Gene Regulation of Biofilm-Associated Functional Amyloids
Abstract
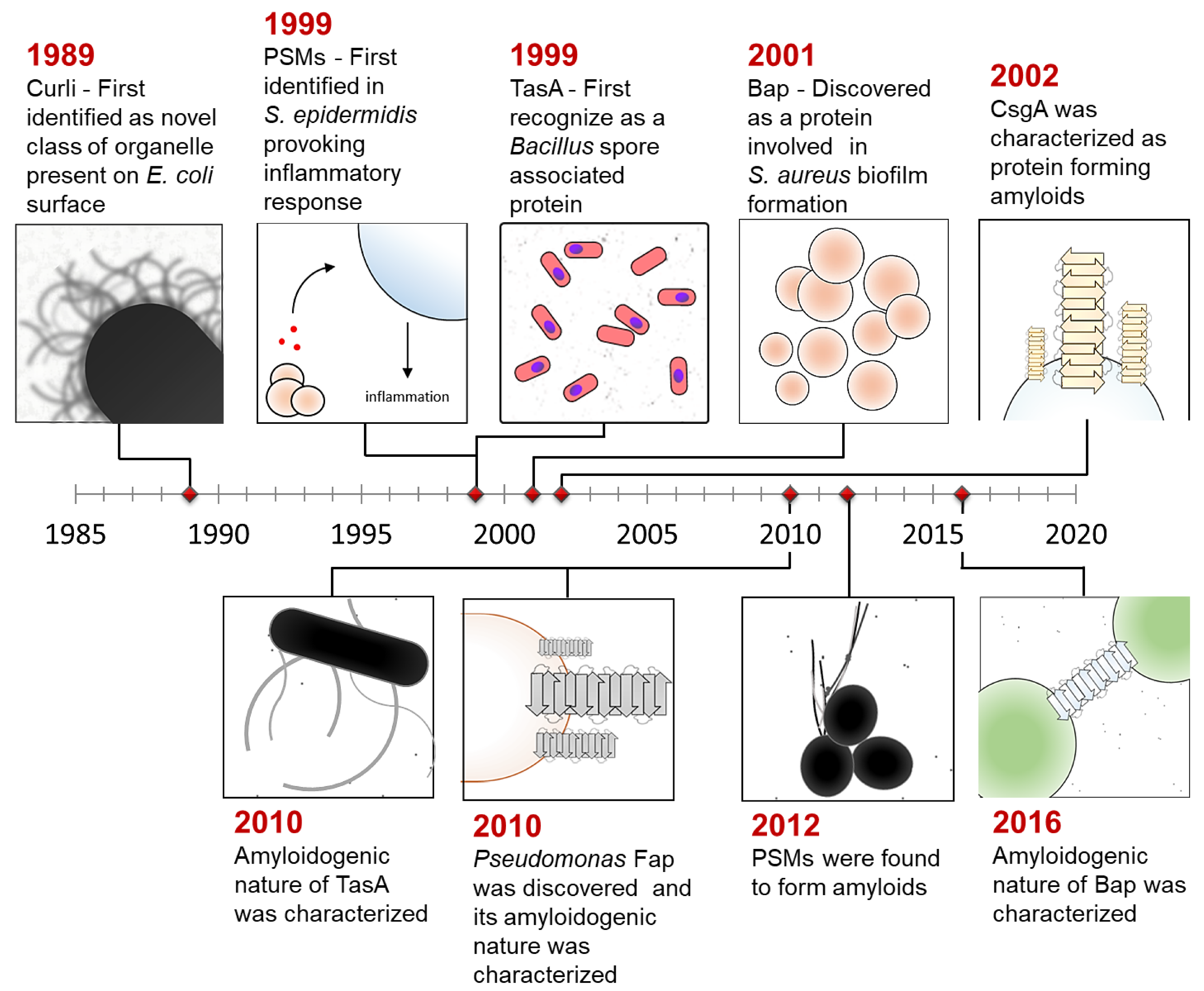
:1. Introduction
2. Biofilm Assembly and Its Gene Regulation
3. Gene Regulation of Bacterial Functional Amyloids during Biofilm Assembly
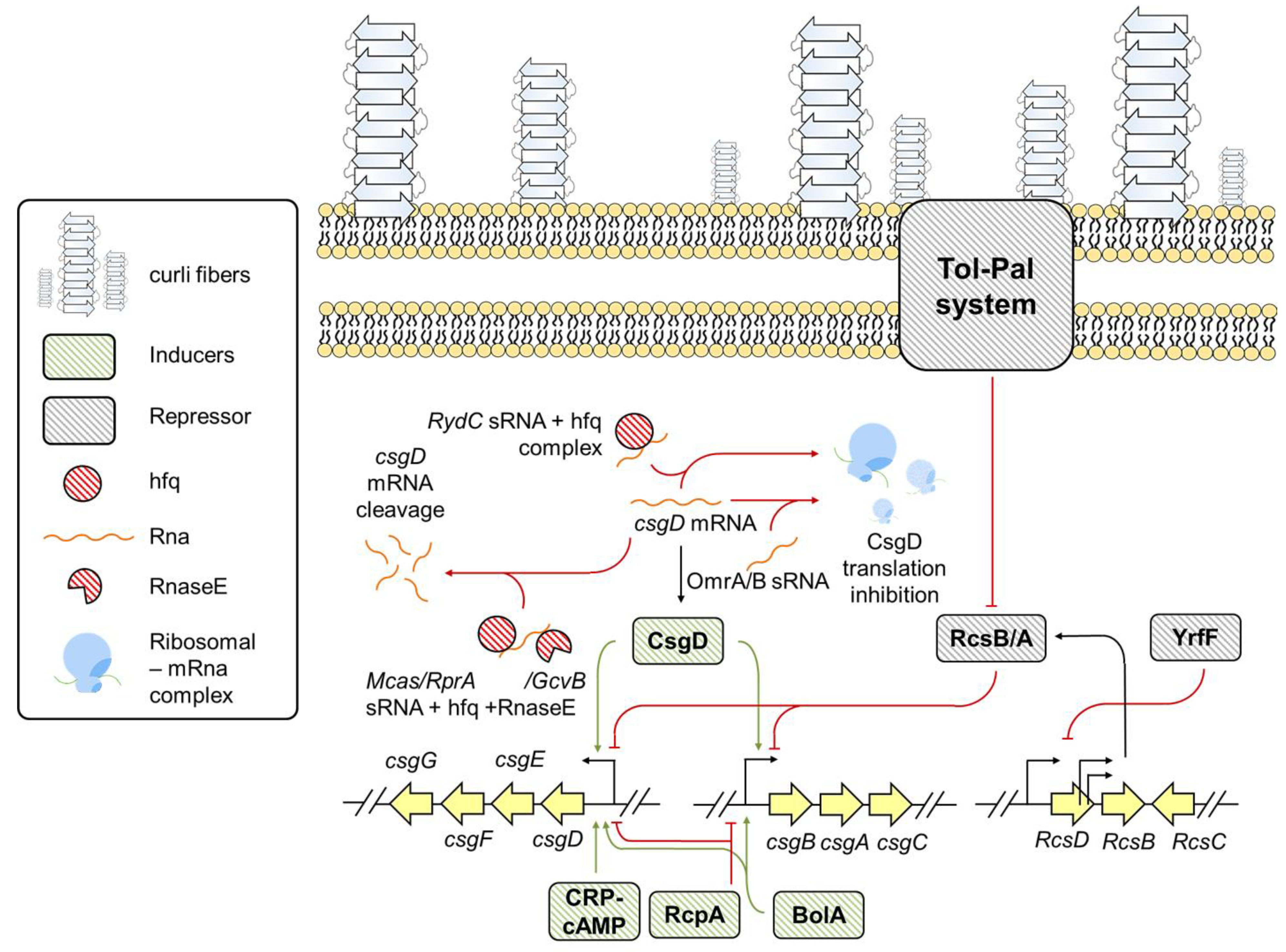
3.1. Escherichia
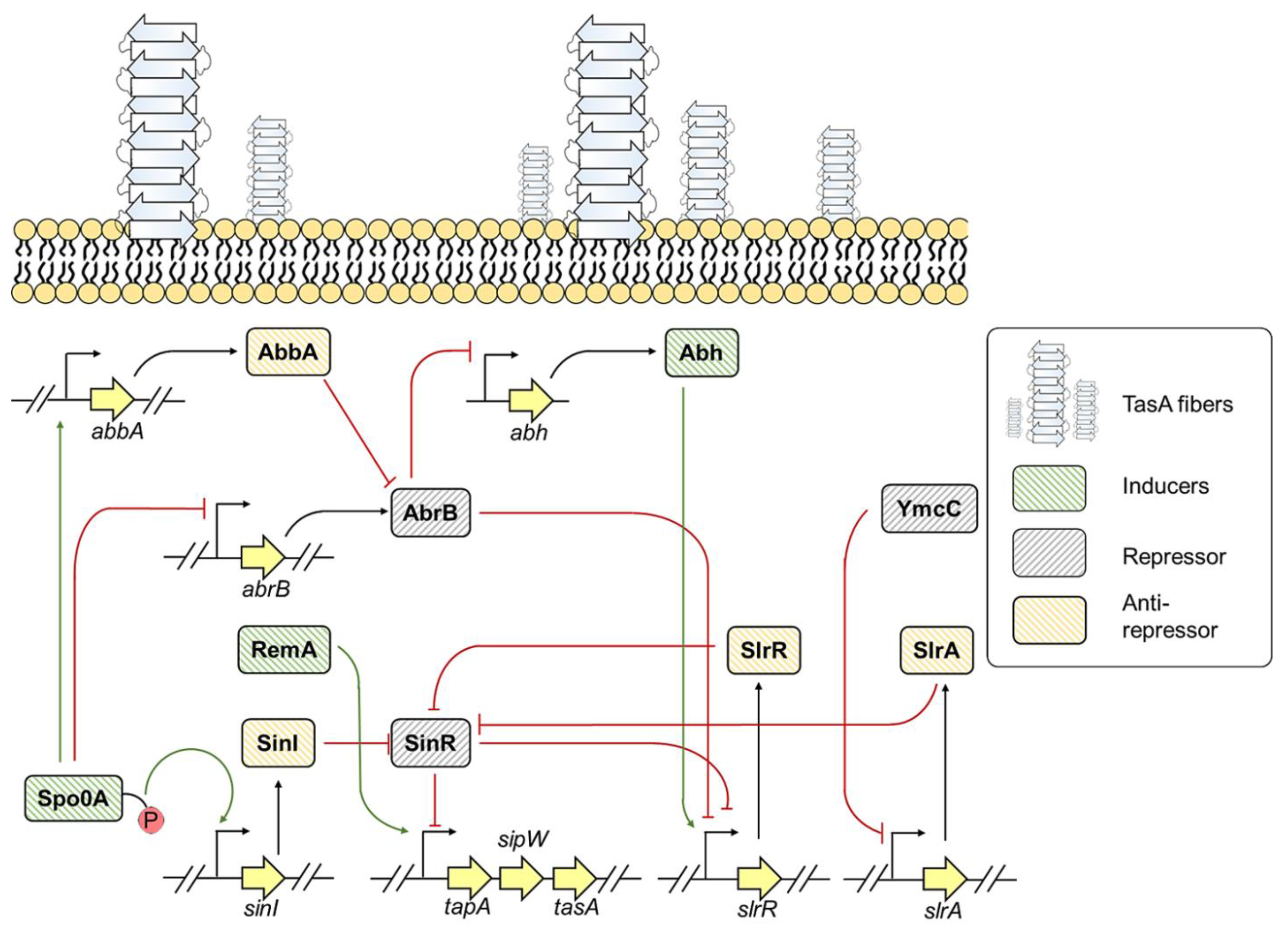
3.2. Bacillus
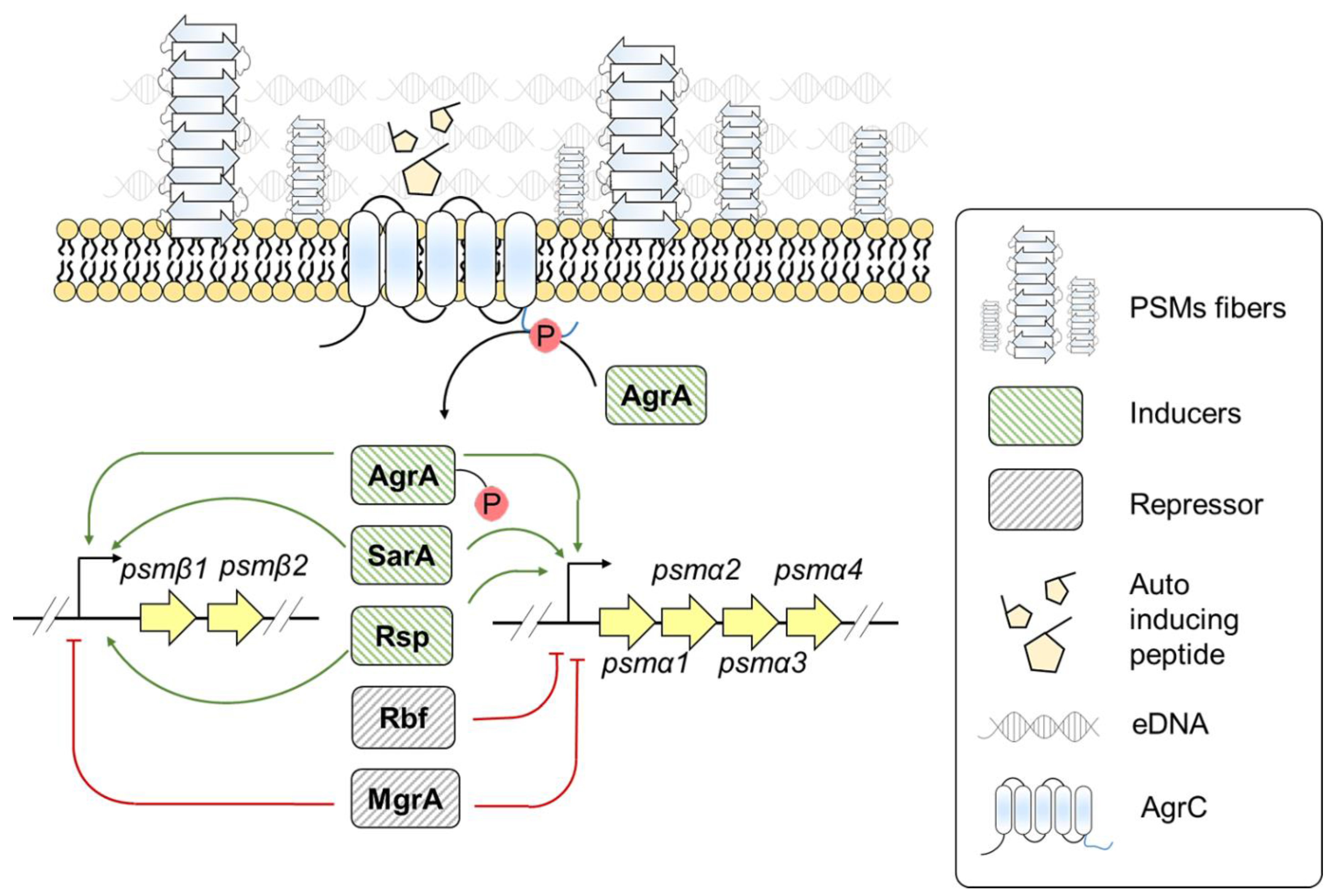
3.3. Staphylococcus
4. Targeting Functional Amyloids Transcriptional Regulation as an Anti-Biofilm Strategy
5. Environmental Factor Regulating Gene Expression of Biofilm-associated Amyloids
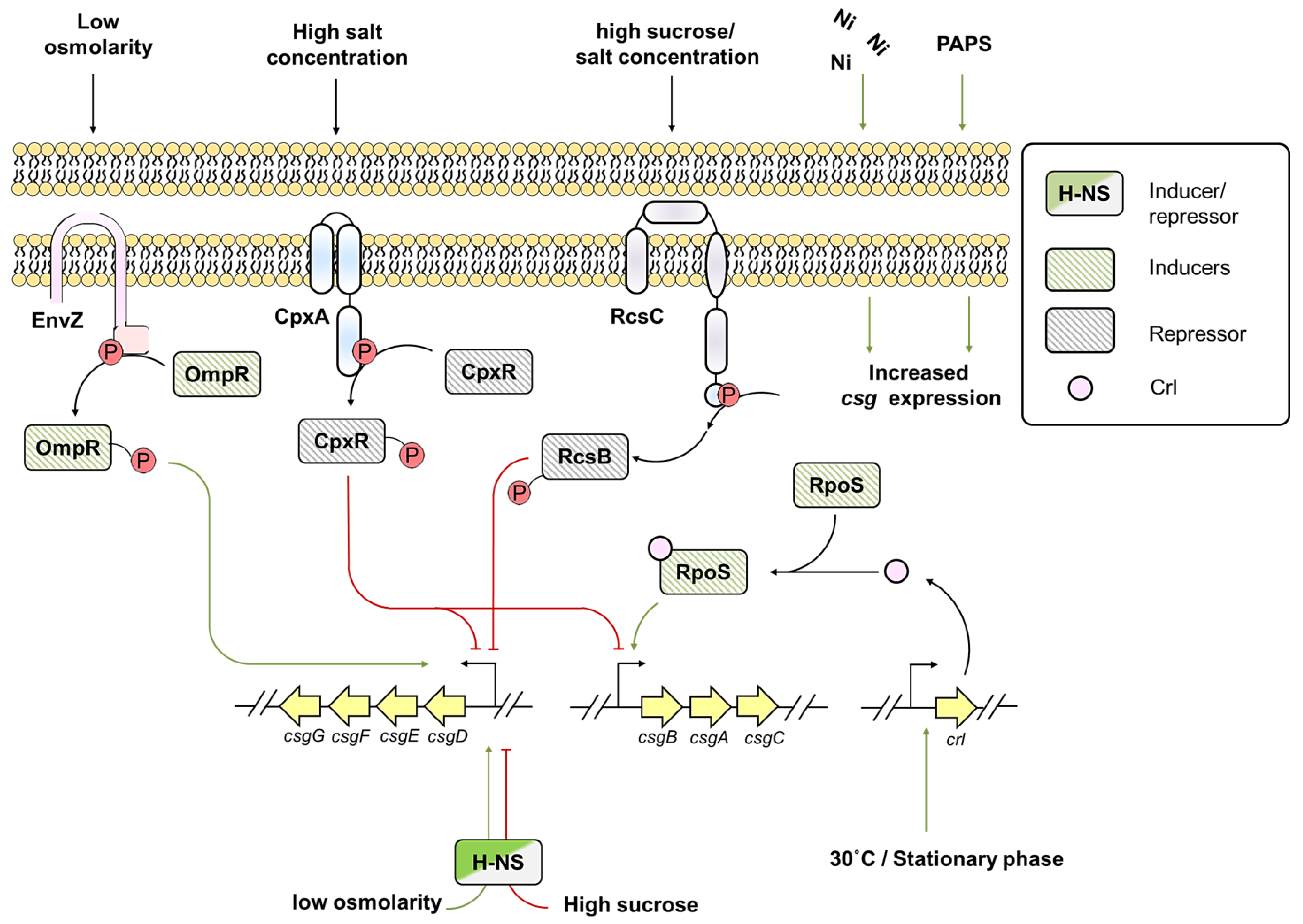
6. Environmental Factors Influencing csg Expression in E. coli
7. Environmental Factors Regulating TasA Expression in B. subtilis
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Nadell, C.D.; Xavier, J.B.; Foster, K.R. The sociobiology of biofilms. FEMS Microbiol. Rev. 2008, 33, 206–224. [Google Scholar] [CrossRef] [Green Version]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Baveye, P.; Neu, T.R.; Stoodley, P.; Szewzyk, U.; Wingender, J.; Wuertz, S. Who put the film in biofilm? the migration of a term from wastewater engineering to medicine and beyond. npj Biofilms Microbiomes 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Fong, J.N.C.; Yildiz, F.H. Biofilm matrix proteins. Microbiol. Spectr. 2015, 3, 201–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gerven, N.; Van der Verren, S.E.; Reiter, D.M.; Remaut, H. The role of functional amyloids in bacterial virulence. J. Mol. Biol. 2018, 430, 3657–3684. [Google Scholar] [CrossRef]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid—From bacteria to humans. Trends Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef]
- Deshmukh, M.; Evans, M.L.; Chapman, M.R. Amyloid by design: Intrinsic regulation of microbial amyloid assembly. J. Mol. Biol. 2018, 430, 3631–3641. [Google Scholar] [CrossRef]
- Otzen, D.; Riek, R. Functional amyloids. Cold Spring Harb. Perspect. Biol. 2019, 11, a033860. [Google Scholar] [CrossRef]
- Balistreri, A.; Goetzler, E.; Chapman, M. Functional amyloids are the rule rather than the exception in cellular biology. Microorganisms 2020, 8, 1951. [Google Scholar] [CrossRef]
- Gebbink, M.F.B.G.; Claessen, D.; Bouma, B.; Dijkhuizen, L.; Wösten, H.A.B. Amyloids--a functional coat for microorganisms. Nat. Rev. Microbiol. 2005, 3, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueholm, M.S.; Petersen, S.V.; Sønderkær, M.; Larsen, P.; Christiansen, G.; Hein, K.L.; Enghild, J.J.; Nielsen, J.L.; Nielsen, K.L.; Nielsen, P.H.; et al. Functional amyloid in Pseudomonas. Mol. Microbiol. 2010, 77, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Romero, D.; Aguilar, C.; Losick, R.; Kolter, R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 2230–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, K.; Syed, A.K.; Stephenson, R.E.; Rickard, A.H.; Boles, B.R. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012, 8, e1002744. [Google Scholar] [CrossRef]
- Taglialegna, A.; Navarro, S.; Ventura, S.; Garnett, J.A.; Matthews, S.; Penades, J.R.; Lasa, I.; Valle, J. Staphylococcal bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog. 2016, 12, e1005711. [Google Scholar] [CrossRef] [Green Version]
