Application of Quantitative-PCR to Monitor Netpen Sites in British Columbia (Canada) for Tenacibaculum Species
Abstract
1. Introduction
2. Results
2.1. Bacterial Isolate Collection
2.2. qPCR Results
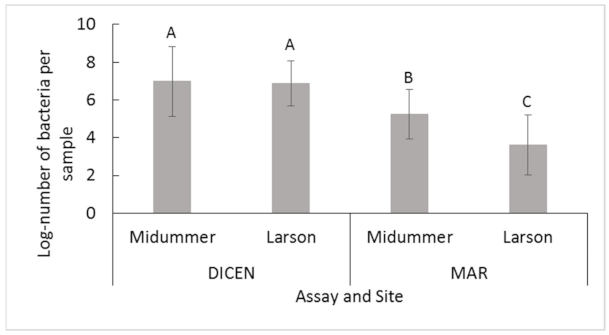
2.2.1. Midsummer and Larson Island
2.2.2. Midsummer
2.2.3. Larson
2.3. Supplemental Data Comparisons
3. Discussion
3.1. Isolates
3.2. Seasonal Comparison
3.3. Netpen Cleaning Comparison
3.4. Outbreak Status Comparison
3.5. The Primary Agent Responsible for the Larson and Midsummer Outbreaks
4. Materials and Methods
4.1. Sites Used in the Study
4.2. Sample Collection, Processing, and Preservation
4.3. Bacterial Isolate Collection
4.4. DNA Extractions and Normalization
4.5. qPCR Application
4.6. Conversion of Cq to the Number of Bacteria Per Sample
4.7. Supplementary Data
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisheries and Oceans Canada (DFO). Aquaculture Production Quantities and Value. Available online: https://www.dfo-mpo.gc.ca/stats/aqua/aqua-prod-eng.htm (accessed on 7 October 2020).
- Nowlan, J.P.; Lumsden, J.S.; Russell, S. Advancements in Characterizing Tenacibaculum Infections in Canada. Pathogens 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R.; Toranzo, A.E.; Magariños, B. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: A review. Dis. Aquat. Org. 2006, 71, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Nondahl, T. Where Does the Money Go? [President’s Message]. IEEE Ind. Appl. Mag. 2009, 15, 4. [Google Scholar] [CrossRef]
- Frisch, K.; Småge, S.B.; Vallestad, C.; Duesund, H.; Brevik, Ø.J.; Klevan, A.; Olsen, R.H.; Sjaatil, S.T.; Gauthier, D.; Brudeseth, B.; et al. Experimental induction of mouthrot in Atlantic salmon smolts usingTenacibaculum maritimumfrom Western Canada. J. Fish. Dis. 2018, 41, 1247–1258. [Google Scholar] [CrossRef]
- Frisch, K.; Småge, S.B.; Johansen, R.; Duesund, H.; Brevik, Ø.J.; Nylund, A. Pathology of experimentally induced mouthrot caused by Tenacibaculum maritimum in Atlantic salmon smolts. PLoS ONE 2018, 13, e0206951. [Google Scholar] [CrossRef]
- Piñeiro-Vidal, M.; Carballas, C.G.; Gómez-Barreiro, O.; Riaza, A.; Santos, Y. Tenacibaculum soleae sp. nov., isolated from diseased sole (Solea senegalensis Kaup). Int. J. Syst. Evol. Microbiol. 2008, 58, 881–885. [Google Scholar] [CrossRef]
- Piñeiro-Vidal, M.; Centeno-Sestelo, G.; Riaza, A.; Santos, Y. Isolation of pathogenic Tenacibaculum maritimum-related or-ganisms from diseased turbot and sole cultured in the Northwest of Spain. Bull. Eur. Assoc. Fish Pathol. 2007, 27, 29–35. [Google Scholar]
