Role of the β-Catenin/REG Iα Axis in the Proliferation of Sessile Serrated Adenoma/Polyps Associated with Fusobacterium nucleatum
Abstract
1. Introduction
2. Results
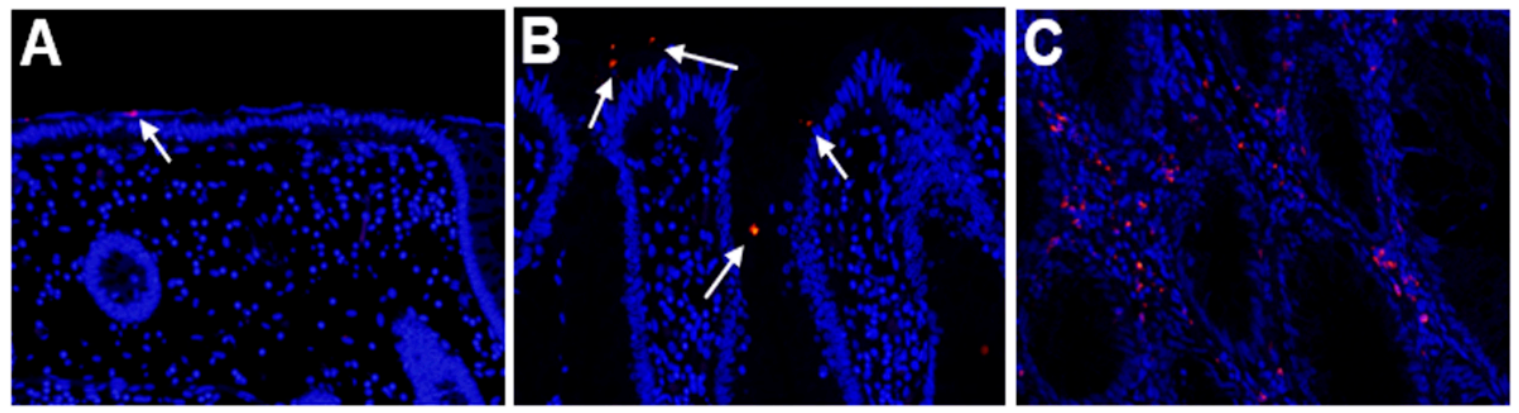
2.1. Detection of Fusobacterium Nucleatum in SSA/P Lesions
2.2. Expression of REG Iα and β-Catenin in SSA/P Lesions
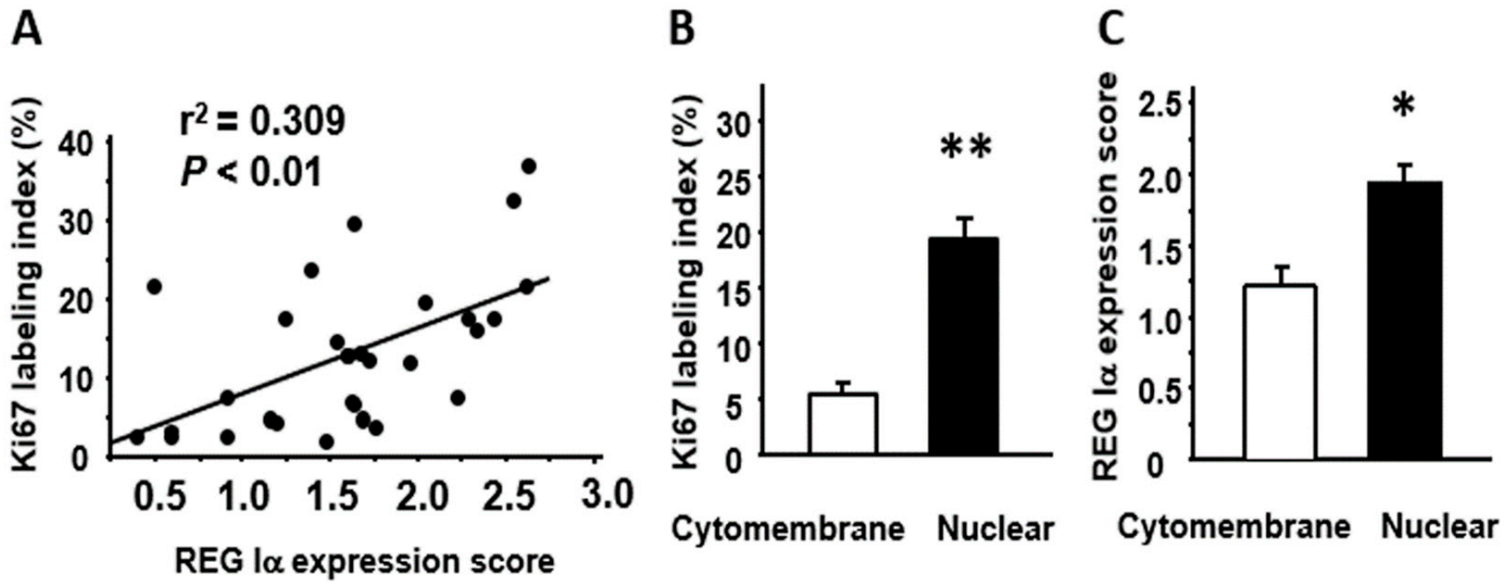
2.3. Relationships among REG Iα/β-Catenin Expression, Fusobacterium Nucleatum Abundance, and Proliferative Activity in SSA/P Lesions
2.4. Relationship among Histology, Tumor Size, and REG Iα/β-catenin/Ki67 Expression in SSA/P Lesions
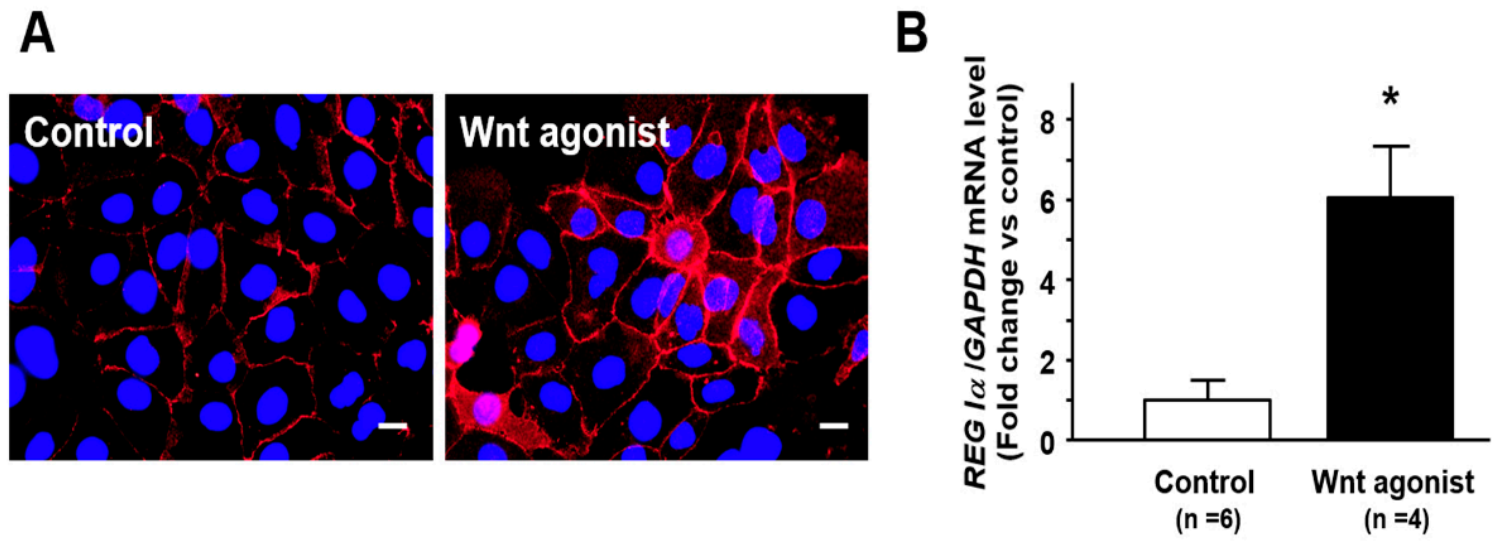
2.5. Effect of Wnt Signaling on Nuclear β-Catenin Translocation and REG Iα Expression in Caco2 Cells
3. Discussion
4. Materials and Methods
4.1. Tissue Specimens and Clinicopathological Examination
4.2. Immunohistochemistry
4.3. Evaluation of Immunostaining
4.4. Fluorescence In Situ Hybridization (FISH)
4.5. Stimulation for Caco2 Cells by Wnt Agonist SKL2001
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powell, S.M.; Zilz, N.; Beazer-Barclay, Y.; Bryan, T.M.; Hamilton, S.R.; Thibodeau, S.N.; Vogelstein, B.; Kinzler, K.W. APC mutations occur early during colorectal tumorigenesis. Nature 1992, 359, 235–237. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Yang, S.; Mack, C.; Xu, H.; Huang, C.S.; Mulcahy, E.; Amorosino, M.; Farraye, F.A. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am. J. Surg. Pathol. 2006, 30, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Leggett, B.; Whitehall, V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010, 138, 2088–2100. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kitamura, S.; Kimura, T.; Nakagawa, T.; Sogabe, M.; Miyamoto, H.; Muguruma, N.; Takayama, T. Clinicopathological characteristics of serrated polyps as precursors to colorectal cancer: Current status and management. J. Gastroenterol. Hepatol. 2017, 32, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Shida, Y.; Ichikawa, K.; Fujimori, T.; Fujimori, Y.; Tomita, S.; Fujii, T.; Sano, Y.; Oda, Y.; Goto, H.; Ohta, A.; et al. Differentiation between sessile serrated adenoma/polyp and non-sessile serrated adenoma/polyp in large hyper plastic polyp: A Japanese collaborative study. Mol. Clin. Oncol. 2013, 1, 53–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, M.; Chan, A.T.; Sun, J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liska, V.; Bruha, J.; Neary, P.; Dezeeuw, N.; Tommasino, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638. [Google Scholar] [CrossRef]