- Dragoš, A.; Kovács, Á.T.; Claessen, D. The role of functional amyloids in multicellular growth and development of gram-positive bacteria. Biomolecules 2017, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Vidakovic, L.; Singh, P.K.; Hartmann, R.; Nadell, C.D.; Drescher, K. Dynamic biofilm architecture confers individual and collective mechanisms of viral protection. Nat. Microbiol. 2018, 3, 26–31. [Google Scholar] [CrossRef]
- Tursi, S.A.; Tükel, Ç. Curli-containing enteric biofilms inside and out: Matrix composition, immune recognition, and disease implications. Microbiol. Mol. Biol. Rev. 2018, 82, e00028-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, L.; Tükel, Ç. Bacterial amyloids: The link between bacterial infections and autoimmunity. Trends Microbiol. 2019, 27, 954–963. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Rudnicka, Z.; Markowska, K. Genetic control of bacterial biofilms. J. Appl. Genet. 2016, 57, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef]
- Prigent-Combaret, C.; Prensier, G.; Le Thi, T.T.; Vidal, O.; Lejeune, P.; Dorel, C. Developmental Pathway for biofilm formation in curli-producing Escherichia coli strains: Role of flagella, curli and colanic acid. Environ. Microbiol. 2000, 2, 450–464. [Google Scholar] [CrossRef]
- Hufnagel, D.A.; Depas, W.H.; Chapman, M.R. The biology of the Escherichia coli extracellular matrix. Microbiol. Spectr. 2015, 3, 249–267. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Chapman, M.R. Bacterial functional amyloids: Order from disorder. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 954–960. [Google Scholar] [CrossRef]
- Bhoite, S.; van Gerven, N.; Chapman, M.R.; Remaut, H. Curli biogenesis: Bacterial amyloid assembly by the Type VIII secretion pathway. Ecosal Plus 2019, 8, 163–171. [Google Scholar]
- Hammer, N.D.; Schmidt, J.C.; Chapman, M.R. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc. Natl. Acad. Sci. USA 2007, 104, 12494–12499. [Google Scholar] [CrossRef] [Green Version]
- Hammer, N.D.; McGuffie, B.A.; Zhou, Y.; Badtke, M.P.; Reinke, A.A.; Brännström, K.; Gestwicki, J.E.; Olofsson, A.; Almqvist, F.; Chapman, M.R. The c-terminal repeating units of CsgB direct bacterial functional amyloid nucleation. J. Mol. Biol. 2012, 422, 376–389. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.L.; Chorell, E.; Taylor, J.D.; Åden, J.; Götheson, A.; Li, F.; Koch, M.; Sefer, L.; Matthews, S.J.; Wittung-Stafshede, P.; et al. The bacterial curli system possesses a potent and selective inhibitor of amyloid formation. Mol. Cell 2015, 57, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, P.; Krasteva, P.V.; Van Gerven, N.; Gubellini, F.; Van den Broeck, I.; Troupiotis-Tsaïlaki, A.; Jonckheere, W.; Péhau-Arnaudet, G.; Pinkner, J.S.; Chapman, M.R.; et al. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature 2014, 516, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Nenninger, A.A.; Robinson, L.S.; Hammer, N.D.; Epstein, E.A.; Badtke, M.P.; Hultgren, S.J.; Chapman, M.R. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Mol. Microbiol. 2011, 81, 486–499. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.D.; Shu, Q.; Cusumano, Z.T.; Nagamatsu, K.; Gualberto, N.C.; Lynch, A.J.L.; Wu, C.; Wang, W.; Jain, N.; Pinkner, J.S.; et al. Structure-function analysis of the curli accessory protein CsgE defines surfaces essential for coordinating amyloid fiber formation. MBio 2018, 9, e01349-18. [Google Scholar] [CrossRef] [Green Version]
