No. 982; World Health Organization: Genova, Switzerland, 2013. [Google Scholar]
- World Health Organization. WHO Expert Committee on Rabies [Meeting Held in Geneva from 24 to 30 September 1991]: Eighth Report; WHO Technical Report Series, No. 824; World Health Organization: Genova, Switzerland, 1992. [Google Scholar]
- World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2013. Available online: https://www.oie.int/standard-setting/terrestrial-manual/access-online/ (accessed on 17 October 2014).
- Aubert, M.F. Practical significance of rabies antibodies in cats and dogs. Rev. Sci. Tech. 1992, 11, 735–760. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Berentsen, A.R.; Dunbar, M.R.; Becker, M.S.; M’soka, J.; Droge, E.; Sakuya, N.M.; Matandiko, W.; McRobb, R.; Hanlon, C.A. Rabies, canine distemper, and canine parvovirus exposure in large carnivore communities from two Zambian ecosystems. Vector-Borne Zoonotic Dis. 2013, 13, 643–649. [Google Scholar] [CrossRef]
- Deem, S.L.; Davis, R.; Pacheco, L.F. Serologic evidence of nonfatal rabies exposure in a free-ranging oncilla (Leopardus tigrinus) in Cotapata National Park, Bolivia. J. Wildl. Dis. 2004, 40, 811–815. [Google Scholar] [CrossRef][Green Version]
- Shipley, R.; Wright, E.; Selden, D.; Wu, G.; Aegerter, J.; Fooks, A.R.; Banyard, A.C. Bats and Viruses: Emergence of Novel Lyssaviruses and Association of Bats with Viral Zoonoses in the EU. Trop. Med. Infect. Dis. 2019, 4, 31. [Google Scholar] [CrossRef]
- Kaneko, C.; Omori, R.; Sasaki, M.; Kataoka-Nakamura, C.; Simulundu, E.; Muleya, W.; Moonga, L.; Ndebe, J.; Hang’ombe, B.M.; Dautu, G.; et al. Domestic dog demographics and estimates of canine vaccination coverage in a rural area of Zambia for the elimination of rabies. PLoS Negl. Trop. Dis. 2021, 15, e0009222. [Google Scholar] [CrossRef]
- Gsell, A.S.; Knobel, D.L.; Kazwala, R.R.; Vounatsou, P.; Zinsstag, J. Domestic dog demographic structure and dynamics relevant to rabies control planning in urban areas in Africa: The case of Iringa, Tanzania. BMC Vet. Res. 2012, 8, 236. [Google Scholar] [CrossRef]
- Kayali, U.; Mindekem, R.; Yémadji, N.; Vounatsou, P.; Kaninga, Y.; Ndoutamia, A.G.; Zinsstag, J. Coverage of pilot parenteral vaccination campaign against canine rabies in N’Djaména, Chad. Bull. World Health Organ. 2003, 81, 739–744. [Google Scholar]
- Suzuki, K.; Pereira, J.A.; Frías, L.A.; López, R.; Mutinelli, L.E.; Pons, E.R. Rabies-vaccination coverage and profiles of the owned-dog population in Santa Cruz de la Sierra, Bolivia. Zoonoses Public Health 2008, 55, 177–183. [Google Scholar] [CrossRef]
- Kongkaew, W.; Coleman, P.; Pfeiffer, D.U.; Antarasena, C.; Thiptara, A. Vaccination coverage and epidemiological parameters of the owned-dog population in Thungsong District, Thailand. Prev. Vet. Med. 2004, 65, 105–115. [Google Scholar] [CrossRef]
- Adeyemi, I.G.; Ikheloa, J.O.; Ogundipe, G.A. Microbial contaminants found in low egg passage rabies vaccine used in Nigeria. Zentralbl. Veterinarmed. B 1993, 40, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, I.; Zessin, K. Retrospective dog rabies vaccination evaluation at the University of Ibadan, Nigeria (1988–1992). Vet. Arhiv. 2000, 70, 223–230. [Google Scholar]
- Sebunya, T.; Ndabambi, N.; Mpuchane, S. A Serosurvey of Rabies Antibodies in Dogs in Gaborone, Botswana. J. Anim. Vet. Adv. 2007, 6, 549–552. [Google Scholar]
- van Sittert, S.J.; Raath, J.; Akol, G.W.; Miyen, J.M.; Mlahlwa, B.; Sabeta, C.T. Rabies in the Eastern Cape Province of South Africa—Where are we going wrong? J. S. Afr. Vet. Assoc. 2010, 81, 207–215. [Google Scholar] [CrossRef]
- Mulipukwa, C.P.; Mudenda, B.; Mbewe, A.R. Insights and efforts to control rabies in Zambia: Evaluation of determinants and barriers to dog vaccination in Nyimba district. PLoS Negl. Trop. Dis. 2017, 11, e0005946. [Google Scholar] [CrossRef]
- Jibat, T.; Hogeveen, H.; Mourits, M.C.M. Review on Dog Rabies Vaccination Coverage in Africa: A Question of Dog Accessibility or Cost Recovery? PLoS Negl. Trop. Dis. 2015, 9, e0003447. [Google Scholar] [CrossRef]
