Comparison of UV, Peracetic Acid and Sodium Hypochlorite Treatment in the Disinfection of Urban Wastewater
Abstract
1. Introduction
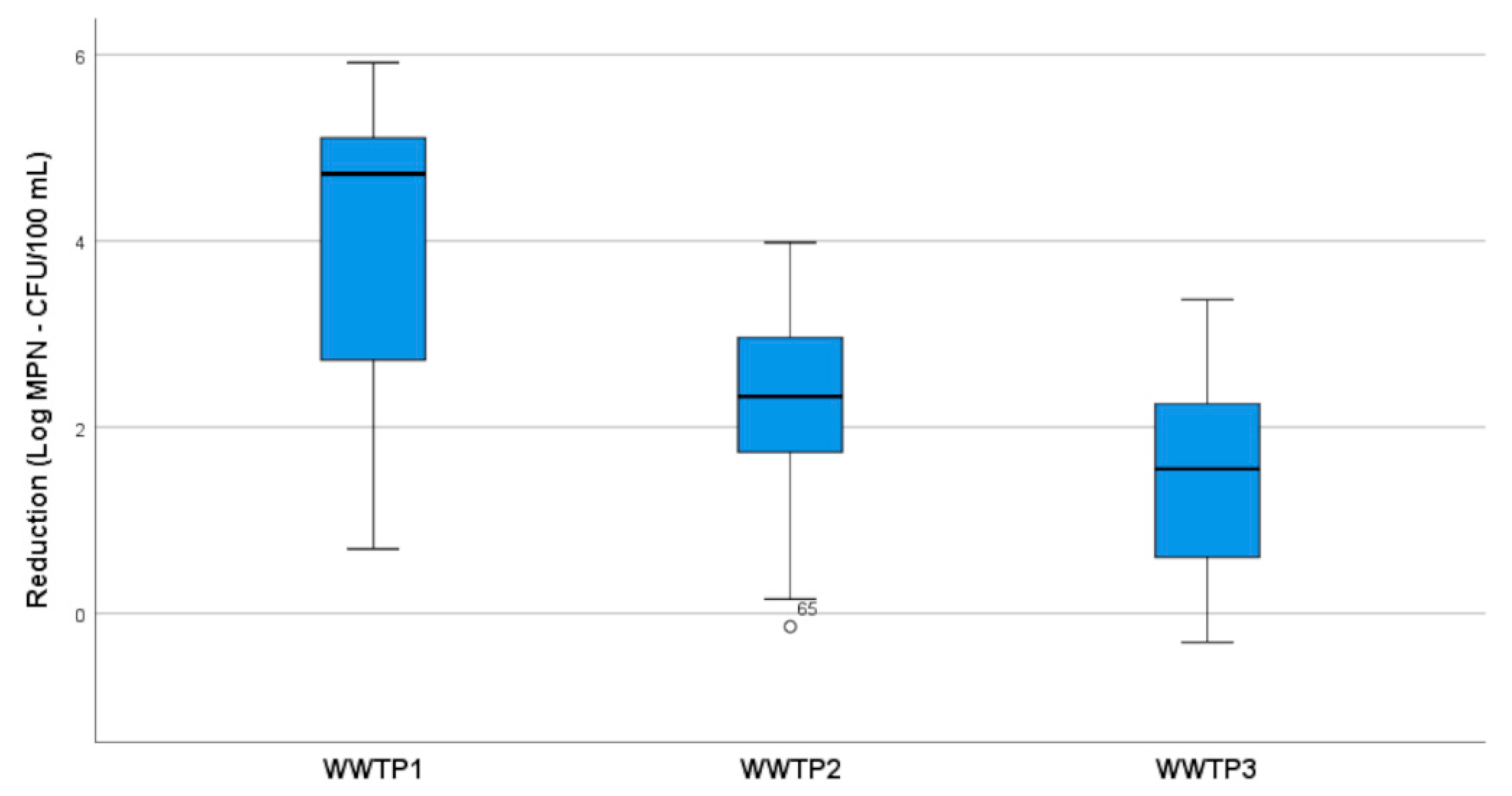
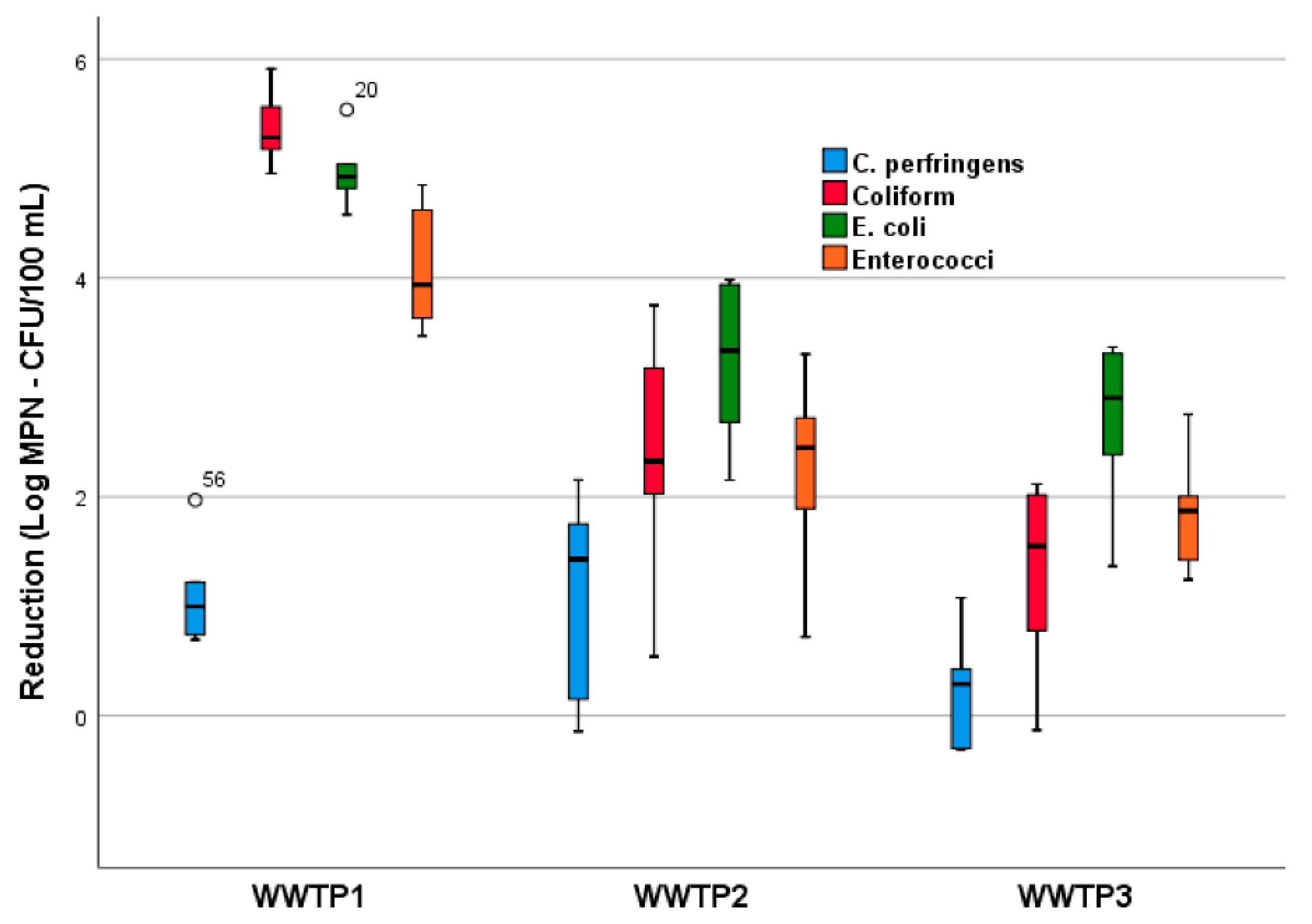
2. Results and Discussion
2.1. Bacterial Indicators
2.2. Pathogenic Bacteria
3. Materials and Methods
3.1. Bacterial Strains and Culture Media
3.2. Sampling
3.3. Microbiological Analyses for Pathogen Detection
3.4. Microbiological Analyses for the Detection of Microbial Indicators
3.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, J.; Matos, A.; Gmurek, M.; Quinta-Ferreira, R.M.; Martins, R.C. Ozone and Photocatalytic Processes for Pathogens Removal from Water: A Review. Catalysts 2019, 9, 46. [Google Scholar] [CrossRef]
- De Souza, B.J.; Valdez, F.Q.; Jeranoski, R.F.; De Sousa Vidal, C.M.; Cavallini, G.S. Water and wastewater disinfection with peracetic acid and UV radiation and using advanced oxidative process PAA/UV. Int. J. Photoenergy 2015. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, H.; Yang, H.W.; Lu, Y.; Chen, Z.; Hu, H.Y. Bacterial removal performance and community changes during advanced treatment process: A case study at a full-scale water reclamation plant. Sci. Total Environ. 2020, 705, 135811. [Google Scholar] [CrossRef]
- Koivunen, J.; Siitonen, A.; Heinonen-Tanski, H. Elimination of enteric bacteria in biological-chemicalwastewater treatment and tertiary filtration units. Water Res. 2003, 37, 690–698. [Google Scholar] [CrossRef]
- López, A.; Rodríguez-Chueca, J.; Mosteo, R.; Gómez, J.; Rubio, E.; Goni, P.; Ormad, M.P. How does urban wastewater treatment affect the microbial quality of treated wastewater? Process. Saf. Environ. 2019, 130, 22–30. [Google Scholar]
