Changes in Rice and Livestock Production and the Potential Emergence of Japanese Encephalitis in Africa
Abstract
1. Introduction
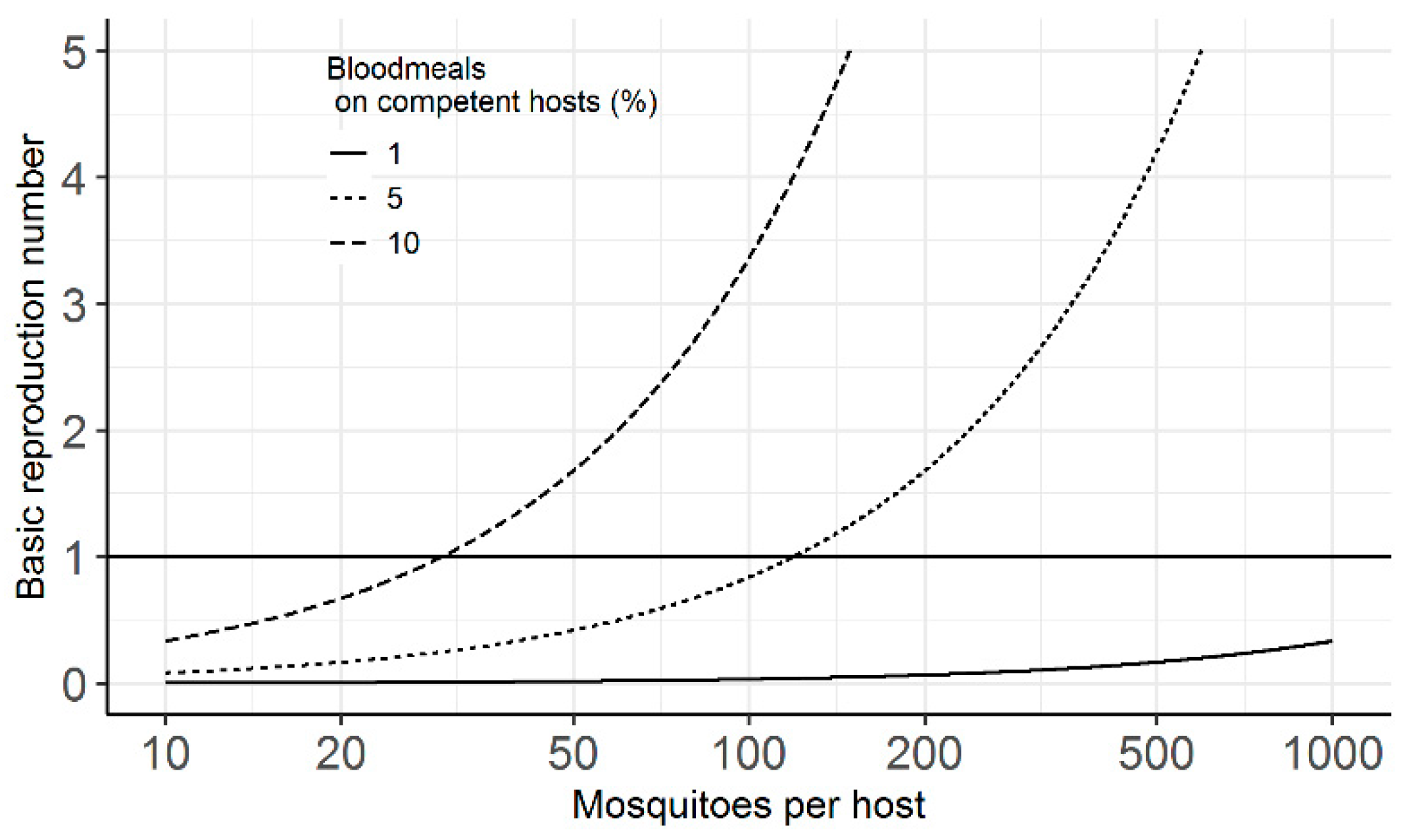
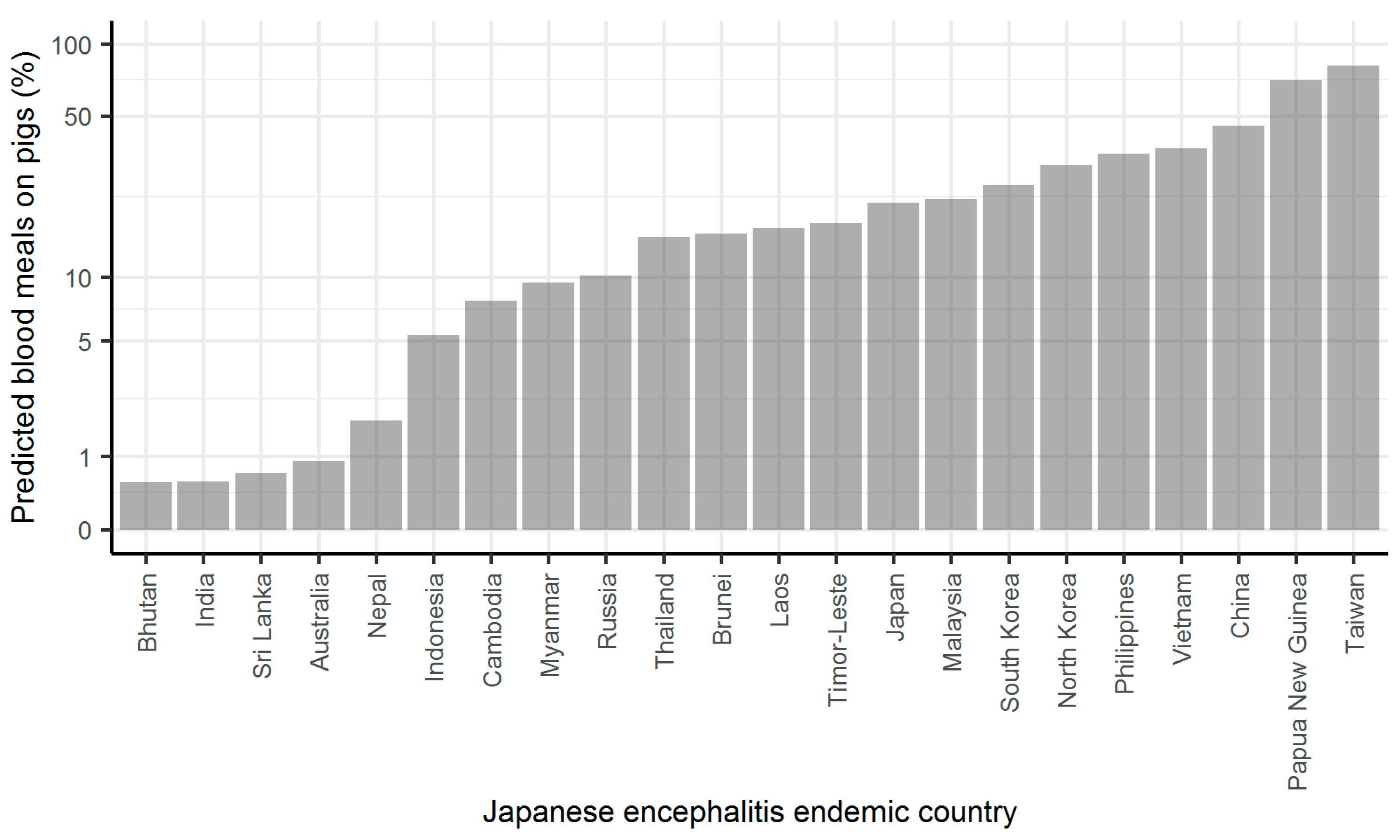
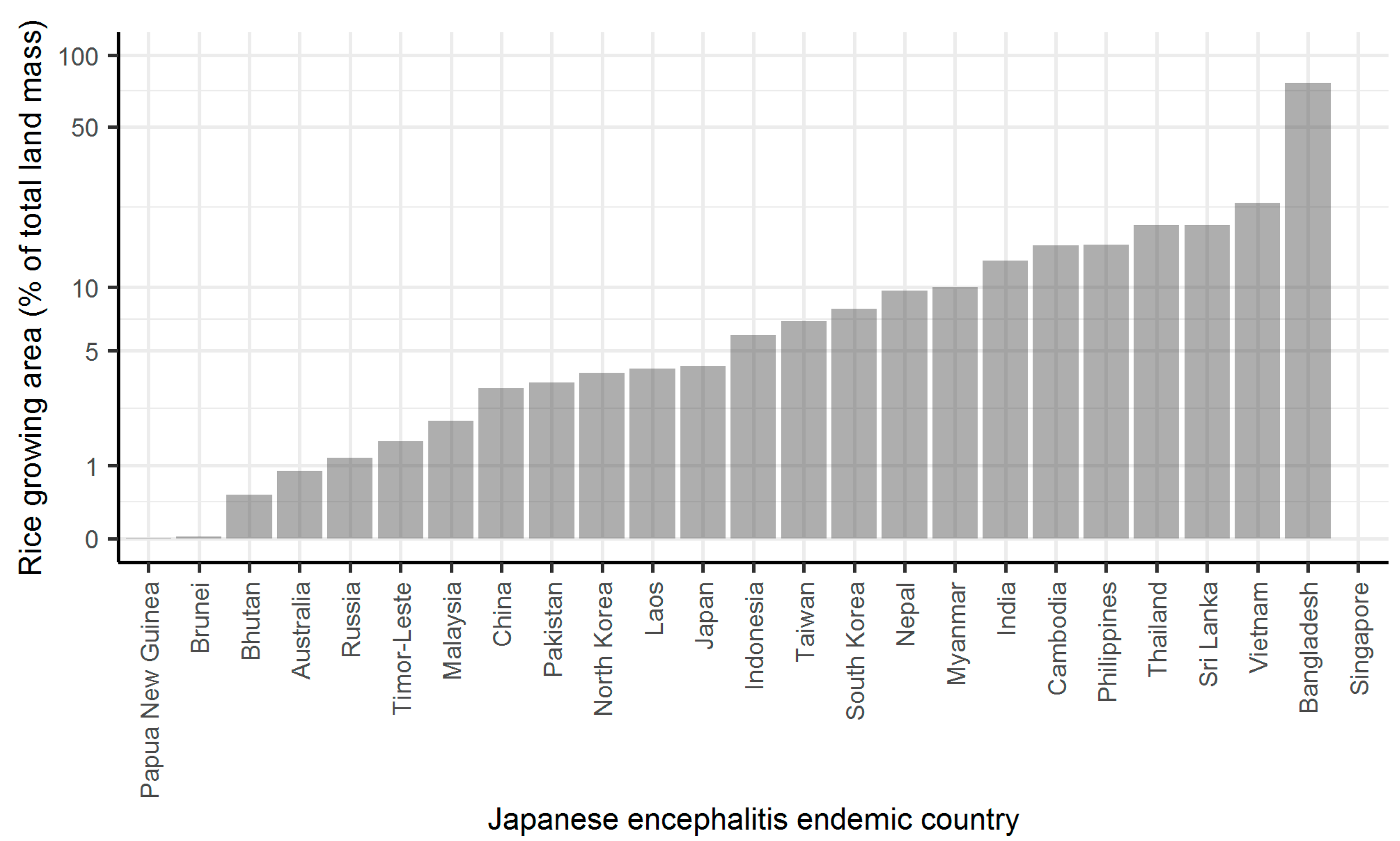
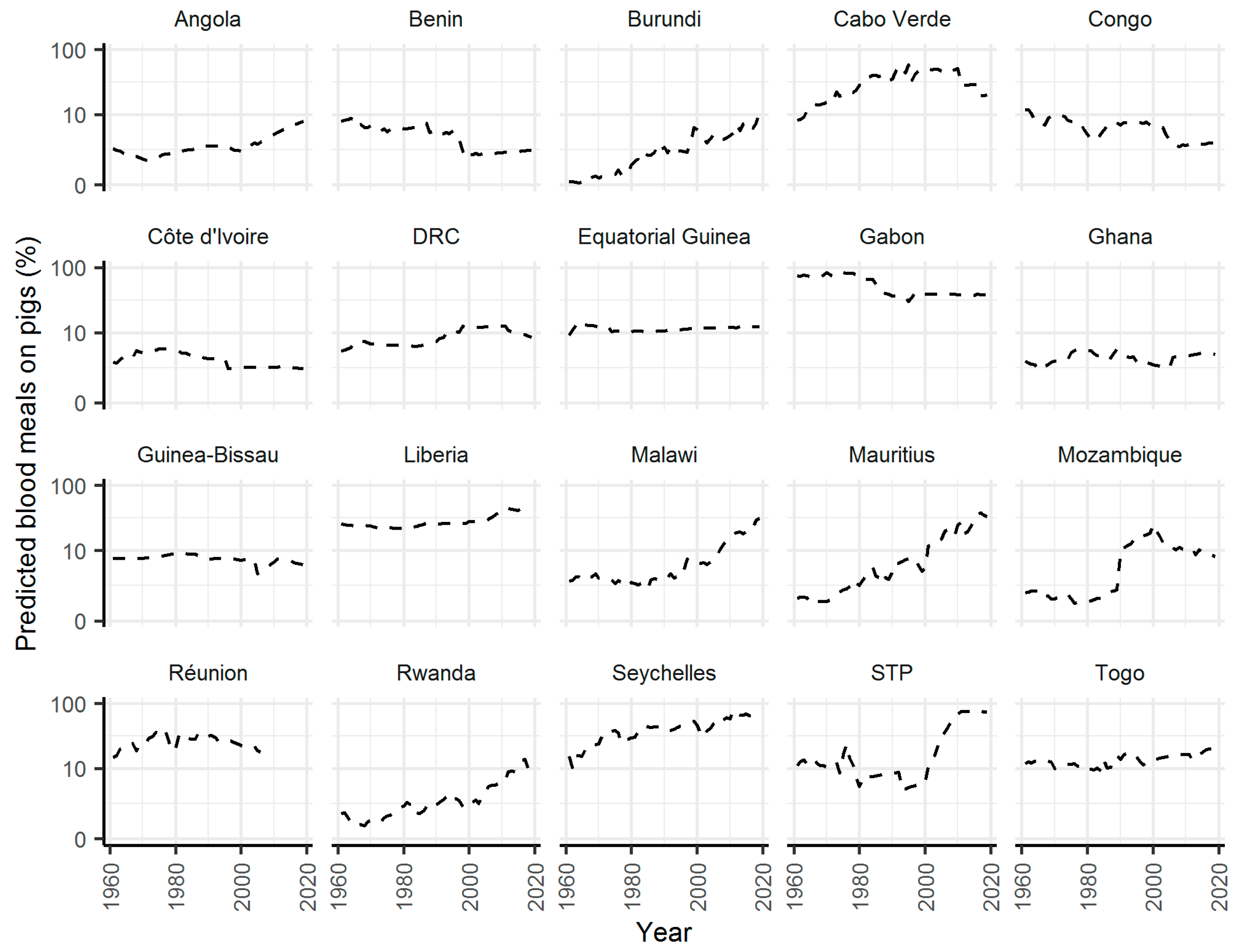
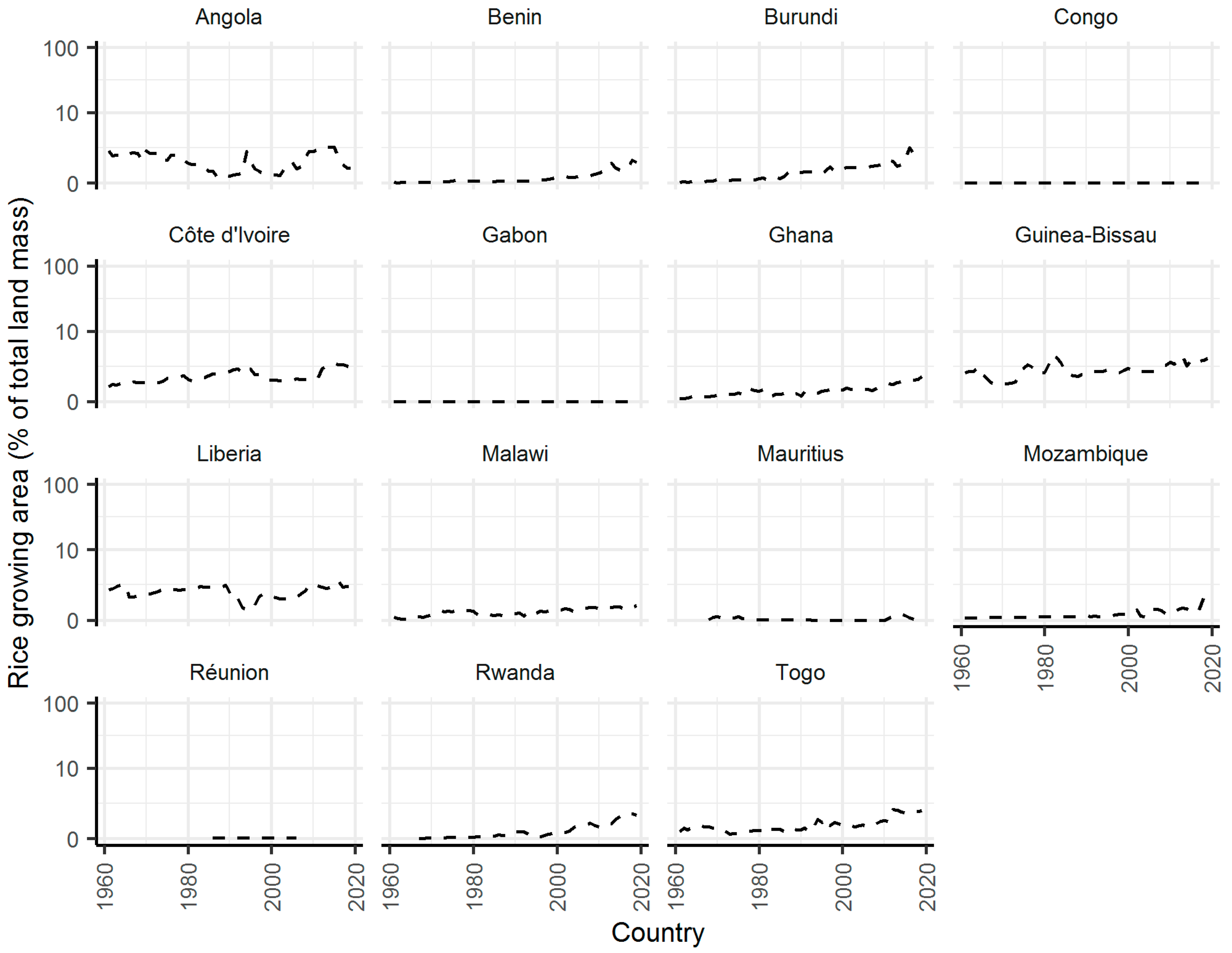
2. Results
3. Discussion
4. Materials and Methods
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quan, T.M.; Thao, T.T.N.; Duy, N.M.; Nhat, T.M.; Clapham, H.E. Estimates of the global burden of japanese encephalitis and the impact of vaccination from 2000-2015. Elife 2020, 9, 1–187. [Google Scholar] [CrossRef]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.E.; Rossi, G.; Karan, L.S.; Mironov, K.O.; Busani, L.; Rezza, G. Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Eurosurveillance 2012, 17, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Simon-Loriere, E.; Faye, O.; Prot, M.; Casdemont, I.; Fall, G.; Fernandez-Garcia, M.; Diagne, M.; Kipela, J.; Fall, I.; Holmes, E.; et al. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N. Engl. J. Med. 2017, 1386–1388. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935-2017. Vector-Borne Zoonotic Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.; Scherer, W. Ecologic studies of Japanese encephalitis virus in Japan. IX. Epidemiologic correlations and conclusions. Am. J. Trop. Med. Hyg. 1959, 8, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Konno, J.; Endo, K.; Agatsuma, H.; Ishida, N. Cyclic outbreaks of japanese encephalitis among pigs and humans. Am. J. Epidemiol. 1966, 84, 292–300. [Google Scholar] [CrossRef]
- Keiser, J.; Maltese, M.F.; Erlanger, T.E.; Bos, R.; Tanner, M.; Singer, B.H.; Utzinger, J. Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop. 2005, 95, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.E.; Masuoka, P.; Brett-Major, D.; Smith, M.; Klein, T.A.; Kim, H.C.; Anyamba, A.; Grieco, J. The relationship between mosquito abundance and rice field density in the Republic of Korea. Int. J. Health Geogr. 2010, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Suwonkerd, W.; Tsuda, Y.; Sugiyama, A.; Wada, Y. Effects of rice culture practices on the abundance of Culex mosquitoes (Diptera: Culicidae) in Northern Thailand. J. Med. Entomol. 1997, 34, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cha, G.W.; Jeong, Y.E.; Lee, W.G.; Chang, K.S.; Roh, J.Y.; Yang, S.C.; Park, M.Y.; Park, C.; Shin, E.H. Detection