- Piñeiro-Vidal, M.; Gijón, D.; Zarza, C.; Santos, Y. Tenacibaculum dicentrarchi sp. nov., a marine bacterium of the family Flavobacteriaceae isolated from European sea bass. Int. J. Syst. Evol. Microbiol. 2012, 62, 425–429. [Google Scholar] [CrossRef]
- Piñeiro-Vidal, M.; Riaza, A.; Santos, Y. Tenacibaculum discolor sp. nov. and Tenacibaculum gallaicum sp. nov., isolated from sole (Solea senegalensis) and turbot (Psetta maxima) culture systems. Int. J. Syst. Evol. Microbiol. 2008, 58, 21–25. [Google Scholar] [CrossRef]
- Burioli, E.A.V.; Varello, K.; Trancart, S.; Bozzetta, E.; Gorla, A.; Prearo, M.; Houssin, M. First description of a mortality event in adult Pacific oysters in Italy associated with infection by a Tenacibaculum soleae strain. J. Fish Dis. 2017, 41, 215–221. [Google Scholar] [CrossRef]
- Sakatoku, A.; Fujimura, T.; Ito, M.; Takashima, S.; Isshiki, T. Newly isolated bacterium Tenacibaculum sp. strain Pbs-1 from diseased pearl oysters is associated with black-spot shell disease. Aquaculture 2018, 493, 61–67. [Google Scholar] [CrossRef]
- Irgang, R.; González-Luna, R.; Gutiérrez, J.; Poblete-Morales, M.; Rojas, V.; Tapia-Cammas, D.; Avendaño-Herrera, R. First identification and characterization ofTenacibaculum dicentrarchiisolated from Chilean red conger eel (Genypterus chilensis,Guichenot 1848). J. Fish Dis. 2017, 40, 1915–1920. [Google Scholar] [CrossRef]
- Barker, D.E.; Braden, L.M.; Coombs, M.P.; Boyce, B. Preliminary studies on the isolation of bacteria from sea lice, Lepeophtheirus salmonis, infecting farmed salmon in British Columbia, Canada. Parasitol. Res. 2009, 105, 1173–1177. [Google Scholar] [CrossRef]
- Chistoserdov, A.Y.; Quinn, R.A.; Gubbala, S.L.; Smolowitz, R. Bacterial communities associated with lesions of shell dis-ease in the American lobster, Homarus americanus Milne-Edwards. J. Shellfish Res. 2012, 31, 449–462. [Google Scholar] [CrossRef]
- Sheu, S.Y.; Lin, K.Y.; Chou, J.H.; Chang, P.S.; Arun, A.B.; Young, C.C.; Chen, W.M. Tenacibaculum litopenaei sp. nov., isolat-ed from a shrimp mariculture pond. Int. J. Syst. Evol. Microbiol. 2007, 57, 1148–1153. [Google Scholar] [CrossRef]
- Delannoy, C.M.; Houghton, J.D.; Fleming, N.E.; Ferguson, H.W. Mauve Stingers (Pelagia noctiluca) as carriers of the bacterial fish pathogen Tenacibaculum maritimum. Aquaculture 2011, 311, 255–257. [Google Scholar] [CrossRef]
- Fringuelli, E.; Savage, P.D.; Gordon, A.; Baxter, E.J.; Rodger, H.D.; Graham, D.A. Development of a quantitative re-al-time PCR for the detection of Tenacibaculum maritimum and its application to field samples. J. Fish Dis. 2012, 35, 579–590. [Google Scholar] [CrossRef]