- Chhetri, R.K.; Klupsch, E.; Andersen, H.R.; Jensen, P.E. Treatment of Arctic wastewater by chemical coagulation, UV and peracetic acid disinfection. Environ. Sci. Pollut. Res. 2018, 25, 32851–32859. [Google Scholar] [CrossRef] [PubMed]
- Luna-Pabello, V.M.; Miranda Ríos, M.; Jiménez, B.; Orta de Velasquez, M.T. Effectiveness of the use of Ag, Cu and PAA to disinfect municipal wastewater. Environ. Technol. 2009, 30, 129–139. [Google Scholar] [CrossRef]
- Du, Y.; Lv, X.T.; Wu, Q.Y.; Zhang, D.Y.; Zhou, Y.T.; Peng, L.; Hu, H.Y. Formation and control of disinfection byproducts and toxicity during reclaimed water chlorination: A review. J. Environ. Sci. 2017, 58, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the Main Disinfection Processes for Wastewater and Drinking Water Treatment Plants. Sustainability 2018, 10, 86. [Google Scholar] [CrossRef]
- Ahmad, S.I. Ultraviolet Light in Human Health, Diseases and Environment; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 996, pp. 267–275. [Google Scholar]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Henao, L.D.; Turolla, A.; Antonelli, M. Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: A review. Chemosphere 2018, 213, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, J.; Heinonen-Tanski, H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Res. 2005, 39, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, T.; Mejia-Tickner, B.; Kissel, J.; Xie, X.; Huang, C.H. Inactivation of Bacteria by Peracetic Acid Combined with Ultraviolet Irradiation: Mechanism and Optimization. Environ. Sci. Technol. 2020, 54, 9652–9661. [Google Scholar] [CrossRef] [PubMed]
