Survey of Viral Reactivations in Elite Athletes: A Case-Control Study
Abstract
1. Introduction
2. Results
2.1. Exercise Load
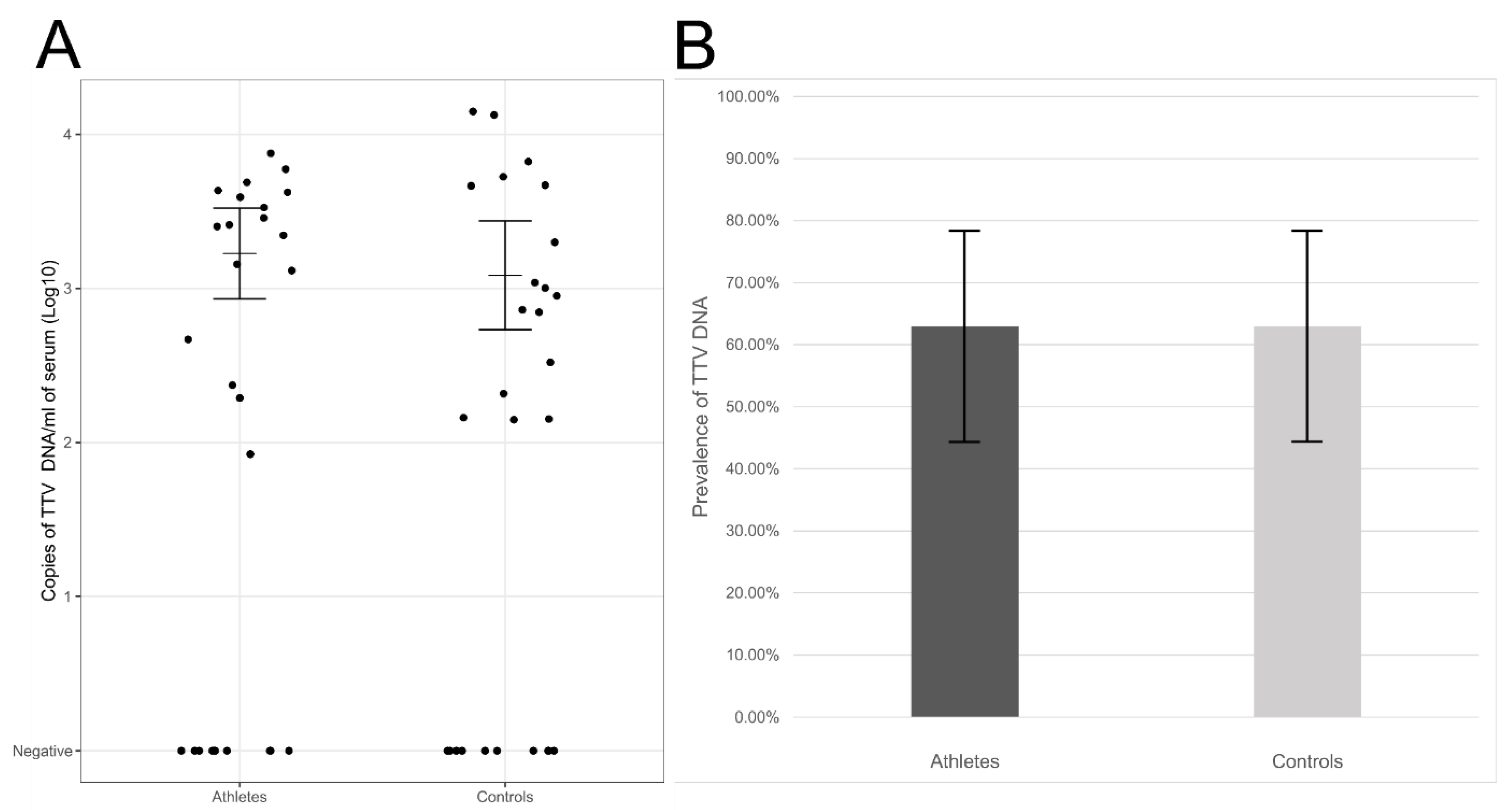
2.2. Detection of Viral DNAs in Sera
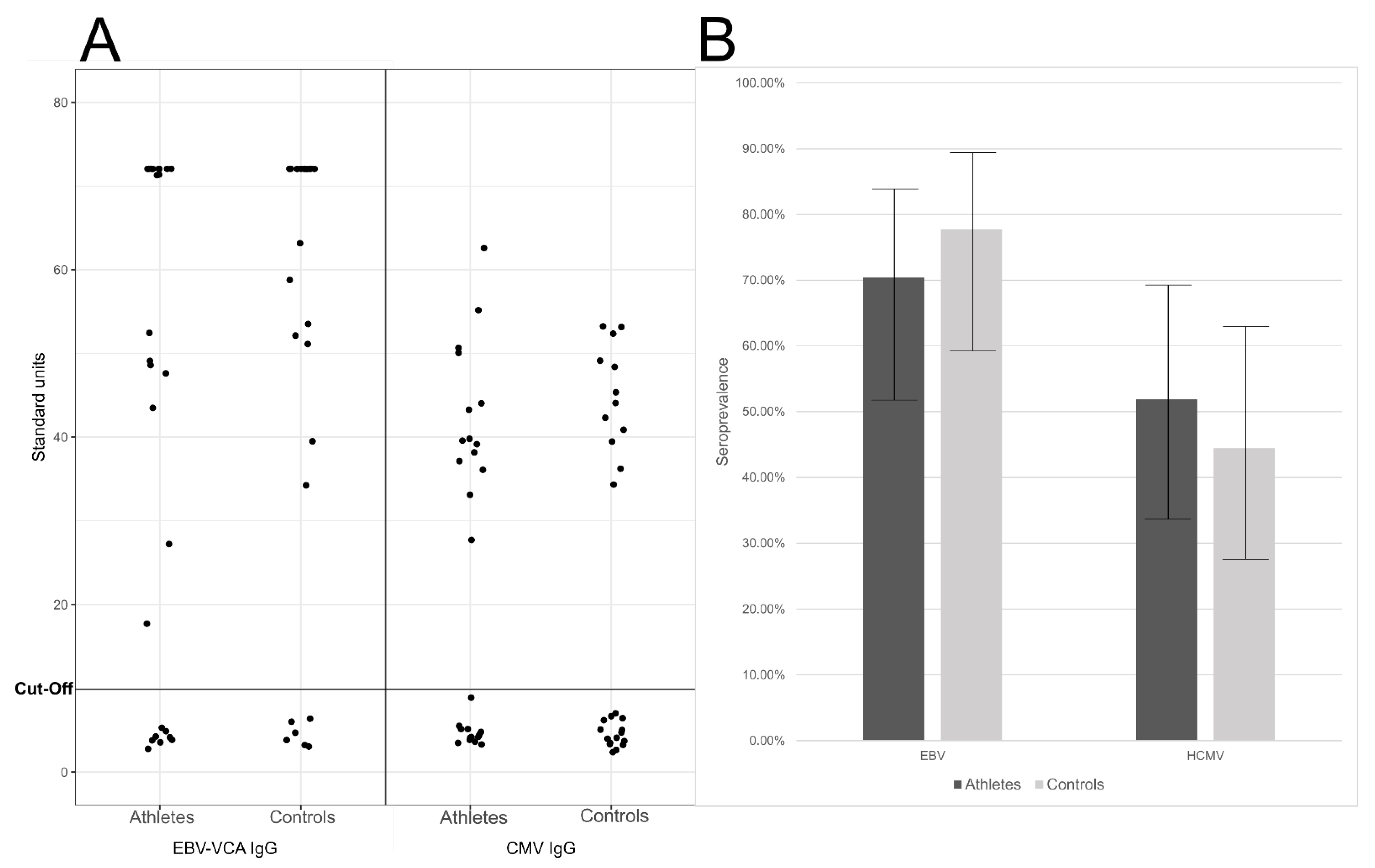
2.3. Past Immunity against Herpesviruses
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleeson, M. Immune Function in Sport and Exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Davison, G. Exercise, Immunity, and Illness. In Muscle and Exercise Physiology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 317–344. [Google Scholar]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious Episodes in Runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fit. 1990, 30, 316–328. [Google Scholar]
- Peters, E.M.; Goetzsche, J.M.; Grobbelaar, B.; Noakes, T.D. Vitamin C Supplementation Reduces the Incidence of Postrace Symptoms of Upper-Respiratory-Tract Infection in Ultramarathon Runners. Am. J. Clin. Nutr. 1993, 57, 170–174. [Google Scholar] [CrossRef]
- Cox, A.J.; Gleeson, M.; Pyne, D.B.; Callister, R.; Hopkins, W.G.; Fricker, P.A. Clinical and Laboratory Evaluation of Upper Respiratory Symptoms in Elite Athletes. Clin. J. Sport Med. 2008, 18, 438–445. [Google Scholar] [CrossRef]
- Svendsen, I.S.; Gleeson, M.; Haugen, T.A.; Tønnessen, E. Effect of an Intense Period of Competition on Race Performance and Self-Reported Illness in Elite Cross-Country Skiers. Scand. J. Med. Sci. Sports 2015, 25, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, M.; Waris, M.; Vuorinen, T.; Eerola, E.; Hakanen, A.J.; Mjosund, K.; Grönroos, W.; Heinonen, O.J.; Ruuskanen, O. Common Cold in Team Finland during 2018 Winter Olympic Games (PyeongChang): Epidemiology, Diagnosis Including Molecular Point-of-Care Testing (POCT) and Treatment. Br. J. Sports Med. 2019, 53, 1093–1098. [Google Scholar] [CrossRef]
- Valtonen, M.; Grönroos, W.; Luoto, R.; Waris, M.; Uhari, M.; Heinonen, O.J.; Ruuskanen, O. Increased Risk of Respiratory Viral Infections in Elite Athletes: A Controlled Study. PLoS ONE 2021, 16, e0250907. [Google Scholar] [CrossRef]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining Chronic Viral Infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef]
- Roberts, M.B.; Fishman, J.A. Immunosuppressive Agents and Infectious Risk in Transplantation: Managing the “Net State of Immunosuppression”. Clin. Infect. Dis. 2020, ciaa1189. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Herpesvirus Latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef]