- Terazono, K.; Yamamoto, H.; Takasawa, S.; Shiga, K.; Yonemura, Y.; Tochino, Y.; Okamoto, H. A novel gene activated in regenerating islets. J. Biol. Chem. 1988, 263, 2111–2114. [Google Scholar] [CrossRef]
- Unno, M.; Yonekura, H.; Nakagawara, K.; Watanabe, T.; Miyashita, H.; Moriizumi, S.; Okamoto, H.; Itoh, T.; Teraoka, H. Structure, chromosomal localization, and expression of mouse reg genes, reg I and reg II. A novel type of reg gene, reg II, exists in the mouse genome. J. Biol. Chem. 1993, 268, 15974–15982. [Google Scholar] [CrossRef]
- Takasawa, S. Regenerating gene (REG) product and its potential clinical usage. Expert Opin. Ther. Targets 2016, 20, 541–550. [Google Scholar] [CrossRef]
- Sekikawa, A.; Fukui, H.; Suzuki, K.; Karibe, T.; Fujii, S.; Ichikawa, K.; Tomita, S.; Imura, J.; Shiratori, K.; Chiba, T.; et al. Involvement of the IL-22/REG Iα axis in ulcerative colitis. Lab. Invest. 2010, 90, 496–505. [Google Scholar] [CrossRef]
- Van Beelen Granlund, A.; Østvik, A.E.; Brenna, Ø.; Torp, S.H.; Gustafsson, B.I.; Sandvik, A.K. REG gene expression in inflamed and healthy colon mucosa explored by in situ hybridization. Cell Tissue Res. 2013, 352, 639–646. [Google Scholar] [CrossRef]
- Sun, C.; Fukui, H.; Hara, K.; Kitayama, Y.; Eda, H.; Yang, M.; Yamagishi, H.; Tomita, T.; Oshima, T.; Watari, J.; et al. Expression of Reg family genes in the gastrointestinal tract of mice treated with indomethacin. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G736–G744. [Google Scholar] [CrossRef]
- Xu, X.; Fukui, H.; Ran, Y.; Wang, X.; Inoue, Y.; Ebisudani, N.; Nishimura, H.; Tomita, T.; Oshima, T.; Watari, J.; et al. The link between type III Reg and STAT3-associated cytokines in inflamed colonic tissues. Mediat. Inflamm. 2019, 2019, 7859460. [Google Scholar] [CrossRef]
- Yamagishi, H.; Fukui, H.; Sekikawa, A.; Kono, T.; Fujii, S.; Ichikawa, K.; Tomita, S.; Imura, J.; Hiraishi, H.; Chiba, T.; et al. Expression profile of REG family proteins REG Iα and REG IV in advanced gastric cancer: Comparison with mucin phenotype and prognostic markers. Mod. Pathol. 2009, 22, 906–913. [Google Scholar] [CrossRef]
- Zheng, H.C.; Sugawara, A.; Okamoto, H.; Takasawa, S.; Takahashi, H.; Masuda, S.; Takano, Y. Expression profile of the REG gene family in colorectal carcinoma. J. Histochem. Cytochem. 2011, 59, 106–115. [Google Scholar] [CrossRef]