- Småge, S.B.; Brevik, Ø.J.; Frisch, K.; Watanabe, K.; Duesund, H.; Nylund, A. Concurrent jellyfish blooms and tenacibac-ulosis outbreaks in Northern Norwegian Atlantic salmon (Salmo salar) farms. PLoS ONE 2017, 12, e0187476. [Google Scholar]
- Hooper, R.; Brealey, J.C.; Van Der Valk, T.; Alberdi, A.; Durban, J.W.; Fearnbach, H.; Robertson, K.M.; Baird, R.W.; Hanson, M.B.; Wade, P.; et al. Host-derived population genomics data provides insights into bacterial and diatom composition of the killer whale skin. Mol. Ecol. 2018, 28, 484–502. [Google Scholar] [CrossRef]
- Frette, L.; Jørgensen, N.O.G.; Irming, H.; Kroer, N. Tenacibaculum skagerrakense sp. nov., a marine bacterium isolated from the pelagic zone in Skagerrak, Denmark. Int. J. Syst. Evol. Microbiol. 2004, 54, 519–524. [Google Scholar] [CrossRef]
- Teramoto, M.; Zhai, Z.; Komatsu, A.; Shibayama, K.; Suzuki, M. Genome sequence of the psychrophilic bacterium Tenaci-baculum ovolyticum strain da5A-8 isolated from deep seawater. Genome Announc. 2016, 4, e00644-16. [Google Scholar] [CrossRef] [PubMed]
- Webb, S. Influence of Eelgrasses on the Microbial Community Structure in San Diego Bay Waters. Master’s Thesis, UC San Diego, San Diego, CA, USA, 2018. [Google Scholar]
- Levipan, H.A.; Irgang, R.; Tapia-Cammas, D.; Avendaño-Herrera, R. A high-throughput analysis of biofilm formation by the fish pathogen Tenacibaculum dicentrarchi. J. Fish Dis. 2019, 42, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Levipan, H.A.; Tapia-Cammas, D.; Molina, V.; Irgang, R.; Toranzo, A.E.; Magariños, B.; Avendaño-Herrera, R. Biofilm development and cell viability: An undervalued mechanism in the persistence of the fish pathogen Tenacibaculum maritimum. Aquaculture 2019, 511, 734267. [Google Scholar] [CrossRef]
- Levipan, H.A.; Avendaño-Herrera, R. Different Phenotypes of Mature Biofilm in Flavobacterium psychrophilum Share a Potential for Virulence That Differs from Planktonic State. Front. Cell. Infect. Microbiol. 2017, 7, 76. [Google Scholar] [CrossRef]
- Grothusen, H.; Castillo, A.; Henríquez, P.; Navas, E.; Bohle, H.; Araya, C.; Bustamante, F.; Bustos, P.; Mancilla, M. First Complete Genome Sequence of Tenacibaculum dicentrarchi, an Emerging Bacterial Pathogen of Salmonids. Genome Announc. 2016, 4, 01756-15. [Google Scholar] [CrossRef]
- Bridel, S.; Olsen, A.-B.; Nilsen, H.; Bernardet, J.-F.; Achaz, G.; Avendaño-Herrera, R.; Duchaud, E. Comparative Genomics of Tenacibaculum dicentrarchi and “Tenacibaculum finnmarkense” Highlights Intricate Evolution of Fish-Pathogenic Species. Genome Biol. Evol. 2018, 10, 452–457. [Google Scholar] [CrossRef]