- Ward, K.N. The Natural History and Laboratory Diagnosis of Human Herpesviruses-6 and -7 Infections in the Immunocompetent. J. Clin. Virol. 2005, 32, 183–193. [Google Scholar] [CrossRef]
- Miller, G.G.; Dummer, J.S. Herpes Simplex and Varicella Zoster Viruses: Forgotten but Not Gone. Am. J. Transpl. 2007, 7, 741–747. [Google Scholar] [CrossRef]
- Fishman, J.A. Overview: Cytomegalovirus and the Herpesviruses in Transplantation. Am. J. Transpl. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Lee, D.H.; Zuckerman, R.A. Herpes Simplex Virus Infections in Solid Organ Transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl. 2019, 33, e13526. [Google Scholar] [CrossRef]
- Focosi, D.; Spezia, P.G.; Macera, L.; Salvadori, S.; Navarro, D.; Lanza, M.; Antonelli, G.; Pistello, M.; Maggi, F. Assessment of Prevalence and Load of Torquetenovirus Viraemia in a Large Cohort of Healthy Blood Donors. Clin. Microbiol. Infect. 2020, 26, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Heilos, A.; Bond, G.; Meyer, E.; Böhm, M.; Puchhammer-Stöckl, E.; Arbeiter, K.; Müller-Sacherer, T.; Csaicsich, D.; Aufricht, C.; et al. Torque Teno Viral Load Reflects Immunosuppression in Paediatric Kidney-Transplanted Patients—A Pilot Study. Pediatr. Nephrol. 2021, 36, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Görzer, I.; Haloschan, M.; Jaksch, P.; Klepetko, W.; Puchhammer-Stöckl, E. Plasma DNA Levels of Torque Teno Virus and Immunosuppression after Lung Transplantation. J. Hear. Lung Transpl. 2014, 33, 320–323. [Google Scholar] [CrossRef]
- Jaksch, P.; Kundi, M.; Görzer, I.; Muraközy, G.; Lambers, C.; Benazzo, A.; Hoetzenecker, K.; Klepetko, W.; Puchhammer-Stöckl, E. Torque Teno Virus as a Novel Biomarker Targeting the Efficacy of Immunosuppression After Lung Transplantation. J. Infect. Dis. 2018, 218, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Maggi, F.; Macera, L.; Spezia, P.G.; Pistello, M.; Provinciali, M.; Piacenza, F.; Basso, A.; Bürkle, A.; Moreno-Villanueva, M.; et al. Prevalence and Loads of Torquetenovirus in the European Mark-Age Study Population. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 1838–1845. [Google Scholar] [CrossRef]