- Schubeis, T.; Spehr, J.; Viereck, J.; Köpping, L.; Nagaraj, M.; Ahmed, M.; Ritter, C. Structural and functional characterization of the curli adaptor protein CsgF. FEBS Lett. 2018, 592, 1020–1029. [Google Scholar] [CrossRef] [Green Version]
- Barnhart, M.M.; Chapman, M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.F.; Knowles, T.P.J.; Dobson, C.M.; Macphee, C.E.; Welland, M.E. Characterization of the nanoscale properties of individual amyloid fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811. [Google Scholar] [CrossRef] [Green Version]
- Brombacher, E.; Baratto, A.; Dorel, C.; Landini, P. Gene expression regulation by the curli activator csgd protein: Modulation of cellulose biosynthesis and control of negative determinants for microbial adhesion. J. Bacteriol. 2006, 188, 2027–2037. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, H.; Ishizuka, T.; Hotta, S.; Aoki, M.; Shimada, T.; Ishihama, A. novel regulators of the CsgD gene encoding the master regulator of biofilm formation in Escherichia coli k-12. Microbiology 2020, 166, 880–890. [Google Scholar] [CrossRef]
- Hufnagel, D.A.; Evans, M.L.; Greene, S.E.; Pinkner, J.S.; Hultgren, S.J.; Chapman, M.R. The catabolite repressor protein-cyclic amp complex regulates CsgD and biofilm formation in uropathogenic Escherichia coli. J. Bacteriol. 2016, 198, 3329–3334. [Google Scholar] [CrossRef] [Green Version]
- Holmqvist, E.; Reimegård, J.; Sterk, M.; Grantcharova, N.; Römling, U.; Wagner, E.G.H. Two antisense RNAs target the transcriptional regulator CsgD to inhibit curli synthesis. EMBO J. 2010, 29, 1840–1850. [Google Scholar] [CrossRef] [Green Version]
- Bordeau, V.; Felden, B. Curli synthesis and biofilm formation in enteric bacteria are controlled by a dynamic small RNA module made up of a pseudoknot assisted by an RNA chaperone. Nucleic Acids Res. 2014, 42, 4682–4696. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, P.R.; Pettersen, J.S.; Szczerba, M.; Valentin-Hansen, P.; Møller-Jensen, J.; Jørgensen, M.G. SRNA-dependent control of curli biosynthesis in Escherichia coli: McaS directs endonucleolytic cleavage of CsgD MRNA. Nucleic Acids Res. 2018, 46, 6746–6760. [Google Scholar] [CrossRef]
- Gerding, M.A.; Ogata, Y.; Pecora, N.D.; Niki, H.; de Boer, P.A.J. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol. Microbiol. 2007, 63, 1008–1025. [Google Scholar] [CrossRef] [Green Version]
- Vianney, A.; Jubelin, G.; Renault, S.; Dorel, C.; Lejeune, P.; Lazzaroni, J.C. Escherichia coli Tol and Rcs genes participate in the complex network affecting curli synthesis. Microbiology 2005, 151, 2487–2497. [Google Scholar] [CrossRef] [Green Version]
- Dressaire, C.; Moreira, R.N.; Barahona, S.; Alves de Matos, A.P.; Arraiano, C.M. BolA is a transcriptional switch that turns off motility and turns on biofilm development. MBio 2015, 6, e02352-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azam, M.W.; Zuberi, A.; Khan, A.U. BolA gene involved in curli amyloids and fimbriae production in E. coli: Exploring pathways to inhibit biofilm and amyloid formation. J. Biol. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Nhu, N.T.K.; Phan, M.-D.; Peters, K.M.; Lo, A.W.; Forde, B.M.; Min Chong, T.; Yin, W.-F.; Chan, K.-G.; Chromek, M.; Brauner, A.; et al. Discovery of new genes involved in curli production by a uropathogenic Escherichia coli strain from the highly virulent O45:K1:H7 lineage. MBio 2018, 9, e01462-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, L.S.; Hobley, L.; Stanley-Wall, N.R. Biofilm formation by Bacillus subtilis: New insights into regulatory strategies and assembly mechanisms. Mol. Microbiol. 2014, 93, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Branda, S.S.; Chu, F.; Kearns, D.B.; Losick, R.; Kolter, R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006, 59, 1229–1238. [Google Scholar] [CrossRef]