- Sekikawa, A.; Fukui, H.; Zhang, X.; Maruo, T.; Tsumura, T.; Okabe, Y.; Wakasa, T.; Osaki, Y.; Chiba, T.; Tomita, T.; et al. REG Iα is a biomarker for predicting response to chemotherapy with S-1 plus cisplatin in patients with unresectable stage IV gastric cancer. Br. J. Cancer 2013, 108, 395–401. [Google Scholar] [CrossRef]
- Bishnupuri, K.S.; Luo, Q.; Korzenik, J.R.; Henderson, J.O.; Houchen, C.W.; Anant, S.; Dieckgraefe, B.K. Dysregulation of Reg gene expression occurs early in gastrointestinal tumorigenesis and regulates anti-apoptotic genes. Cancer Biol. Ther. 2006, 5, 1714–1720. [Google Scholar] [CrossRef]
- Nanakin, A.; Fukui, H.; Fujii, S.; Sekikawa, A.; Kanda, N.; Hisatsune, H.; Seno, H.; Konda, Y.; Fujimori, T.; Chiba, T. Expression of the REG IV gene in ulcerative colitis. Lab. Invest. 2007, 87, 304–314. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Wang, J.; Guo, J. Role of regenerating islet-derived protein 3A in gastrointestinal cancer. Front. Oncol. 2019, 9, 1449. [Google Scholar] [CrossRef]
- Fukui, H.; Kinoshita, Y.; Maekawa, T.; Okada, A.; Waki, S.; Hassan, S.; Okamoto, H.; Chiba, T. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology 1998, 115, 1483–1493. [Google Scholar] [CrossRef]
- Sekikawa, A.; Fukui, H.; Fujii, S.; Takeda, J.; Nanakin, A.; Hisatsune, H.; Seno, H.; Takasawa, S.; Okamoto, H.; Fujimori, T.; et al. REG Iα protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology 2005, 128, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Fukui, H.; Fujii, S.; Nanakin, A.; Kanda, N.; Uenoyama, Y.; Sawabu, T.; Hisatsune, H.; Kusaka, T.; Ueno, S.; et al. Possible role of REG Iα protein in ulcerative colitis and colitic cancer. Gut 2005, 54, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Fukui, H.; Fujii, S.; Ichikawa, K.; Tomita, S.; Imura, J.; Chiba, T.; Fujimori, T. REG Iα protein mediates an anti-apoptotic effect of STAT3 signaling in gastric cancer cells. Carcinogenesis 2008, 29, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Mitomi, H.; Saito, T.; Takahashi, M.; Sakamoto, N.; Fukui, N.; Yao, T.; Watanabe, S. Distinct WNT/β-catenin signaling activation in the serrated neoplasia pathway and the adenoma-carcinoma sequence of the colorectum. Mod. Pathol. 2015, 28, 146–158. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Mansoor, A.; Koro, K.; Zhang, Q.; Minoo, P. Expression profiling reveals involvement of Wnt pathway in the malignant progression of sessile serrated adenomas. Am. J. Pathol. 2019, 189, 1732–1743. [Google Scholar] [CrossRef]