- Westman, G.; Schoofs, C.; Ingelsson, M.; Järhult, J.D.; Muradrasoli, S. Torque Teno Virus Viral Load Is Related to Age, CMV Infection and HLA Type but Not to Alzheimer’s Disease. PLoS ONE 2020, 15, e0227670. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Khush, K.K.; Strehl, C.; Kohli, B.; Luikart, H.; Neff, N.F.; Okamoto, J.; Snyder, T.M.; Cornfield, D.N.; Nicolls, M.R.; et al. Temporal Response of the Human Virome to Immunosuppression and Antiviral Therapy. Cell 2013, 155, 1178. [Google Scholar] [CrossRef]
- Solli, G.S.; Tønnessen, E.; Sandbakk, Ø. The Training Characteristics of the World’s Most Successful Female Cross-Country Skier. Front. Physiol. 2017, 8, 1069. [Google Scholar] [CrossRef]
- Komatsu, H.; Inui, A.; Sogo, T.; Kuroda, K.; Tanaka, T.; Fujisawa, T. TTV Infection in Children Born to Mothers Infected with TTV but Not with HBV, HCV, or HIV. J. Med. Virol. 2004, 74, 499–506. [Google Scholar] [CrossRef]
- Görzer, I.; Jaksch, P.; Kundi, M.; Seitz, T.; Klepetko, W.; Puchhammer-Stöckl, E. Pre-Transplant Plasma Torque Teno Virus Load and Increase Dynamics after Lung Transplantation. PLoS ONE 2015, 10, e0122975. [Google Scholar]
- Haloschan, M.; Bettesch, R.; Görzer, I.; Weseslindtner, L.; Kundi, M.; Puchhammer-Stöckl, E. TTV DNA Plasma Load and Its Association with Age, Gender, and HCMV IgG Serostatus in Healthy Adults. Age Omaha 2014, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.D.; Eugen-Olsen, J.; Kirk, O.; Parner, J.; Christensen, J.K.; Brasholt, M.S.; Nielsen, J.O.; Krogsgaard, K. TTV Viral Load as a Marker for Immune Reconstitution after Initiation of HAART in HIV-Infected Patients. HIV Clin. Trials 2002, 3, 287–295. [Google Scholar] [PubMed]
- Masouridi-Levrat, S.; Pradier, A.; Simonetta, F.; Kaiser, L.; Chalandon, Y.; Roosnek, E. Torque Teno Virus in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation for Hematological Malignancies. Bone Marrow Transpl. 2016, 51, 440–442. [Google Scholar] [CrossRef]
- Strassl, R.; Schiemann, M.; Doberer, K.; Görzer, I.; Puchhammer-Stöckl, E.; Eskandary, F.; Kikić, Ž.; Gualdoni, G.A.; Vossen, M.G.; Rasoul-Rockenschaub, S.; et al. Quantification of Torque Teno Virus Viremia as a Prospective Biomarker for Infectious Disease in Kidney Allograft Recipients. J. Infect. Dis. 2018, 218, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, M.; Albert, E.; Fernández-Ruiz, M.; González-Álvaro, I.; Rodríguez, E.; Aguado, J.M.; Navarro, D.; Pablos, J.L. Torque Teno Virus Viremia in Patients with Chronic Arthritis: Influence of Biologic Therapies. Semin. Arthritis Rheum. 2020, 50, 166–171. [Google Scholar] [CrossRef]