of Japanese encephalitis virus genotype V in Culex orientalis and Culex pipiens (Diptera: Culicidae) in Korea. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, J.; Choeung, R.; Peng, B.; Pring, L.; Pang, S.; Ong, S.; Sorn, S.; Tarantola, A.; Fontenille, D.; Cappelle, J. Comparison of the dynamics of Japanese encephalitis virus circulation in sentinel pigs between a rural and a peri-urban setting in Cambodia. PLoS Negl. Trop. Dis. 2018, 12, 1–18. [Google Scholar] [CrossRef]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.-P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef]
- Lord, J.S.; Gurley, E.S.; Pulliam, J.R.C. Rethinking Japanese encephalitis virus transmission: A framework for implicating host and vector species. PLoS Negl. Trop. Dis. 2015, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ladreyt, H.; Durand, B.; Dussart, P.; Chevalier, V. How central is the domestic pig in the epidemiological cycle of Japanese encephalitis virus? A review of scientific evidence and implications for disease control. Viruses 2019, 11, 949. [Google Scholar] [CrossRef]
- Walter Reed Biosystematics Unit. Culex Tritaeniorhynchus Species Page. Available online: https://www.wrbu.si.edu/vectorspecies/mosquitoes/tritaeniorhynchus (accessed on 14 February 2021).
- Mwandawiro, C.; Tuno, N.; Suwonkerd, W.; Tsuda, Y.; Yanagi, T.; Takagi, M. Host preference of Japanese encephalitis vectors in Chiangmai, Northern Thailand. Med. Entomol. Zool. 1999, 50, 323–333. [Google Scholar] [CrossRef]
- Ilkal, M.A.; Dhanda, V.; Rae, B.U.; George, S.; Mishra, A.C.; Prasanna, Y.; Gopall, S. Absence of viraemia in cattle after experimental infection with Japanese encephalitis virus. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 628–631. [Google Scholar] [CrossRef]
- Longbottom, J.; Browne, A.J.; Pigott, D.M.; Sinka, M.E.; Golding, N.; Hay, S.I.; Moyes, C.L.; Shearer, F.M. Mapping the spatial distribution of the Japanese encephalitis vector, Culex tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) within areas of Japanese encephalitis risk. Parasites Vectors 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; Van Boeckel, T.P.; Vanwambeke, S.O.; Wint, G.R.W.; Robinson, T.P. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci. Data 2018, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Database. Available online: http://www.fao.org/faostat/en/#data/RF (accessed on 5 February 2018).
