Nitroreductase Activites in Giardia lamblia: ORF 17150 Encodes a Quinone Reductase with Nitroreductase Activity
Abstract
1. Introduction
2. Results
2.1. Size Distribution of NAD(P)H-Dependent NR Activities in G. lamblia Extracts
2.2. Identification of Proteins in Fractions with Reductase Activities
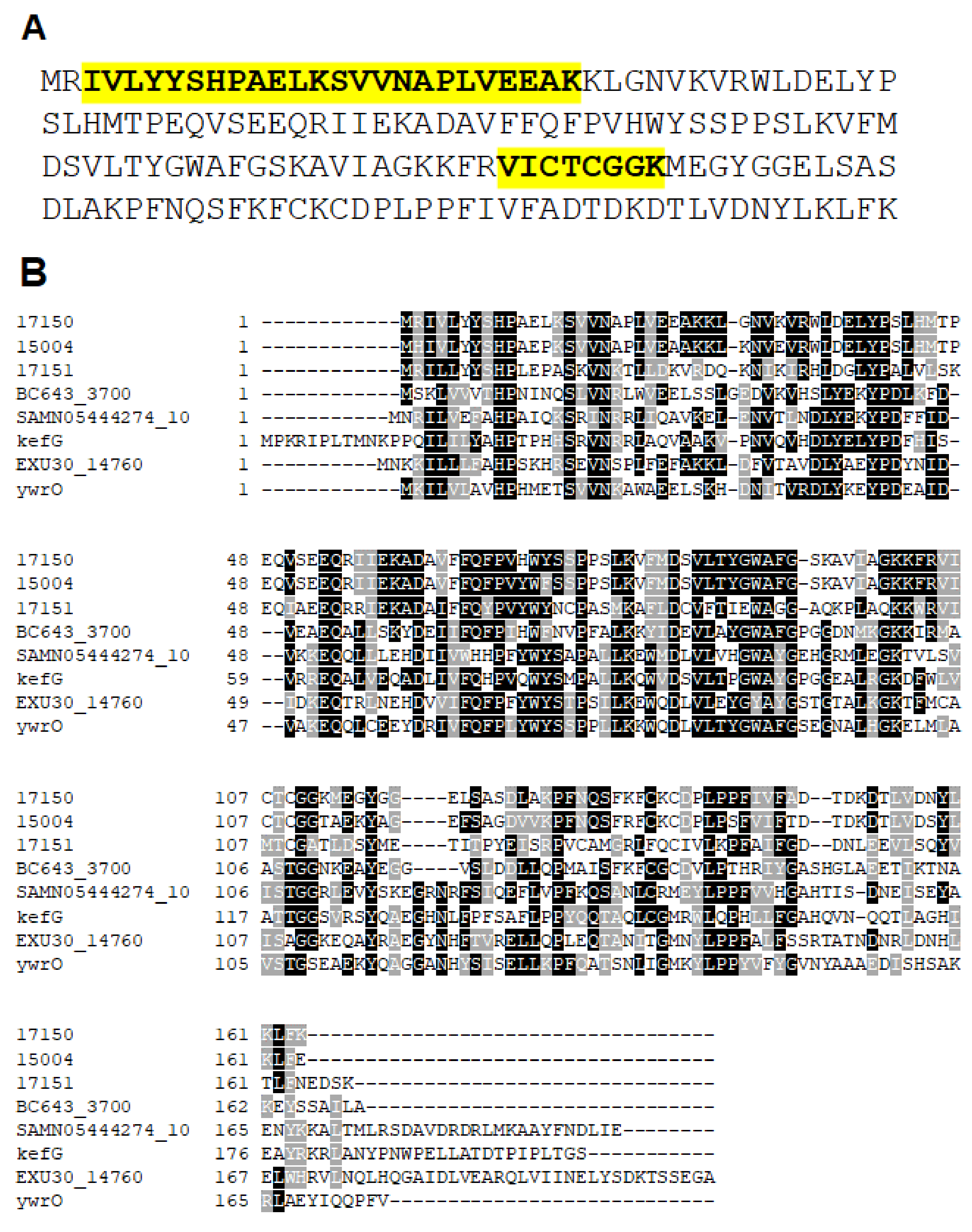