- Romero, D.; Vlamakis, H.; Losick, R.; Kolter, R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol. Microbiol. 2011, 80, 1155–1168. [Google Scholar] [CrossRef] [Green Version]
- Terra, R.; Stanley-Wall, N.R.; Cao, G.; Lazazzera, B.A. Identification of Bacillus subtilis SipW as a bifunctional signal peptidase that controls surface-adhered biofilm formation. J. Bacteriol. 2012, 194, 2781–2790. [Google Scholar] [CrossRef] [Green Version]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, F.; Kearns, D.B.; McLoon, A.; Chai, Y.; Kolter, R.; Losick, R. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol. Microbiol. 2008, 68, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, J.A.; Rodrigues, C.; Lewis, R.J. Molecular basis of the activity of SinR Protein, the master regulator of biofilm formation in Bacillus subtilis. J. Biol. Chem. 2013, 288, 10766–10778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol. Microbiol. 2008, 69, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Norman, T.; Kolter, R.; Losick, R. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev. 2010, 24, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumsakul, O.; Takahashi, H.; Oshima, T.; Hishimoto, T.; Kanaya, S.; Ogasawara, N.; Ishikawa, S. Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog abh reveals their interactive role in transcriptional regulation. Nucleic Acids Res. 2011, 39, 414–428. [Google Scholar] [CrossRef]
- Tucker, A.T.; Bobay, B.G.; Banse, A.V.; Olson, A.L.; Soderblom, E.J.; Moseley, M.A.; Thompson, R.J.; Varney, K.M.; Losick, R.; Cavanagh, J. A DNA mimic: The structure and mechanism of action for the anti-repressor protein AbbA. J. Mol. Biol. 2014, 426, 1911–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauch, M.; Webb, V.; Spiegelman, G.; Hoch, J.A. The SpoOA protein of Bacillus subtilis is a repressor of the AbrB gene. Proc. Natl. Acad. Sci. USA 1990, 87, 1801–1805. [Google Scholar] [CrossRef] [Green Version]
- Murray, E.J.; Strauch, M.A.; Stanley-Wall, N.R. SigmaX is involved in controlling Bacillus subtilis biofilm architecture through the AbrB homologue Abh. J. Bacteriol. 2009, 191, 6822–6832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Y.; Kolter, R.; Losick, R. Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis. Mol. Microbiol. 2009, 74, 876–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkelman, J.T.; Blair, K.M.; Kearns, D.B. RemA (YlzA) and RemB (YaaB) regulate extracellular matrix operon expression and biofilm formation in Bacillus subtilis. J. Bacteriol. 2009, 191, 3981–3991. [Google Scholar] [CrossRef] [Green Version]
- Winkelman, J.T.; Bree, A.C.; Bate, A.R.; Eichenberger, P.; Gourse, R.L.; Kearns, D.B. RemA is a DNA-binding protein that activates biofilm matrix gene expression in Bacillus subtilis. Mol. Microbiol. 2013, 88, 984–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal biofilm: Adhesins, regulation, and host response. In Virulence Mechanisms of Bacterial Pathogens; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2016; pp. 529–566. ISBN 9781683670711. [Google Scholar]