- Schmidt, L.; Jensen, B.E.O.; Walker, A.; Keitel-Anselmino, V.; di Cristanziano, V.; Böhm, M.; Knops, E.; Heger, E.; Kaiser, R.; de Luca, A.; et al. Torque Teno Virus Plasma Level as Novel Biomarker of Retained Immunocompetence in HIV-Infected Patients. Infection 2021, 1, 3. [Google Scholar]
- Rueschenbaum, S.; Ciesek, S.; Queck, A.; Widera, M.; Schwarzkopf, K.; Brüne, B.; Welsch, C.; Wedemeyer, H.; Zeuzem, S.; Weigert, A.; et al. Dysregulated Adaptive Immunity Is an Early Event in Liver Cirrhosis Preceding Acute-on-Chronic Liver Failure. Front. Immunol. 2021, 11, 534731. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Macera, L.; Pistello, M.; Maggi, F. Torque Teno Virus Viremia Correlates With Intensity of Maintenance Immunosuppression in Adult Orthotopic Liver Transplant. J. Infect. Dis. 2014, 210, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Wolfarth, B.; Hörterer, H.G.; Halle, M.; Reichhuber, C.; Nadas, K.; Tora, C.; Erfle, V.; Protzer, U.; Schätzl, H.M. Elevated Epstein-Barr Virus Loads and Lower Antibody Titers in Competitive Athletes. J. Med. Virol. 2010, 82, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Pyne, D.B.; Austin, J.P.; Francis, J.L.; Clancy, R.L.; McDonald, W.A.; Fricker, P.A. Epstein-Barr Virus Reactivation and Upper-Respiratory Illness in Elite Swimmers. Med. Sci. Sports Exerc. 2002, 34, 411–417. [Google Scholar] [CrossRef]
- Reid, V.L.; Gleeson, M.; Williams, N.; Clancy, R.L. Clinical Investigation of Athletes with Persistent Fatigue and/or Recurrent Infections. Br. J. Sports Med. 2004, 38, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.L.; Gleeson, M.; Cox, A.; Callister, R.; Dorrington, M.; D’Este, C.; Pang, G.; Pyne, D.; Fricker, P.; Henriksson, A. Reversal in Fatigued Athletes of a Defect in Interferon γ Secretion after Administration of Lactobacillus Acidophilus. Br. J. Sports Med. 2006, 40, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Venables, P.J.W.; Teo, C.G.; Baboonian, C.; Griffin, B.E.; Hughes, R.A.; Maini, R.N. Persistence of Epstein-Barr Virus in Salivary Gland Biopsies from Healthy Individuals and Patients with Sjogren’s Syndrome. Clin. Exp. Immunol. 1989, 75, 359–364. [Google Scholar]
- Maurmann, S.; Fricke, L.; Wagner, H.J.; Schlenke, P.; Hennig, H.; Steinhoff, J.; Jabs, W.J. Molecular Parameters for Precise Diagnosis of Asymptomatic Epstein-Barr Virus Reactivation in Healthy Carriers. J. Clin. Microbiol. 2003, 41, 5419–5428. [Google Scholar] [CrossRef]