- Cavard, C.; Terris, B.; Grimber, G.; Christa, L.; Audard, V.; Radenen-Bussiere, B.; Simon, M.T.; Renard, C.A.; Buendia, M.A.; Perret, C. Overexpression of regenerating islet-derived 1 alpha and 3 alpha genes in human primary liver tumors with beta-catenin mutations. Oncogene 2006, 25, 599–608. [Google Scholar] [CrossRef]
- Hakata, Y.; Fukui, H.; Sekikawa, A.; Yamagishi, H.; Ichikawa, K.; Tomita, S.; Imura, J.; Kawamata, H.; Imai, Y.; Fujimori, T. Expression of β-catenin and REG Iα in relation to cell proliferative ability in salivary gland tumors. Exp. Ther. Med. 2010, 1, 437–443. [Google Scholar] [CrossRef]
- Sayed, I.M.; Sahan, A.Z.; Venkova, T.; Chakraborty, A.; Mukhopadhyay, D.; Bimczok, D.; Beswick, E.J.; Reyes, V.E.; Pinchuk, I.; Sahoo, D.; et al. Helicobacter pylori infection downregulates the DNA glycosylase NEIL2, resulting in increased genome damage and inflammation in gastric epithelial cells. J. Biol. Chem. 2020, 295, 11082–11098. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Fu, X.; Zhou, X.; Peng, Y.; Shi, L.; Chen, T.; Wu, Y. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int. J. Cancer 2016, 139, 1318–1326. [Google Scholar] [CrossRef]
- Sayed, I.M.; Chakraborty, A.; Abd El-Hafeez, A.A.; Sharma, A.; Sahan, A.Z.; Huang, W.J.M.; Sahoo, D.; Ghosh, P.; Hazra, T.K.; Das, S. The DNA glycosylase NEIL2 suppresses Fusobacterium-infection-induced inflammation and DNA damage in colonic epithelial cells. Cells 2020, 9, 1980. [Google Scholar] [CrossRef]
- Fujita, K.; Yamamoto, H.; Matsumoto, T.; Hirahashi, M.; Gushima, M.; Kishimoto, J.; Nishiyama, K.; Taguchi, T.; Yao, T.; Oda, Y. Sessile serrated adenoma with early neoplastic progression: A clinicopathologic and molecular study. Am. J. Surg. Pathol. 2011, 35, 295–304. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Y.; Yu, J.; Chen, T.; Wu, Y.; Shi, L.; Li, Q.; Wu, J.; Fu, X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 2017, 8, 31802–31814. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Vishnoi, K.; Viswakarma, N.; Rana, A.; Rana, B. Transcription factors in cancer development and therapy. Cancers 2020, 12, 2296. [Google Scholar] [CrossRef]
- Yachida, S.; Mudali, S.; Martin, S.A.; Montgomery, E.A.; Iacobuzio-Donahue, C.A. Beta-catenin nuclear labeling is a common feature of sessile serrated adenomas and correlates with early neoplastic progression after BRAF activation. Am. J. Surg. Pathol. 2009, 33, 1823–1832. [Google Scholar] [CrossRef]