- Manoli, K.; Sarathy, S.; Maffettone, R.; Santoro, D. Detailed modeling and advanced control for chemical disinfection of secondary effluent wastewater by peracetic acid. Water Res. 2019, 153, 251–262. [Google Scholar] [CrossRef]
- Moreira, N.A.; Bondelind, M. Safe drinking water and waterborne outbreaks. J. Water Health 2017, 15, 83–96. [Google Scholar] [CrossRef]
- Levantesi, C.; Bonadonna, L.; Briancesco, R.; Grohmann, E.; Toze, S.; Tandoi, V. Salmonella in surface and drinking water: Occurrence and water-mediated transmission. Food Res. Int. 2012, 45, 587–602. [Google Scholar] [CrossRef]
- Bronowsky, C.; James, C.E.; Winstanley, C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef]
- Pennington, H. Escherichia coli O157. Lancet 2010, 376, 1428–1435. [Google Scholar] [CrossRef]
- Muniesa, M.; Jofre, J.; Garcia-Aliaro, C.; Blanch, A.R. Occurrence of Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environ. Sci. Technol. 2006, 40, 7141–7149. [Google Scholar] [CrossRef]
- Li, B.; Liu, H.; Wang, W. Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E. coli. BMC Microbiol. 2017, 17, 215. [Google Scholar] [CrossRef]
- McCarthy, T.A.; Barrett, N.L.; Hadler, J.L.; Salsbury, B.; Howard, R.T.; Dingman, D.W.; Brinkman, C.D.; Bibb, W.F.; Cartter, M.L. Hemolytic-Uremic Syndrome and Escherichia coli O121 at a Lake in Connecticut, 1999. Pediatrics 2001, 108, e59. [Google Scholar] [CrossRef] [PubMed]
- Mauer, A.M.; Sturchler, D. A waterborne outbreak of small round structured virus, campylobacter and shigella co-infections in La Neuveville, Switzerland, 1998. Epidemiol. Infect. 2000, 125, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Pedati, C.; Koirala, S.; Safranek, T.; Buss, B.F.; Carlson, A.V. Campylobacteriosis Outbreak Associated with Contaminated Municipal Water Supply-Nebraska, 2017. MMWR 2019, 68, 169–173. [Google Scholar] [CrossRef]