- Hadinoto, V.; Shapiro, M.; Sun, C.C.; Thorley-Lawson, D.A. The Dynamics of EBV Shedding Implicate a Central Role for Epithelial Cells in Amplifying Viral Output. PLoS Pathog. 2009, 5, e1000496. [Google Scholar] [CrossRef]
- Pyöriä, L.; Toppinen, M.; Mäntylä, E.; Hedman, L.; Aaltonen, L.M.; Vihinen-Ranta, M.; Ilmarinen, T.; Söderlund-Venermo, M.; Hedman, K.; Perdomo, M.F. Extinct Type of Human Parvovirus B19 Persists in Tonsillar B Cells. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Hara, S.; Kimura, H.; Hoshino, Y.; Tanaka, N.; Nishikawa, K.; Ihira, M.; Yoshikawa, T.; Morishima, T. Detection of Herpesvirus DNA in the Serum of Immunocompetent Children. Microbiol. Immunol. 2002, 46, 177–180. [Google Scholar] [CrossRef]
- Aalto, S.M.; Juvonen, E.; Tarkkanen, J.; Volin, L.; Ruutu, T.; Mattila, P.S.; Piiparinen, H.; Knuutila, S.; Hedman, K. Lymphoproliferative Disease after Allogeneic Stem Cell Transplantation—Pre-Emptive Diagnosis by Quantification of Epstein-Barr Virus DNA in Serum. J. Clin. Virol. 2003, 28, 275–283. [Google Scholar] [CrossRef]
- Kullberg-Lindh, C.; Olofsson, S.; Brune, M.; Lindh, M. Comparison of Serum and Whole Blood Levels of Cytomegalovirus and Epstein-Barr Virus DNA. Transpl. Infect. Dis. 2008, 10, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Puhakka, L.; Sarvikivi, E.; Lappalainen, M.; Surcel, H.M.; Saxen, H. Decrease in Seroprevalence for Herpesviruses among Pregnant Women in Finland: Cross-Sectional Study of Three Time Points 1992, 2002 and 2012. Infect. Dis. Auckl. 2016, 48, 406–410. [Google Scholar] [CrossRef]
- Focosi, D.; Antonelli, G.; Pistello, M.; Maggi, F. Torquetenovirus: The Human Virome from Bench to Bedside. Clin. Microbiol. Infect. 2016, 22, 589–593. [Google Scholar] [CrossRef]
- Balfour, H.H.; Sifakis, F.; Sliman, J.A.; Knight, J.A.; Schmeling, D.O.; Thomas, W. Age-Specific Prevalence of Epstein-Barr Virus Infection among Individuals Aged 6-19 Years in the United States and Factors Affecting Its Acquisition. J. Infect. Dis. 2013, 208, 1286–1293. [Google Scholar] [CrossRef]
- Rostgaard, K.; Balfour, H.H.; Jarrett, R.; Erikstrup, C.; Pedersen, O.; Ullum, H.; Nielsen, L.P.; Voldstedlund, M.; Hjalgrim, H. Primary Epstein-Barr Virus Infection with and without Infectious Mononucleosis. PLoS ONE 2019, 14, e0226436. [Google Scholar] [CrossRef]