- Murakami, T.; Akazawa, Y.; Yatagai, N.; Hiromoto, T.; Sasahara, N.; Saito, T.; Sakamoto, N.; Nagahara, A.; Yao, T. Molecular characterization of sessile serrated adenoma/polyps with dysplasia/carcinoma based on immunohistochemistry, next-generation sequencing, and microsatellite instability testing: A case series study. Diagn. Pathol. 2018, 13, 88. [Google Scholar] [CrossRef]
- Murakami, T.; Sakamoto, N.; Nagahara, A. Clinicopathological features, diagnosis, and treatment of sessile serrated adenoma/polyp with dysplasia/carcinoma. J. Gastroenterol. Hepatol. 2019, 34, 1685–1695. [Google Scholar] [CrossRef]
- Chen, Z.; Downing, S.; Tzanakakis, E.S. Four decades after the discovery of regenerating islet-derived (Reg) proteins: Current understanding and challenges. Front. Cell Dev. Biol. 2019, 7, 235. [Google Scholar] [CrossRef]
- Zenilman, M.E.; Kim, S.; Levine, B.A.; Lee, C.; Steinberg, J.J. Ectopic expression of reg protein: A marker of colorectal mucosa at risk for neoplasia. J. Gastrointest. Surg. 1997, 1, 194–202. [Google Scholar] [CrossRef]
- Rechreche, H.; Montalto, G.; Mallo, G.V.; Vasseur, S.; Marasa, L.; Soubeyran, P.; Dagorn, J.C.; Iovanna, J.L. pap, reg I alpha and reg I beta mRNAs are concomitantly up-regulated during human colorectal carcinogenesis. Int. J. Cancer 1999, 81, 688–694. [Google Scholar] [CrossRef]
- Jing, D.; Kehoe, D.E.; Tzanakakis, E.S. Expression of Reg family proteins in embryonic stem cells and its modulation by Wnt/beta-catenin signaling. Stem Cells Dev. 2010, 19, 1307–1319. [Google Scholar] [CrossRef]
- Lin, R.; Han, C.; Ding, Z.; Shi, H.; He, R.; Liu, J.; Qian, W.; Zhang, Q.; Fu, X.; Deng, X.; et al. Knock down of BMSC-derived Wnt3a or its antagonist analogs attenuate colorectal carcinogenesis induced by chronic Fusobacterium nucleatum infection. Cancer Lett. 2020, 495, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, J.; Yu, T.; Fang, X.; Lou, L.; Xin, S.; Ji, L.; Jiang, F.; Lou, Y. Fusobacterium nucleatum promotes the progression of colorectal cancer through Cdk5-activated Wnt/β-catenin signaling. Front. Microbiol. 2021, 11, 545251. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Fukui, H.; Sun, C.; Kitayama, Y.; Eda, H.; Yamasaki, T.; Kondo, T.; Tomita, T.; Oshima, T.; Watari, J.; et al. Effect of REG Iα protein on angiogenesis in gastric cancer tissues. Oncol. Rep. 2015, 33, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Fujimori, T.; Yamaguchi, T.; Ichikawa, K.; Tomita, S.; Sugai, T.; Imura, J.; Ohkura, Y.; Yao, T.; Fujii, S.; et al. Overexpression of regenerating gene Iα appears to reflect aberration of crypt cell compartmentalization in sessile serrated adenoma/polyps of the colon. Diagn. Pathol. 2013, 8, 187. [Google Scholar] [CrossRef]