- Habib, C.; Houel, A.; Lunazzi, A.; Bernardet, J.F.; Olsen, A.B.; Nilsen, H.; Toranzo, A.E.; Castro, N.; Nicolas, P.; Duchaud, E. Multilocus sequence analysis of the marine bacterial genus Tenacibaculum suggests parallel evolution of fish patho-genicity and endemic colonization of aquaculture systems. Appl. Environ. Microbiol. 2014, 80, 5503–5514. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Collarte, C.; Saldarriaga-Córdoba, M.; Irgang, R. New salmonid hosts for Tenacibaculum species: Expansion of tenacibaculosis in Chilean aquaculture. J. Fish Dis. 2020, 43, 1077–1085. [Google Scholar] [CrossRef]
- Nowlan, J. Quantitative-PCR Detection of Tenacibaculum Maritimum and Tenacibaculum Dicentrarchi at Netpen Sites in British Columbia (Canada). Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2020. [Google Scholar]
- Nowlan, J.P.; Lumsden, J.S.; Russell, S. Quantitative PCR for Tenacibaculum dicentrarchi and T. finnmarkense. J. Fish Dis. 2021, 00, 1–5. [Google Scholar] [CrossRef]
- Masuda, Y.; Tajima, K.; Ezura, Y. Resuscitation of Tenacibaculum sp., the causative bacterium of spotting disease of sea urchin Strongylocentroutus intermedius, from the viable but non-culturable state. Fish Sci. 2004, 70, 277–284. [Google Scholar] [CrossRef]
- Fernández-Álvarez, C.; Santos, Y. Identification and typing of fish pathogenic species of the genus Tenacibaculum. Appl. Microbiol. Biot. 2017, 102, 9973–9989. [Google Scholar] [CrossRef]
- Henson, M.W.; Pitre, D.M.; Weckhorst, J.L.; Lanclos, V.C.; Webber, A.T.; Thrash, J.C. Artificial seawater media facili-tate cultivating members of the microbial majority from the Gulf of Mexico. MSphere 2016, 1, e00028-16. [Google Scholar]
- Spearman, M.; Chan, S.; Jung, V.; Kowbel, V.; Mendoza, M.; Miranda, V.; Butler, M. Components of yeast (Sacchromyces cervisiae) extract as defined media additives that support the growth and productivity of CHO cells. J. Biotechnol. 2016, 233, 129–142. [Google Scholar] [CrossRef]
- Spearman, M.; Lodewyks, C.; Richmond, M.; Butler, M. The bioactivity and fractionation of peptide hydrolysates in cul-tures of CHO cells. Biotechnol. Prog. 2014, 30, 584–593. [Google Scholar] [CrossRef]
- Madetoja, J.; Wiklund, T. Detection of the Fish Pathogen Flavobacterium psychrophilum in Water from Fish Farms. Syst. Appl. Microbiol. 2002, 25, 259–266. [Google Scholar] [CrossRef]
- Sugahara, K.; Fujiwara-Nagata, E.; Fukuda, A.; Eguchi, M. Viable but Non-culturable State of Bacterial Cold-water Disease Pathogen Flavobacterium psychrophilum at Various Temperatures. Fish Pathol. 2010, 45, 158–163. [Google Scholar] [CrossRef]