- Liu, H.; Whitehouse, C.A.; Li, B. Presence and Persistence of Salmonella in Water: The Impact on Microbial Quality of Water and Food Safety. Front. Public Health 2018, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Mathur, N.; Singh, A.; Pareek, H.; Bhatnagar, P. Evaluation of Factors Influencing the Environmental Spread of Pathogens by Wastewater Treatment Plants. Water Air Soil Pollut. 2020, 231, 440. [Google Scholar] [CrossRef]
- Hassaballah, A.H.; Bhatt, T.; Nyitrai, J.; Dai, N.; Sassoubre, L. Inactivation of E. coli, Enterococcus spp., somatic coliphage, and Cryptosporidium parvum in wastewater by peracetic acid (PAA), sodium hypochlorite, and combined PAA-ultraviolet disinfection. Environ. Sci. Water Res. Technol. 2020, 6, 197. [Google Scholar] [CrossRef]
- Bonetta, S.; Pignata, C.; Lorenzi, E.; De Ceglia, M.; Meucci, L.; Bonetta, S.; Gilli, G.; Carraro, E. Peracetic Acid (PAA) Disinfection: Inactivation of Microbial Indicators and Pathogenic Bacteria in a Municipal Wastewater Plant. Water 2017, 9, 427. [Google Scholar] [CrossRef]
- Gehr, R.; Wagner, M.; Veerasubramanian, P.; Payment, P. Disinfection efficiency of peracetic acid, UV and ozone after enhanced primary treatment of municipal wastewater. Water Res. 2003, 37, 4573–4586. [Google Scholar] [CrossRef]
- Decreto Legislativo 3.04.2006, n. 152: Norme in Materia Ambientale; GU Repubblica Italiana n. 88 del 14.04.2006; Ministero della Giustizia: Roma, Italia, 2006.
- Decreto Ministero dell’Ambiente e della Tutela del Territorio. Norme Tecniche per il Riutilizzo delle Acque Reflue, ai Sensi Dell’articolo 99, Comma 1, del Decreto Legislativo 3 Aprile 2006, n. 152; Ministero dell’Ambiente e Tutela del Territorio: Roma, Italia, 2006.