- Loy, A.; Arnold, R.; Tischler, P.; Rattei, T.; Wagner, M.; Horn, M. ProbeCheck-a central resource for evaluating oligonucleotide probe coverage and specificity. Environ. Microbiol. 2008, 10, 2894–2898. [Google Scholar] [CrossRef]

| Fusobacterium nucleatum | p Value | ||
|---|---|---|---|
| Negative | Positive | ||
| β-catenin expression | 0.001 | ||
| Cytomembrane type | 10 | 2 | |
| Nuclear type | 4 | 14 | |
| REG Iα expression score | 1.23 ± 0.15 | 1.85 ± 0.14 | <0.01 |
| Ki67 labeling index | 7.0 ± 1.3 | 16.8 ± 2.3 | <0.01 |
| Dysplastic Change | p Value | ||
|---|---|---|---|
| (−) | (+) | ||
| β-catenin expression | 0.025 | ||
| Cytomembrane type | 9 | 3 | |
| Nuclear type | 6 | 12 | |
| REG Iα expression score | 1.35 ± 0.16 | 1.85 ± 0.15 | 0.019 |
| Ki67 labeling index | 10.7 ± 2.5 | 15.1 ± 2.3 | 0.130 |
| Gender | ||
|---|---|---|
| Male | 16 | |
| Female | 14 | |
| Age (years, mean ± SE, range) | 64.5 ± 2.2 (31–83) | |
| Tumor location | ||
| Right side | 26 | |
| Left side | 4 | |
| Tumor size (mm, mean ± SE, range) | 21.1 ± 1.5 (6–40) | |
| Endoscopic morphology | ||
| Is/Isp | 2 | |
| IIa | 14 | |
| LST-NG | 14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, H.; Fukui, H.; Wang, X.; Ebisutani, N.; Nakanishi, T.; Tomita, T.; Oshima, T.; Hirota, S.; Miwa, H. Role of the β-Catenin/REG Iα Axis in the Proliferation of Sessile Serrated Adenoma/Polyps Associated with Fusobacterium nucleatum. Pathogens 2021, 10, 434. https://doi.org/10.3390/pathogens10040434
Nishimura H, Fukui H, Wang X, Ebisutani N, Nakanishi T, Tomita T, Oshima T, Hirota S, Miwa H. Role of the β-Catenin/REG Iα Axis in the Proliferation of Sessile Serrated Adenoma/Polyps Associated with Fusobacterium nucleatum. Pathogens. 2021; 10(4):434. https://doi.org/10.3390/pathogens10040434
Chicago/Turabian StyleNishimura, Heihachiro, Hirokazu Fukui, Xuan Wang, Nobuhiko Ebisutani, Takashi Nakanishi, Toshihiko Tomita, Tadayuki Oshima, Seiichi Hirota, and Hiroto Miwa. 2021. "Role of the β-Catenin/REG Iα Axis in the Proliferation of Sessile Serrated Adenoma/Polyps Associated with Fusobacterium nucleatum" Pathogens 10, no. 4: 434. https://doi.org/10.3390/pathogens10040434
APA StyleNishimura, H., Fukui, H., Wang, X., Ebisutani, N., Nakanishi, T., Tomita, T., Oshima, T., Hirota, S., & Miwa, H. (2021). Role of the β-Catenin/REG Iα Axis in the Proliferation of Sessile Serrated Adenoma/Polyps Associated with Fusobacterium nucleatum. Pathogens, 10(4), 434. https://doi.org/10.3390/pathogens10040434