- Arias, C.R.; LaFrentz, S.; Cai, W.; Olivares-Fuster, O. Adaptive response to starvation in the fish pathogen Flavobacterium columnare: Cell viability and ultrastructural changes. BMC Microbiol. 2012, 12, 266. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Irgang, R.; Sandoval, C.; Moreno-Lira, P.; Houel, A.; Duchaud, E.; Poblete-Morales, M.; Nicolas, P.; Ilardi, P. Isolation, Characterization and Virulence Potential of Tenacibaculum dicentrarchi in Salmonid Cultures in Chile. Transbound. Emerg. Dis. 2016, 63, 121–126. [Google Scholar] [CrossRef]
- Brosnahan, C.L.; Munday, J.S.; Ha, H.J.; Preece, M.; Jones, J.B. New Zealand rickettsia-like organism (NZ-RLO) and Te-nacibaculum maritimum: Distribution and phylogeny in farmed Chinook salmon (Oncorhynchus tshawytscha). J. Fish Dis. 2019, 42, 85–95. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Almeda, R.; Messmer, A.M.; Sampedro, N.; Gosselin, L.A. Feeding rates and abundance of marine invertebrate planktonic larvae under harmful algal bloom conditions off Vancouver Island. Harmful Algae 2011, 10, 194–206. [Google Scholar] [CrossRef]
- Floerl, O.; Sunde, L.; Bloecher, N. Potential environmental risks associated with biofouling management in salmon aquaculture. Aquac. Environ. Interact. 2016, 8, 407–417. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Leadbeater, S.; Garcia, C.; Sylvain, F.-E.; Custodio, M.; Ang, K.P.; Powell, F.; Carvalho, G.R.; Creer, S.; Elliot, J.; et al. Parasitism perturbs the mucosal microbiome of Atlantic Salmon. Sci. Rep. 2017, 7, 43465. [Google Scholar] [CrossRef]
- Derome, N.; Gauthier, J.; Boutin, S.; Llewellyn, M. Bacterial Opportunistic Pathogens of Fish. Adv. Environ. Microbiol. 2016, 3, 81–108. [Google Scholar] [CrossRef]
- Wedekind, C.; Gessner, M.O.; Vazquez, F.; Maerki, M.; Steiner, D. Elevated resource availability sufficient to turn opportunistic into virulent fish pathogens. Ecology 2010, 91, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.; Gardiner, M. Microbial Dysbiosis: Rethinking Disease in Marine Ecosystems. Front. Microbiol. 2016, 7, 991. [Google Scholar] [CrossRef] [PubMed]
- Wynne, J.W.; Thakur, K.K.; Slinger, J.; Samsing, F.; Milligan, B.; Powell, J.F.F.; McKinnon, A.; Nekouei, O.; New, D.; Richmond, Z.; et al. Microbiome Profiling Reveals a Microbial Dysbiosis During a Natural Outbreak of Tenacibaculosis (Yellow Mouth) in Atlantic Salmon. Front. Microbiol. 2020, 11, 586387. [Google Scholar] [CrossRef] [PubMed]
- Slinger, J.; Adams, M.B.; Wynne, J.W. Bacteriomic profiling of branchial lesions induced by Neoparamoeba perurans chal-lenge reveals commensal dysbiosis and an association with Tenacibaculum dicentrarchi in AGD-affected Atlantic salmon (Salmo salar L.). Microorganisms 2020, 8, 1189. [Google Scholar] [CrossRef]