- Veschetti, E.; Cutilli, D.; Bonadonna, L.; Briancesco, R.; Martini, C.; Cecchini, G.; Anastasi, P.; Ottaviani, M. Pilot-plant comparative study of peracetic acid and sodium hypochlorite wastewater disinfection. Water Res. 2003, 37, 78–94. [Google Scholar] [CrossRef]
- Ostoich, M.; Aimo, E.; Vazzoler, M.; Stradella, S.; Osti, P. Integrated approach for microbiological impact assessment of public wastewater treatment plants. Chem. Ecol. 2007, 23, 43–62. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Kauppinen, A.; Martikainen, K.; Pitkänen, T.; Kusnetsov, J.; Miettinen, I.T.; Pessi, M.; Poutiainen, H.; Heinonen-Tanski, H. Microbial reduction in wastewater treatment using Fe3+ and Al3+ bcoagulants and PAA disinfectant. J. Water Health 2013, 11, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Pignata, C.; Lorenzi, E.; De Ceglia, M.; Meucci, L.; Bonetta, S.; Gilli, G.; Carraro, E. Detection of pathogenic Campylobacter, E. coli O157:H7 and Salmonella spp. in wastewater by PCR assay. Environ. Sci. Pollut. Res. 2016, 23, 15302–15309. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Stanley, K.N.; Allison, H.E.; Flint, H.J.; Stewart, C.S.; Sharp, R.J.; Saunders, J.R.; McCarthy, A.J. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 2001, 67, 4335–4337. [Google Scholar] [CrossRef]
- Strauch, E.; Hammerl, J.A.; Konietzny, A.; Schneiker-Bekel, S.; Arnold, W.; Goesmann, A.; Pühler, A.; Beutin, L. Bacteriophage 2851 is a prototype phage for dissemination of the Shiga toxin variant gene 2c in Escherichia coli O157:H7. Infect. Immun. 2008, 76, 5466–5477. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Castillo, A.; Allue-Guardia, A.; Dahbi, G.; Blanco, J.; Creuzburg, K.; Schmidt, H.; Muniesa, M. Type III effector genes and other virulence factors of Shiga toxin-encoding Escherichia coli isolated from wastewater. Environ. Microbiol. Rep. 2012, 4, 147–155. [Google Scholar] [CrossRef]
- Li, D.; Zeng, S.; Gu, A.Z.; He, M.; Shi, H. Inactivation, reactivation and regrowth of indigenous bacteria in reclaimed water after chlorine disinfection of a municipal wastewater treatment plant. J. Environ. Sci. 2013, 25, 1319–1325. [Google Scholar] [CrossRef]
- Wery, N.; Lhoutellier, C.; Ducray, F.; Delgenes, J.; Godon, J. Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res. 2008, 42, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Borelli, E.; Bonetta, S.; Conio, O.; Palumbo, F.; Carraro, E. Development of a PCR protocol for the detection of Escherichia coli O157:H7 and Salmonella spp. in surface water. Environ. Monit. Assess. 2011, 177, 493–503. [Google Scholar] [CrossRef]
- Amagliani, G.; Rotundo, L.; Carloni, E.; Omiccioli, E.; Magnani, M.; Brandi, G.; Fratamico, P. Detection of Shiga toxin-producing Escherichia coli (STEC) in ground beef and bean sprouts: Evaluation of culture enrichment conditions. Food Res. Int. 2018, 103, 398–405. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Microbiological Parameters for the Analysis of Sludge and Similar Products: Analytical Reference Methods; Rapporti ISTISAN 14/18; Bonadonna, L., Musmeci, L., Eds.; Istituto Superiore di Sanità: Roma, Italy, 2014; Volume vii, p. 153. (In Italian)