- Durr, S.; Mindekem, R.; Kaninga, Y.; Doumagoum Moto, D.; Meltzer, M.I.; Vounatsou, P.; Zinsstag, J. Effectiveness of dog rabies vaccination programmes: Comparison of owner-charged and free vaccination campaigns. Epidemiol. Infect. 2009, 137, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Morters, M.K.; McKinley, T.J.; Horton, D.L.; Cleaveland, S.; Schoeman, J.P.; Restif, O.; Whay, H.R.; Goddard, A.; Fooks, A.R.; Damriyasa, I.M.; et al. Achieving population-level immunity to rabies in free-roaming dogs in Africa and Asia. PLoS Negl. Trop. Dis. 2014, 8, e3160. [Google Scholar] [CrossRef]
- Sugiyama, M.; Yoshiki, R.; Tatsuno, Y.; Hiraga, S.; Itoh, O.; Gamoh, K.; Minamoto, N. A new competitive enzyme-linked immunosorbent assay demonstrates adequate immune levels to rabies virus in compulsorily vaccinated Japanese domestic dogs. Clin. Diagn. Lab. Immunol. 1997, 4, 727. [Google Scholar] [CrossRef]
- Pimburage, R.M.S.; Gunatilake, M.; Wimalaratne, O.; Balasuriya, A.; Perera, K.A.D.N. Sero-prevalence of virus neutralizing antibodies for rabies in different groups of dogs following vaccination. BMC Vet. Res. 2017, 13, 133. [Google Scholar] [CrossRef]
- Lankester, F.J.; Wouters, P.A.; Czupryna, A.; Palmer, G.H.; Mzimbiri, I.; Cleaveland, S.; Francis, M.J.; Sutton, D.J.; Sonnemans, D.G. Thermotolerance of an inactivated rabies vaccine for dogs. Vaccine 2016, 34, 5504–5511. [Google Scholar] [CrossRef]

| Male | Age (Months) | Female |
|---|---|---|
| 15 | 3–11 | 23 |
| 17 | 12–23 | 22 |
| 11 | 24–35 | 10 |
| 18 | 36–47 | 12 |
| 9 | 48–59 | 9 |
| 16 | 60–71 | 12 |
| 5 | 72–83 | 4 |
| 5 | 84–95 | 2 |
| 8 | Over 96 | 0 |
| 24 | Unidentified | 29 |
| 128 | Total | 123 |
| a. Seropositivity with a threshold of 0.5 IU/mL | |||||
|---|---|---|---|---|---|
| Valid | Uncertain | Expired | Never Vaccinated Before | Total | |
| Seropositive | 40 | 38 | 24 | 4 | 106 (42.2) |
| Seronegative | 10 | 34 | 23 | 78 | 145 (57.8) |
| Total | 50 (19.9) | 72 (28.7) | 47 (18.7) | 82 (32.7) | 251 |
| Values in parentheses are the proportion of the corresponding status (%). | |||||
| b. Seropositivity with a threshold of 0.2 IU/mL | |||||
| Valid | Uncertain | Expired | Never Vaccinated Before | Total | |
| Seropositive | 43 | 45 | 32 | 12 | 132 (52.6) |
| Seronegative | 7 | 27 | 15 | 70 | 119 (47.4) |
| Total | 50 (19.9) | 72 (28.7) | 47 (18.7) | 82 (32.7) | 251 |
| Values in parentheses are the proportion of the corresponding status (%). | |||||
| Immunization Coverage (n = 251) | Minimum Immunization Coverage (n = 366) † | |||
|---|---|---|---|---|
| Threshold: 0.5 IU/mL | Threshold: 0.2 IU/mL | Threshold: 0.5 IU/mL | Threshold: 0.2 IU/mL | |
| Coverage (%) | 42.2 (33.6–50.9) | 52.6 (43.9–61.3) | 29.0 (22.4–35.5) | 36.1 (29.1–43.0) |
| Values in parentheses are obtained at 95% confidence intervals. | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneko, C.; Sasaki, M.; Omori, R.; Nakao, R.; Kataoka-Nakamura, C.; Moonga, L.; Ndebe, J.; Muleya, W.; Simulundu, E.; Hang’ombe, B.M.; et al. Immunization Coverage and Antibody Retention against Rabies in Domestic Dogs in Lusaka District, Zambia. Pathogens 2021, 10, 738. https://doi.org/10.3390/pathogens10060738
Kaneko C, Sasaki M, Omori R, Nakao R, Kataoka-Nakamura C, Moonga L, Ndebe J, Muleya W, Simulundu E, Hang’ombe BM, et al. Immunization Coverage and Antibody Retention against Rabies in Domestic Dogs in Lusaka District, Zambia. Pathogens. 2021; 10(6):738. https://doi.org/10.3390/pathogens10060738
Chicago/Turabian StyleKaneko, Chiho, Michihito Sasaki, Ryosuke Omori, Ryo Nakao, Chikako Kataoka-Nakamura, Ladslav Moonga, Joseph Ndebe, Walter Muleya, Edgar Simulundu, Bernard M. Hang’ombe, and et al. 2021. "Immunization Coverage and Antibody Retention against Rabies in Domestic Dogs in Lusaka District, Zambia" Pathogens 10, no. 6: 738. https://doi.org/10.3390/pathogens10060738
APA StyleKaneko, C., Sasaki, M., Omori, R., Nakao, R., Kataoka-Nakamura, C., Moonga, L., Ndebe, J., Muleya, W., Simulundu, E., Hang’ombe, B. M., Dautu, G., Kajihara, M., Mori-Kajihara, A., Qiu, Y., Ito, N., Chambaro, H. M., Sugimoto, C., Higashi, H., Takada, A., ... Isoda, N. (2021). Immunization Coverage and Antibody Retention against Rabies in Domestic Dogs in Lusaka District, Zambia. Pathogens, 10(6), 738. https://doi.org/10.3390/pathogens10060738