- Huq, A.; Huq, S.A.; Grimes, D.J.; O’Brien, M.; Chu, K.H.; Capuzzo, J.M.; Colwell, R.R. Colonization of the gut of the blue crab (Callinectes sapidus) by Vibrio cholerae. Appl. Environ. Microbiol. 1986, 52, 586–588. [Google Scholar] [CrossRef]
- Kueh, C.S.; Chan, K.-Y. Bacteria in bivalve shellfish with special reference to the oyster. J. Appl. Bacteriol. 1985, 59, 41–47. [Google Scholar] [CrossRef]
- Cavallo, R.A.; Acquaviva, M.I.; Stabili, L. Culturable heterotrophic bacteria in seawater and Mytilus galloprovincialis from a Mediterranean area (Northern Ionian Sea—Italy). Environ. Monit. Assess. 2008, 149, 465–475. [Google Scholar] [CrossRef]
- McEdward, L. Ecology of Marine Invertebrate Larvae; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Siddig, A.A.; Ellison, A.M.; Ochs, A.; Villar-Leeman, C.; Lau, M.K. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef]
- Zong, H.; Ma, D.; Wang, J.; Hu, J. Research on florfenicol residue in coastal area of Dalian (northern China) and analy-sis of functional diversity of the microbial community in marine sediment. Bull. Environ. Contam. Toxicol. 2010, 84, 245–249. [Google Scholar] [CrossRef]
- Miranda, C.D.; Rojas, R. Occurrence of florfenicol resistance in bacteria associated with two Chilean salmon farms with different history of antibacterial usage. Aquaculture 2007, 266, 39–46. [Google Scholar] [CrossRef]
- Madetoja, J.; Nyman, P.; Wiklund, T. Flavobacterium psychrophilum, invasion into and shedding by rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 2000, 43, 27–38. [Google Scholar] [CrossRef]
- Kunttu, H.M.T.; Valtonen, E.T.; Jokinen, E.I.; Suomalainen, L.R. Saprophytism of a fish pathogen as a transmission strat-egy. Epidemics 2009, 1, 96–100. [Google Scholar] [CrossRef]
- King, W.L.; Siboni, N.; Kahlke, T.; Green, T.J.; Labbate, M.; Seymour, J.R. A New High Throughput Sequencing Assay for Characterizing the Diversity of Natural Vibrio Communities and Its Application to a Pacific Oyster Mortality Event. Front. Microbiol. 2019, 10, 2907. [Google Scholar] [CrossRef]
- Suzuki, K.I.; Sasaki, J.; Uramoto, M.; Nakase, T.; Komagata, K. Agromyces mediolanus sp. nov., nom. rev., comb. nov., a species for “Corynebacterium mediolanum” Mamoli 1939 and for some aniline-assimilating bacteria which contain 2, 4-diaminobutyric acid in the cell wall peptidoglycan. Int. J. Syst. Evol. Microbiol. 1996, 46, 88–93. [Google Scholar] [CrossRef]
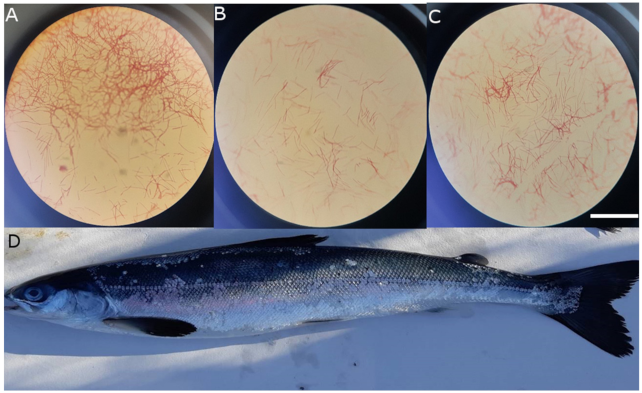