- ISO 14189:2013. Water Quality—Enumeration of Clostridium Perfringens—Method Using Membrane Filtration; ISO: Geneva, Switzerland, 2013. [Google Scholar]
| Sample | Sampling Month | WWTP | Salmonella | E. coli O157:H7 | Campylobacter | Salmonella | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| invA | O157 | H7 | Intimin | SLT-I | SLT-II | Genus | C. jejuni | C. coli | Culture Method | |||
| E | September 2017 | 1 | + | + | + | − | + | − | − | − | − | + |
| DE | September 2017 | 1 | − | − | − | − | − | + | − | − | − | − |
| E | November 2017 | 1 | + | + | + | − | + | − | − | − | − | − * |
| DE | November 2017 | 1 | + | − | + | − | − | − | − | − | − | − |
| E | January 2018 | 1 | + | + | + | − | − | − | − | − | − | + |
| DE | January 2018 | 1 | − | − | + | − | − | − | − | − | − | − |
| E | March 2018 | 1 | + | − | + | − | + | − | − | − | − | + |
| DE | March 2018 | 1 | − | − | − | + | − | − | − | − | − | − |
| E | May 2018 | 1 | + | − | − | − | + | − | − | − | − | + |
| DE | May 2018 | 1 | − | − | − | − | − | + | − | − | − | − |
| E | July 2018 | 1 | + | − | − | − | − | − | − | − | − | + |
| DE | July 2018 | 1 | + | − | − | − | − | − | − | − | − | − |
| E | September 2017 | 2 | + | + | + | − | + | − | − | − | − | + |
| DE | September 2017 | 2 | − | − | + | − | + | + | − | − | − | − |
| E | November 2017 | 2 | + | − | + | + | + | − | − | − | − | − * |
| DE | November 2017 | 2 | − | − | + | − | − | − | − | − | − | − |
| E | January 2018 | 2 | + | − | + | − | − | + | − | − | − | − * |
| DE | January 2018 | 2 | − | − | − | − | − | − | − | − | − | − |
| E | March 2018 | 2 | + | − | + | − | + | − | − | − | − | + |
| DE | March 2018 | 2 | + | − | + | − | − | − | − | − | − | − |
| E | May 2018 | 2 | + | − | − | − | + | − | − | − | − | + |
| DE | May 2018 | 2 | + | − | − | − | + | − | − | − | − | + |
| E | July 2018 | 2 | + | − | − | − | − | − | − | − | − | + |
| DE | July 2018 | 2 | + | − | − | − | − | − | − | − | − | − |
| E | September 2017 | 3 | + | + | + | − | + | − | − | − | − | + |
| DE | September 2017 | 3 | − | − | − | − | − | + | − | − | − | − |
| E | November 2017 | 3 | + | − | + | − | + | − | − | − | − | − * |
| DE | November 2017 | 3 | − | + | − | − | − | − | − | − | − | − |
| E | January 2018 | 3 | − | − | + | − | + | − | − | − | − | − * |
| DE | January 2018 | 3 | − | + | + | − | + | − | − | − | − | − |
| E | March 2018 | 3 | + | + | + | − | + | − | − | − | − | − * |
| DE | March 2018 | 3 | − | − | + | − | − | − | − | − | − | − |
| E | May 2018 | 3 | + | − | − | − | + | − | − | − | − | + |
| DE | May 2018 | 3 | − | − | − | − | − | − | − | − | − | − |
| E | July 2018 | 3 | + | − | − | − | − | − | − | − | − | + |
| DE | July 2018 | 3 | + | − | − | − | − | − | − | − | − | − |
| Sample | Sampling Month | WWTP | E. coli Serogroup | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O157 | O103 | O26 | O145 | O111 | O104 | SLT-I | SLT-II | |||
| DE | September 2017 | 1 | − | − | − | − | − | − | − | + |
| E | March 2018 | 1 | − | + | + | − | + | + | + | − |