- Theall, B.; Wang, H.; Kuremsky, C.A.; Cho, E.; Hardin, K.; Robelot, L.; Marucci, J.; Mullenix, S.; Lemoine, N.; Johannsen, N.M.; et al. Allostatic Stress Load and CMV Serostatus Impact Immune Response to Maximal Exercise in Collegiate Swimmers. J. Appl. Physiol. 2020, 128, 178–188. [Google Scholar] [CrossRef]
- Mabilangan, C.; Burton, C.; O’brien, S.; Plitt, S.; Eurich, D.; Preiksaitis, J. Using Blood Donors and Solid Organ Transplant Donors and Recipients to Estimate the Seroprevalence of Cytomegalovirus and Epstein–Barr Virus in Canada: A Cross-Sectional Study. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2020, 5, 158–176. [Google Scholar] [CrossRef]
- Pyöriä, L.; Jokinen, M.; Toppinen, M.; Salminen, H.; Vuorinen, T.; Hukkanen, V.; Schmotz, C.; Elbasani, E.; Ojala, P.M.; Hedman, K.; et al. HERQ-9 Is a New Multiplex PCR for Differentiation and Quantification of All Nine Human Herpesviruses. mSphere 2020, 5, e00265-20. [Google Scholar] [CrossRef] [PubMed]
- Toppinen, M.; Pratas, D.; Väisänen, E.; Söderlund-Venermo, M.; Hedman, K.; Perdomo, M.F.; Sajantila, A. The Landscape of Persistent Human DNA Viruses in Femoral Bone. Forensic Sci. Int. Genet. 2020, 48, 102353. [Google Scholar] [CrossRef]
| Athletes (n = 27) | Controls (n = 27) | |
|---|---|---|
| Age | 27.1 (20.0–40.9) | 27.4 (20.7–40.4) |
| Female | 13 (48%) | 13 (48%) |
| BMI | 22.05 (18.0–24.8) | 24.0 (18.4–35.2) |
| Children (<5 years) at home | 3 (11%) | 3 (11%) |
| Training load, h/week (n = 26) | 15 (11–17) | <6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyöriä, L.; Valtonen, M.; Luoto, R.; Grönroos, W.; Waris, M.; Heinonen, O.J.; Ruuskanen, O.; Perdomo, M.F. Survey of Viral Reactivations in Elite Athletes: A Case-Control Study. Pathogens 2021, 10, 666. https://doi.org/10.3390/pathogens10060666
Pyöriä L, Valtonen M, Luoto R, Grönroos W, Waris M, Heinonen OJ, Ruuskanen O, Perdomo MF. Survey of Viral Reactivations in Elite Athletes: A Case-Control Study. Pathogens. 2021; 10(6):666. https://doi.org/10.3390/pathogens10060666
Chicago/Turabian StylePyöriä, Lari, Maarit Valtonen, Raakel Luoto, Wilma Grönroos, Matti Waris, Olli J. Heinonen, Olli Ruuskanen, and Maria F. Perdomo. 2021. "Survey of Viral Reactivations in Elite Athletes: A Case-Control Study" Pathogens 10, no. 6: 666. https://doi.org/10.3390/pathogens10060666
APA StylePyöriä, L., Valtonen, M., Luoto, R., Grönroos, W., Waris, M., Heinonen, O. J., Ruuskanen, O., & Perdomo, M. F. (2021). Survey of Viral Reactivations in Elite Athletes: A Case-Control Study. Pathogens, 10(6), 666. https://doi.org/10.3390/pathogens10060666