| Isolate Name | Most Similar BLAST Match | Alignment Length (bp) | Query Cover (%) | E-Value | Percent Identity (%) |
|---|---|---|---|---|---|
| MS C7 M2 | T. dicentrarchi QCR29 | 1326 | 100 | 0 | 99.4 |
| MS C9 F1 | T. dicentrarchi TdChD06 | 1335 | 100 | 0 | 99.63 |
| MS C10 M2 | T. finnmarkense AY7486TD | 1335 | 100 | 0 | 100 |
| LI C6 FM1-G | T. finnmarkense AY7486TD | 1018 | 100 | 0 | 100 |
| LI C6 FM1-F | T. finnmarkense AY7486TD | 1330 | 100 | 0 | 99.92 |
| LI C6 FM2-G | T. finnmarkense AY7486TD | 1335 | 100 | 0 | 100 |
| LI C6 FM3-F | T. finnmarkense AY7486TD | 1335 | 100 | 0 | 99.85 |
| Site | T. dicentrarchi | T. maritimum | Both |
|---|---|---|---|
| Midsummer | Total: 131 (16.9) | Total: 82 (10.6) | Total: 31 (3.99) |
| Fish: 71 (17.9) | Fish: 76 (19.2) | Fish: 30 (7.59) | |
| Env.: 60 (15.7) | Env.: 6 (1.57) | Env.: 1 (0.262) | |
| Larson Island | Total: 34 (8.79) | Total: 24 (6.20) | Total: 6 (1.55) |
| Fish: 23 (13.8) | Fish: 9 (5.39) | Fish: 6 (3.59) | |
| Env.: 11 (5.09) | Env.: 15 (6.94) | Env.: 0 |
| LNOB Comparison | Mean LNOB ± SD * | F Value | p |
|---|---|---|---|
| 1. Between sites using both assays | MS: 6.3 ± 1.7 A LI: 5.6 ± 2.0 B | F1,95.18 = 7.7 | 0.0068 |
| 2. Within/between sites comparing DICEN and MAR assays | MS DICEN: 7.1 ± 1.5 A MS MAR: 5.2 ± 1.3 B LI DICEN: 6.8 ± 1.2 A LI MAR: 3.9 ± 1.7 C | F3,91.88 = 63 | <2.2 × 10−16 |
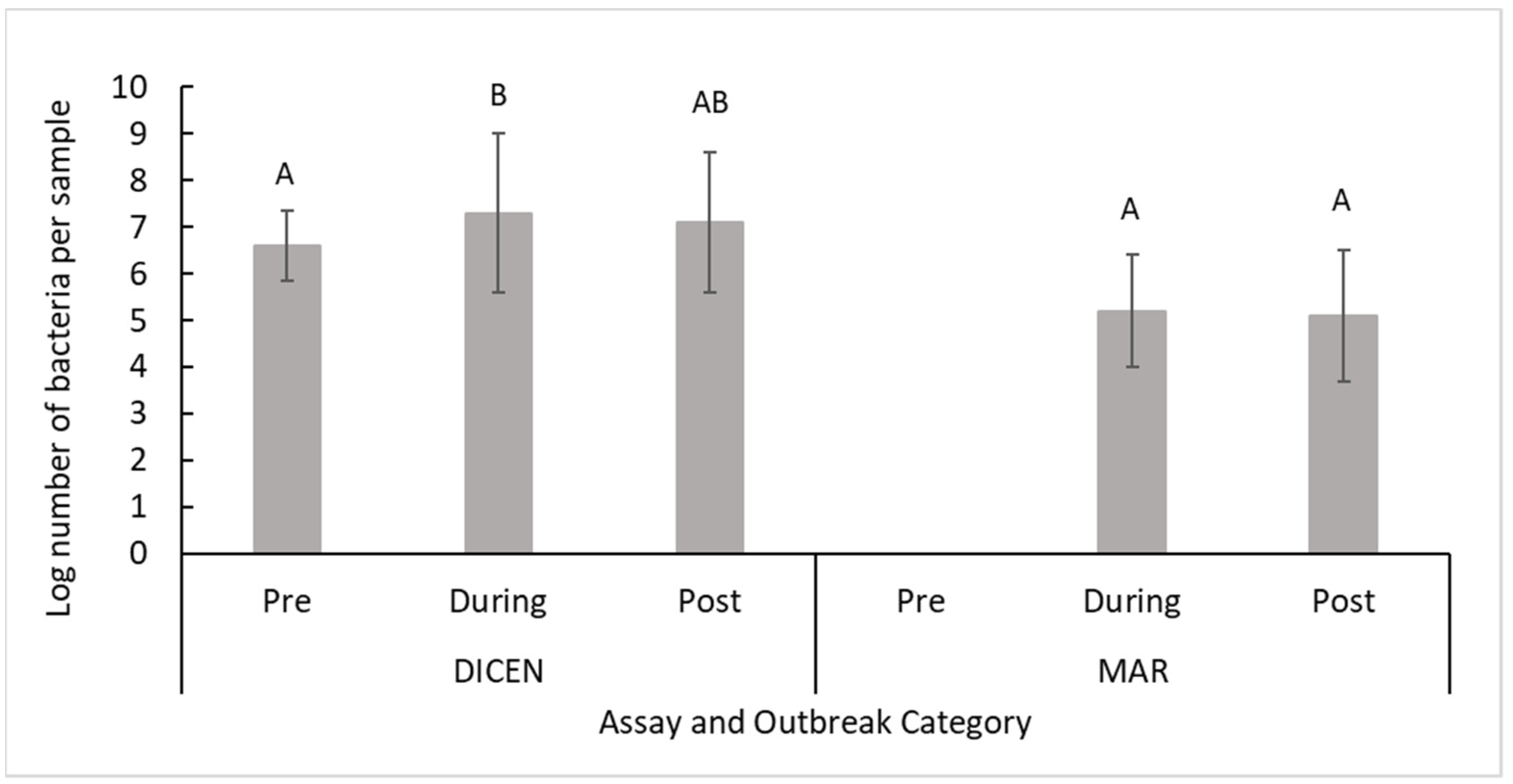
| 3. Throughout an outbreak using both sites and assays | Pre: 5.7 ± 1.9 A During: 6.5 ± 1.8 B Post: 5.4 ± 1.4 A | F2,110.44 = 13 | 1.2 × 10−5 |
| 4. Between fish and the env. using both sites and assays | Fish: 6.5 ± 1.7 A Env.: 5.3 ± 1.7 B | F1,164.11 = 33 | 4.9 × 10−8 |
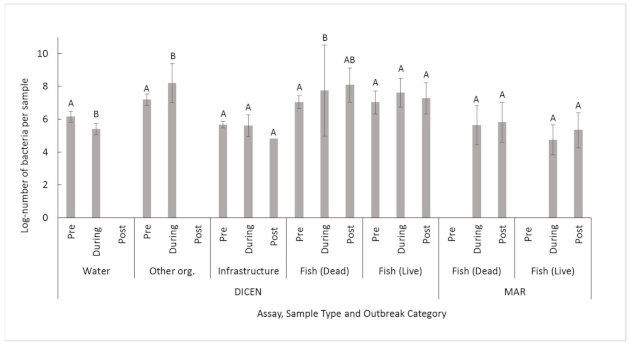
| 5. Between sample types using both sites and assays | Fish-Live: 6.0 ± 1.5 A Fish-Dead: 6.8 ± 1.8 B OO: 7.0 ± 1.7 A,B Water: 4.5 ± 1.5 C Infrastructure: 5.0 ± 1.3 C | F4,80.43 = 23 | 7.8 × 10−13 |
| 6. Throughout an outbreak at MS using the DICEN assay | Pre: 6.6 ± 0.74 A During: 7.3 ± 1.7 B Post: 7.1 ± 1.5 AB | F2,13.481 = 7.0 | 0.0082 |
| 7. Throughout an outbreak at MS using the MAR assay | Pre: NA During: 5.2 ± 1.2 A Post: 5.1 ± 1.4 A | F1,77.501 = 0.053 | 0.82 |
| 8. Between sample types at MS using the DICEN assay | Fish-Live: 7.3 ± 0.81 A Fish-Dead: 7.9 ± 1.6 A OO: 7.8 ± 0.50 A Water: 5.6 ± 1.5 B Infrastructure: 5.6 ± 0.49 B | F4,49.523 = 60 | <2.2 × 10−16 |
| 9. Between sample types at MS using the MAR assay | Fish-Live: 4.9 ± 1.1 A Fish-Dead: 5.7 ± 1.2 B OO: 3.8 ± 0.65 A,B,C Water: 2.8 ± 0.91 C | F3,6.73 = 15 | 0.0021 |
| 10. In live fish throughout an outbreak at MS using the DICEN assay | Pre: 7.1 ± 0.78 A During: 7.5 ± 0.81 A Post: 6.7 ± 0.30 A | F2,4.5048 = 4.08 | 0.097 |