| E | May 2018 | 1 | − | + | + | + | + | + | + | − |
| DE | May 2018 | 1 | − | − | − | − | − | − | − | + |
| E | November 2017 | 2 | − | + | + | − | + | + | + | − |
| E | January 2018 | 2 | − | + | + | − | + | + | − | + |
| E | March 2018 | 2 | − | + | + | + | + | + | + | − |
| E | May 2018 | 2 | − | + | + | + | + | + | + | − |
| DE | May 2018 | 2 | − | + | + | + | + | + | + | − |
| DE | September 2017 | 3 | − | − | − | − | − | − | − | + |
| E | November 2017 | 3 | − | + | − | − | + | + | + | − |
| E | January 2018 | 3 | − | + | + | + | + | + | + | − |
| E | May 2018 | 3 | − | + | + | + | + | + | + | − |
| WWTP | TSS (mg/L) | COD (mg/L) | pH |
|---|---|---|---|
| 1 | 11.04 ± 4.53 | 28.38 ± 8.86 | 6.55 ± 0.57 |
| 2 | 11.22 ± 5.53 | 25.32 ± 9.02 | 6.86 ± 0.32 |
| 3 | 10.08 ± 5.77 | 21.38 ± 7.93 | 6.90 ± 0.31 |
| WWTP | Sampling Period | Flow (m3/die) | Medium Flow (m3/h) | Contact Time (min) | Disinfectant Agent | Quantity * (mg/L) or (mJ/cm2) |
|---|---|---|---|---|---|---|
| 1 | September 2017 | 16,431 | 685 | 36.81 | NaClO | 0.69 |
| 1 | November 2017 | 11,720 | 488 | 51.60 | NaClO | 1.50 |
| 1 | January 2018 | 13,088 | 545 | 46.21 | NaClO | 1.34 |
| 1 | March 2018 | 14,126 | 589 | 42.81 | NaClO | 1.24 |
| 1 | May 2018 | 15,448 | 644 | 39.15 | NaClO | 0.73 |
| 1 | July 2018 | 17,774 | 741 | 34.03 | NaClO | 0.64 |
| 2 | September 2017 | 2451 | 102 | / | UV | 54 |
| 2 | November 2017 | 1605 | 67 | / | UV | 54 |
| 2 | January 2018 | 1638 | 68 | / | UV | 54 |
| 2 | March 2018 | 1394 | 58 | / | UV | 54 |
| 2 | May 2018 | 2851 | 119 | / | UV | 54 |
| 2 | July 2018 | 1485 | 62 | / | UV | 54 |
| 3 | September 2017 | 1556 | 65 | 18.66 | PAA | 3.93 |
| 3 | November 2017 | 1710 | 71 | 16.98 | PAA | 3.58 |
| 3 | January 2018 | 1528 | 64 | 19.01 | PAA | 4.01 |
| 3 | March 2018 | 1920 | 80 | 15.12 | PAA | 3.19 |
| 3 | May 2018 | 2436 | 102 | 11.92 | PAA | 2.51 |
| 3 | July 2018 | 2218 | 92 | 13.09 | PAA | 2.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetta, S.; Pignata, C.; Bonetta, S.; Amagliani, G.; Brandi, G.; Gilli, G.; Carraro, E. Comparison of UV, Peracetic Acid and Sodium Hypochlorite Treatment in the Disinfection of Urban Wastewater. Pathogens 2021, 10, 182. https://doi.org/10.3390/pathogens10020182
Bonetta S, Pignata C, Bonetta S, Amagliani G, Brandi G, Gilli G, Carraro E. Comparison of UV, Peracetic Acid and Sodium Hypochlorite Treatment in the Disinfection of Urban Wastewater. Pathogens. 2021; 10(2):182. https://doi.org/10.3390/pathogens10020182
Chicago/Turabian StyleBonetta, Silvia, Cristina Pignata, Sara Bonetta, Giulia Amagliani, Giorgio Brandi, Giorgio Gilli, and Elisabetta Carraro. 2021. "Comparison of UV, Peracetic Acid and Sodium Hypochlorite Treatment in the Disinfection of Urban Wastewater" Pathogens 10, no. 2: 182. https://doi.org/10.3390/pathogens10020182
APA StyleBonetta, S., Pignata, C., Bonetta, S., Amagliani, G., Brandi, G., Gilli, G., & Carraro, E. (2021). Comparison of UV, Peracetic Acid and Sodium Hypochlorite Treatment in the Disinfection of Urban Wastewater. Pathogens, 10(2), 182. https://doi.org/10.3390/pathogens10020182