- Peschel, A.; Otto, M. Phenol-soluble modulins and Staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef]
- Le, K.Y.; Villaruz, A.E.; Zheng, Y.; He, L.; Fisher, E.L.; Nguyen, T.H.; Ho, T.V.; Yeh, A.J.; Joo, H.-S.; Cheung, G.Y.C.; et al. Role of phenol-soluble modulins in Staphylococcus epidermidis biofilm formation and infection of indwelling medical devices. J. Mol. Biol. 2019, 431, 3015–3027. [Google Scholar] [CrossRef]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.-H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Dastgheyb, S.S.; Villaruz, A.E.; Le, K.Y.; Tan, V.Y.; Duong, A.C.; Chatterjee, S.S.; Cheung, G.Y.C.; Joo, H.-S.; Hickok, N.J.; Otto, M. Role of phenol-soluble modulins in formation of Staphylococcus aureus biofilms in synovial fluid. Infect. Immun. 2015, 83, 2966–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Khan, B.A.; Cheung, G.Y.C.; Bach, T.-H.L.; Jameson-Lee, M.; Kong, K.-F.; Queck, S.Y.; Otto, M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Invest. 2011, 121, 238–248. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Kretschmer, D.; Queck, S.Y.; Joo, H.-S.; Wang, R.; Duong, A.C.; Nguyen, T.H.; Bach, T.-H.L.; Porter, A.R.; DeLeo, F.R.; et al. Insight into structure-function relationship in phenol-soluble modulins using an alanine screen of the phenol-soluble modulin (PSM) A3 peptide. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Kizaki, H.; Omae, Y.; Tabuchi, F.; Saito, Y.; Sekimizu, K.; Kaito, C. Cell-surface phenol soluble modulins regulate Staphylococcus aureus colony spreading. PLoS ONE 2016, 11, e0164523. [Google Scholar] [CrossRef]
- Marinelli, P.; Pallares, I.; Navarro, S.; Ventura, S. Dissecting the contribution of Staphylococcus aureus α-phenol-soluble modulins to biofilm amyloid structure. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Tayeb-Fligelman, E.; Tabachnikov, O.; Moshe, A.; Goldshmidt-Tran, O.; Sawaya, M.R.; Coquelle, N.; Colletier, J.-P.; Landau, M. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science 2017, 355, 831–833. [Google Scholar] [CrossRef]
- Salinas, N.; Colletier, J.-P.; Moshe, A.; Landau, M. Extreme amyloid polymorphism in Staphylococcus aureus virulent PSMα peptides. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, K.; Ganesan, M.; Payne, D.E.; Solomon, M.J.; Boles, B.R. Extracellular DNA facilitates the formation of functional amyloids in Staphylococcus aureus biofilms. Mol. Microbiol. 2016, 99, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Trotonda, M.P.; Tamber, S.; Memmi, G.; Cheung, A.L. MgrA represses biofilm formation in Staphylococcus aureus. Infect. Immun. 2008, 76, 5645–5654. [Google Scholar] [CrossRef] [Green Version]
- Paulander, W.; Varming, A.N.; Bojer, M.S.; Friberg, C.; Bæk, K.; Ingmer, H. The Agr quorum sensing system in Staphylococcus aureus cells mediates death of sub-population. BMC Res. Notes 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Fang, B.; Liu, B.; Sun, B. Transcriptional regulation of virulence factors Hla and phenol-soluble modulins α by AraC-type regulator Rbf in Staphylococcus aureus. Int. J. Med. Microbiol. 2020, 310, 151436. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, B. Rsp promotes the transcription of virulence factors in an agr-independent manner in Staphylococcus aureus. Emerg. Microbes Infect. 2020, 9, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, M.; Fang, X.; Zhen, S.; Zhang, J.; Yang, X.; Qiao, L.; Yang, Y.; Zhang, C. Expression and regulation of phenol-soluble modulins and enterotoxins in foodborne Staphylococcus aureus. AMB Express 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Jiang, Q.; Jin, Z.; Sun, B. MgrA negatively regulates biofilm formation and detachment by repressing the expression of Psm operons in Staphylococcus aureus. Appl. Environ. Microbiol. 2018, 84, e01008-18. [Google Scholar] [CrossRef] [Green Version]
- Cucarella, C.; Solano, C.; Valle, J.; Amorena, B.; Lasa, I.; Penadés, J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001, 183, 2888–2896. [Google Scholar] [CrossRef] [Green Version]
- Ubeda, C.; Tormo, M.A.; Cucarella, C.; Trotonda, P.; Foster, T.J.; Lasa, I.; Penadés, J.R. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol. Microbiol. 2003, 49, 193–210. [Google Scholar] [CrossRef]
- Trotonda, M.P.; Manna, A.C.; Cheung, A.L.; Lasa, I.; Penadés, J.R. SarA positively controls bap-dependent biofilm formation in Staphylococcus Aureus. J. Bacteriol. 2005, 187, 5790–5798. [Google Scholar] [CrossRef] [Green Version]
- Ziebandt, A.-K.; Becher, D.; Ohlsen, K.; Hacker, J.; Hecker, M.; Engelmann, S. The Influence of Agr and SigmaB in growth phase dependent regulation of virulence factors in Staphylococcus aureus. Proteomics 2004, 4, 3034–3047. [Google Scholar] [CrossRef]
- Ziebuhr, W.; Krimmer, V.; Rachid, S.; Lössner, I.; Götz, F.; Hacker, J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: Evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol. Microbiol. 1999, 32, 345–356. [Google Scholar] [CrossRef]
- Tormo, M.Á.; Úbeda, C.; Martí, M.; Maiques, E.; Cucarella, C.; Valle, J.; Foster, T.J.; Lasa, Í.; Penadés, J.R. Phase-variable expression of the biofilm-associated protein (Bap) in Staphylococcus aureus. Microbiology 2007, 153, 1702–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Bowler, P.G. Antibiotic resistance and biofilm tolerance: A combined threat in the treatment of chronic infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Ravikumar, R.; Santhosh, R.S.; Princy, S. SarA based novel therapeutic candidate against Staphylococcus aureus associated with vascular graft infections. Front. Microbiol. 2015, 6, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balamurugan, P.; Praveen Krishna, V.; Bharath, D.; Lavanya, R.; Vairaprakash, P.; Adline Princy, S. Staphylococcus aureus quorum regulator SarA targeted compound, 2-[(Methylamino) Methyl] phenol inhibits biofilm and down-regulates virulence genes. Front. Microbiol. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xue, Y.J.; Duan, X.J.; Shao, S.H. Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Valliammai, A.; Selvaraj, A.; Yuvashree, U.; Aravindraja, C.; Karutha Pandian, S. SarA-dependent antibiofilm activity of thymol enhances the antibacterial efficacy of rifampicin against Staphylococcus aureus. Front. Microbiol. 2020, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Valliammai, A.; Sethupathy, S.; Priya, A.; Selvaraj, A.; Bhaskar, J.P.; Krishnan, V.; Pandian, S.K. 5-Dodecanolide interferes with biofilm formation and reduces the virulence of Methicillin-resistant Saphylococcus aureus (MRSA) through up regulation of Agr system. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Joo, H.-S.; Duong, A.C.; Bach, T.-H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the ΣE-dependent sRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [CrossRef] [Green Version]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A gut bacterial amyloid promotes a-synuclein aggregation and motor impairment in mice. Elife 2020, 9, e53111. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558. [Google Scholar] [CrossRef]
- Ferreira, N.; Cardoso, I.; Domingues, M.R.; Vitorino, R.; Bastos, M.; Bai, G.; Saraiva, M.J.; Almeida, M.R. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett. 2009, 583, 3569–3576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzen, N.; Nielsen, S.B.; Yoshimura, Y.; Vad, B.S.; Andersen, C.B.; Betzer, C.; Kaspersen, J.D.; Christiansen, G.; Pedersen, J.S.; Jensen, P.H. How epigallocatechin gallate can inhibit α-synuclein oligomer toxicity in vitro. J. Biol. Chem. 2014, 289, 21299–21310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, N.; Pereira-Henriques, A.; Almeida, M.R. Transthyretin chemical chaperoning by flavonoids: Structure–activity insights towards the design of potent amyloidosis inhibitors. Biochem. Biophys. Rep. 2015, 3, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndjoko, K.; Wolfender, J.-L.; Hostettmann, K. Determination of trace amounts of ginkgolic acids in Ginkgo biloba L. leaf extracts and phytopharmaceuticals by liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 249–255. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Ryu, S.Y.; Cho, M.H.; Lee, J. Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157: H7 and Staphylococcus aureus biofilm formation. Int. J. Food Microbiol. 2014, 174, 47–55. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157: H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157: H7. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Regmi, S.C.; Kim, J.-A.; Cho, M.H.; Yun, H.; Lee, C.-S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Park, J.-H.; Kim, J.-A.; Neupane, G.P.; Cho, M.H.; Lee, C.-S.; Lee, J. Low concentrations of honey reduce biofilm formation, quorum sensing, and virulence in Escherichia coli O157: H7. Biofouling 2011, 27, 1095–1104. [Google Scholar] [CrossRef]
- Dorel, C.; Vidal, O.; Prigent-Combaret, C.; Vallet, I.; Lejeune, P. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 1999, 178, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougdour, A.; Lelong, C.; Geiselmann, J. Crl, A low temperature-induced protein in Escherichia coli that binds directly to the stationary phase sigma subunit of RNA polymerase. J. Biol. Chem. 2004, 279, 19540–19550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jubelin, G.; Vianney, A.; Beloin, C.; Ghigo, J.-M.; Lazzaroni, J.-C.; Lejeune, P.; Dorel, C. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 2005, 187, 2038–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigent-Combaret, C.; Brombacher, E.; Vidal, O.; Ambert, A.; Lejeune, P.; Landini, P.; Dorel, C. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the CsgD gene. J. Bacteriol. 2001, 183, 7213–7223. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, H.; Yamada, K.; Kori, A.; Yamamoto, K.; Ishihama, A. Regulation of the Escherichia coli CsgD promoter: Interplay between five transcription factors. Microbiology 2010, 156, 2470–2483. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Bayles, D.O.; Alt, D.P.; Looft, T.; Brunelle, B.W.; Stasko, J.A. Disruption of RcsB by a duplicated sequence in a curli-producing Escherichia coli O157:H7 results in differential gene expression in relation to biofilm formation, stress responses and metabolism. BMC Microbiol. 2017, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.Q.; Parker, C.T.; Louie, J.W.; Huynh, S.; Fagerquist, C.K.; Mandrell, R.E. RcsB contributes to the distinct stress fitness among Escherichia coli O157:H7 curli variants of the 1993 hamburger-associated outbreak strains. Appl. Environ. Microbiol. 2012, 78, 7706–7719. [Google Scholar] [CrossRef] [Green Version]
- May, T.; Okabe, S. Escherichia coli harboring a natural IncF conjugative F plasmid develops complex mature biofilms by stimulating synthesis of colanic acid and curli. J. Bacteriol. 2008, 190, 7479–7490. [Google Scholar] [CrossRef] [Green Version]
- Perrin, C.; Briandet, R.; Jubelin, G.; Lejeune, P.; Mandrand-Berthelot, M.-A.; Rodrigue, A.; Dorel, C. Nickel promotes biofilm formation by Escherichia coli K-12 strains that produce curli. Appl. Environ. Microbiol. 2009, 75, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.; Motta, S.; Mauri, P.; Landini, P. Sulfate assimilation pathway intermediate phosphoadenosine 59-phosphosulfate acts as a signal molecule affecting production of curli fibres in Escherichia coli. Microbiology 2014, 160, 1832–1844. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Martin, E.; Leyh, T.S. GTPase activation of ATP sulfurylase: The Mechanism. Biochemistry 1994, 33, 2042–2047. [Google Scholar] [CrossRef]
- Shivaprasad, D.P.; Taneja, N.K.; Lakra, A.; Sachdev, D. In vitro and in situ abrogation of biofilm formation in E. coli by vitamin C through ROS generation, disruption of quorum sensing and exopolysaccharide production. Food Chem. 2021, 341, 128171. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, S.M.; Kolodkin-Gal, I.; McLoon, A.; Chai, L.; Kolter, R.; Losick, R.; Weitz, D.A. Osmotic pressure can regulate matrix gene expression in Bacillus subtilis. Mol. Microbiol. 2012, 86, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, D.; Vlamakis, H.; Losick, R.; Kolter, R. Cannibalism enhances biofilm development in Bacillus subtilis. Mol. Microbiol. 2009, 74, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, C.; Vlamakis, H.; Guzman, A.; Losick, R.; Kolter, R. KinD is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms. MBio 2010, 1, e00035-10. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khambhati, K.; Patel, J.; Saxena, V.; A, P.; Jain, N. Gene Regulation of Biofilm-Associated Functional Amyloids. Pathogens 2021, 10, 490. https://doi.org/10.3390/pathogens10040490
Khambhati K, Patel J, Saxena V, A P, Jain N. Gene Regulation of Biofilm-Associated Functional Amyloids. Pathogens. 2021; 10(4):490. https://doi.org/10.3390/pathogens10040490
Chicago/Turabian StyleKhambhati, Khushal, Jaykumar Patel, Vijaylaxmi Saxena, Parvathy A, and Neha Jain. 2021. "Gene Regulation of Biofilm-Associated Functional Amyloids" Pathogens 10, no. 4: 490. https://doi.org/10.3390/pathogens10040490
APA StyleKhambhati, K., Patel, J., Saxena, V., A, P., & Jain, N. (2021). Gene Regulation of Biofilm-Associated Functional Amyloids. Pathogens, 10(4), 490. https://doi.org/10.3390/pathogens10040490