| 11. In infrastructure swabs throughout an outbreak at MS using the DICEN assay | Pre: 5.6 ± 0.15 A During: 5.6 ± 0.61 A Post: 4.8 | F1,25.651 = 0.29 | 0.59 |
| 12. In dead fish throughout an outbreak at MS using the DICEN assay | Pre: 6.9 + 0.270 A During: 8.2 + 1.75 B Post: 8.1 + 1.04 A,B | F2,5.27 = 12 | 0.011 |
| 13. In OO throughout an outbreak at MS using the DICEN assay | Pre: 7.1 ± 0.316 A During: 8.2 ± 1.18 B Post: NA | F1,8.76 = 5.3 | 0.047 |
| 14. In water throughout an outbreak at MS using the DICEN assay | Pre: 6.1 ± 0.316 A During: 5.3 ± 0.361 B Post: NA | F1,11.50 = 19 | 0.0011 |
| 15. In dead fish throughout an outbreak at MS using the MAR assay | Pre: NA During: 5.7 ± 1.22 A Post: 5.7 ± 1.14 A | F1,48.76 = 0.00077 | 0.98 |
| 16. In live fish throughout an outbreak at MS using the MAR assay | Pre: NA During: 4.9 ± 1.14 A Post: 4.9 ± 0.837 A | F1,27.155 = 0.011 | 0.91 |
| 17. Throughout an outbreak at LI using the DICEN assay | Pre: 9.4 During: 6.8 ± 1.14 Post: 6.1 | NA | NA |
| 18. Throughout an outbreak at LI using the MAR assay | Pre: 2.7 ± 0.831 During: 5.0 ± 1.65 Post: 5.3 ± 1.10 | F2,9.6835 = 17 | 6.4 × 10−4 |
| 19. Between sample types at LI using the DICEN assay | Fish-Live: 6.5 ± 0.469 A Fish-Dead: 7.7 ± 1.21 B OO: 7.1 ± 0.678 A,B Water: NA Infrastructure: 5.2 ± 0.469 C | F3,12.80 = 20 | 4.6 × 10−5 |
| 20. Between sample types at LI using the MAR assay | Fish-Live: NA Fish-Dead: 5.1 ± 1.41 A OO: NA Water: 3.0 ± 0.71 B Infrastructure: 2.3 ± 0.77 B | F2,16.597 = 19 | 4.7 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowlan, J.P.; Britney, S.R.; Lumsden, J.S.; Russell, S. Application of Quantitative-PCR to Monitor Netpen Sites in British Columbia (Canada) for Tenacibaculum Species. Pathogens 2021, 10, 414. https://doi.org/10.3390/pathogens10040414
Nowlan JP, Britney SR, Lumsden JS, Russell S. Application of Quantitative-PCR to Monitor Netpen Sites in British Columbia (Canada) for Tenacibaculum Species. Pathogens. 2021; 10(4):414. https://doi.org/10.3390/pathogens10040414
Chicago/Turabian StyleNowlan, Joseph P., Scott R. Britney, John S. Lumsden, and Spencer Russell. 2021. "Application of Quantitative-PCR to Monitor Netpen Sites in British Columbia (Canada) for Tenacibaculum Species" Pathogens 10, no. 4: 414. https://doi.org/10.3390/pathogens10040414
APA StyleNowlan, J. P., Britney, S. R., Lumsden, J. S., & Russell, S. (2021). Application of Quantitative-PCR to Monitor Netpen Sites in British Columbia (Canada) for Tenacibaculum Species. Pathogens, 10(4), 414. https://doi.org/10.3390/pathogens10040414