- Smith, D.L.; McKenzie, F.E. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar. J. 2004, 3, 13. [Google Scholar] [CrossRef]
- Khan, S.U.; Salje, H.; Hannan, A.; Islam, M.A.; Bhuyan, A.A.M.; Islam, M.A.; Rahman, M.Z.; Nahar, N.; Hossain, M.J.; Luby, S.P.; et al. Dynamics of Japanese Encephalitis Virus Transmission among Pigs in Northwest Bangladesh and the Potential Impact of Pig Vaccination. PLoS Negl. Trop. Dis. 2014, 8, e3166. [Google Scholar] [CrossRef]
- Buescher, E.; Scherer, W.; Rosenberg, M.; Gresser, I.; Hardy, J.L.; Bullock, H.R. Ecologic studies of Japanese encephalitis virus in Japan. II. Mosquito infection. Am. J. Trop. Med. Hyg. 1959, 8, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Gajanana, A.; Rajendran, R.; Samuel, P.P.; Thenmozhi, V.; Tsai, T.F.; Kimura-Kuroda, J.; Reuben, R. Japanese Encephalitis in South Arcot District, Tamil Nadu, India: A Three-Year Longitudinal Study of Vector Abundance and Infection Frequency. J. Med. Entomol. 1997, 34, 651–659. [Google Scholar] [CrossRef]
- Cleton, N.B.; Bosco-Lauth, A.; Page, M.J.; Bowen, R. A Age-related susceptibility to Japanese encephalitis virus in domestic ducklings and chicks. Am. J. Trop. Med. Hyg. 2014, 90, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Ladreyt, H.; Auerswald, H.; Tum, S.; Ken, S.; Heng, L.; In, S.; Lay, S.; Top, C.; Ly, S.; Duong, V.; et al. Comparison of Japanese encephalitis force of infection in pigs, poultry and dogs in cambodian villages. Pathogens 2020, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Williams, D.T.; Smith, D.W. Japanese Encephalitis Virus: The Geographic Distribution, Incidence, and Spread of a Virus with a Propensity to Emerge in New Areas. Perspect. Med. Virol. 2006, 16, 201–268. [Google Scholar] [CrossRef]
- Sule, W.F.; Oluwayelu, D.O.; Hernández-Triana, L.M.; Fooks, A.R.; Venter, M.; Johnson, N. Epidemiology and ecology of West Nile virus in sub-Saharan Africa. Parasites Vectors 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Oliveira, A.R.S.; Strathe, E.; Etcheverry, L.; Cohnstaedt, L.W.; Mcvey, D.S. Assessment of data on vector and host competence for Japanese encephalitis virus: A systematic review of the literature. Prev. Vet. Med. 2018, 154, 71–89. [Google Scholar] [CrossRef]
- Seo, H.J.; Kim, H.C.; Klein, T.A.; Ramey, A.M.; Lee, J.H.; Kyung, S.G.; Park, J.Y.; Cho, Y.S.; Cho, I.S.; Yeh, J.Y. Molecular Detection and Genotyping of Japanese Encephalitis Virus in Mosquitoes during a 2010 Outbreak in the Republic of Korea. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Heal. 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Forestry. National Rice Development Strategy (NRDS) July 2018 Executive Summary; Ministry of Agriculture and Forestry: Luanda, Angola, 2018; Available online: https://riceforafrica.net/images/pdf/NRDS_Eng_Angola_20180910.pdf (accessed on 28 February 2021).
- Ricklin, M.E.; Garcìa-Nicolàs, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Posthaus, H.; Oevermann, A.; Summerfield, A. Japanese encephalitis virus tropism in experimentally infected pigs. Vet. Res. 2016, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lord, J.S. Changes in Rice and Livestock Production and the Potential Emergence of Japanese Encephalitis in Africa. Pathogens 2021, 10, 294. https://doi.org/10.3390/pathogens10030294
Lord JS. Changes in Rice and Livestock Production and the Potential Emergence of Japanese Encephalitis in Africa. Pathogens. 2021; 10(3):294. https://doi.org/10.3390/pathogens10030294
Chicago/Turabian StyleLord, Jennifer S. 2021. "Changes in Rice and Livestock Production and the Potential Emergence of Japanese Encephalitis in Africa" Pathogens 10, no. 3: 294. https://doi.org/10.3390/pathogens10030294
APA StyleLord, J. S. (2021). Changes in Rice and Livestock Production and the Potential Emergence of Japanese Encephalitis in Africa. Pathogens, 10(3), 294. https://doi.org/10.3390/pathogens10030294