2.3. Identification of a Putative NADPH Oxidase Encoded by ORF 17150
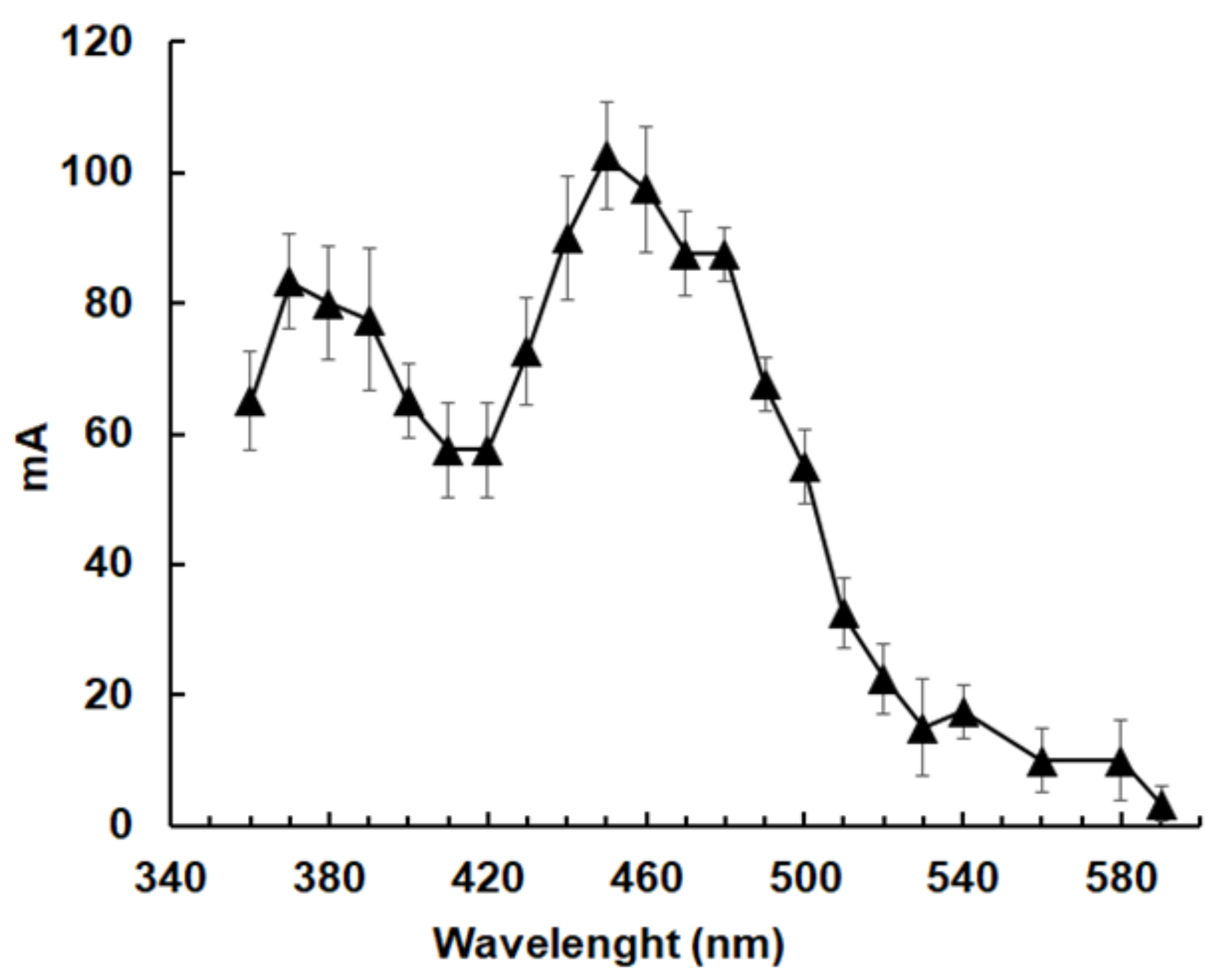
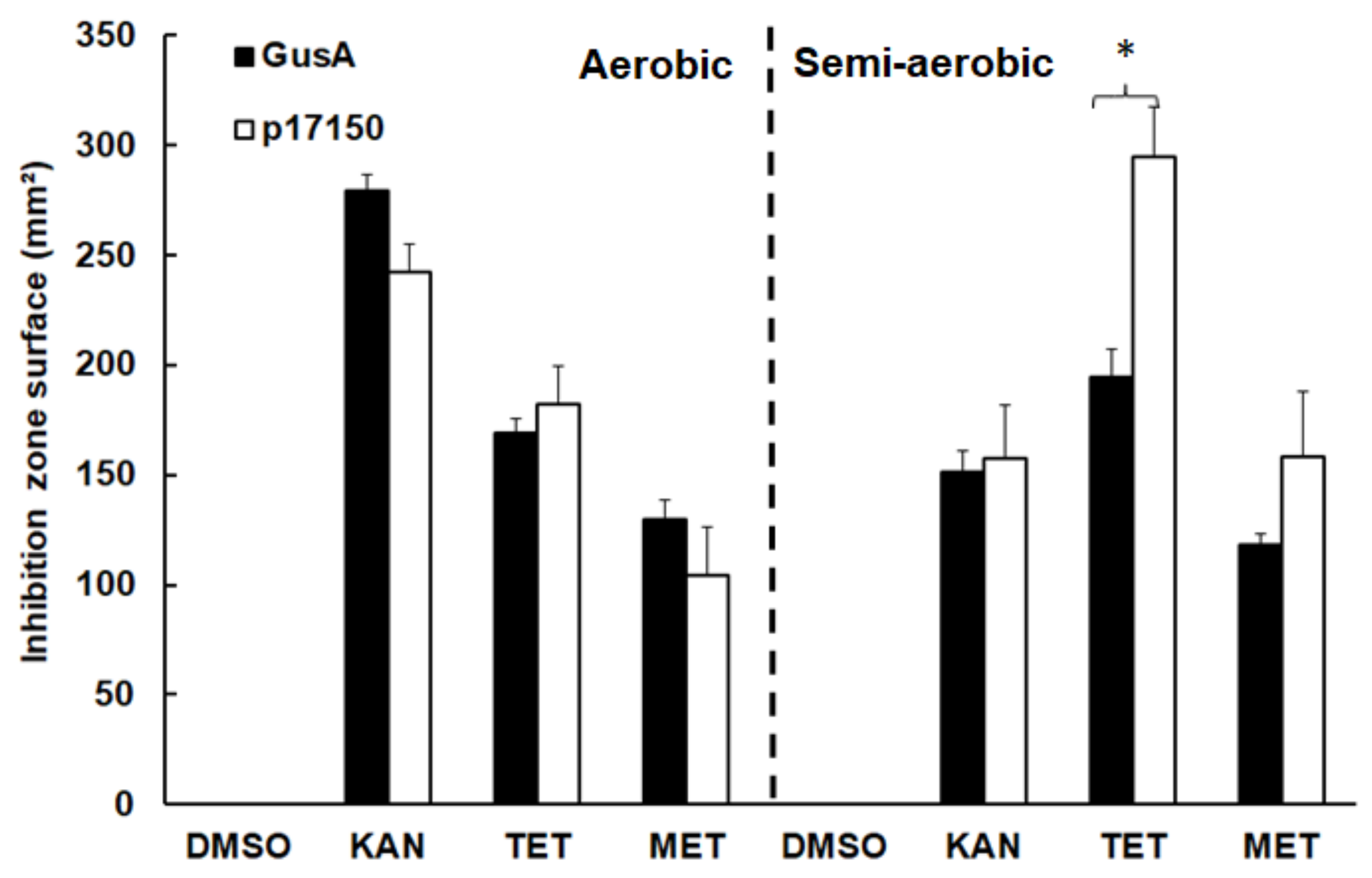
2.4. The Polypeptide Encoded by ORF 17150 Is a NADPH-Dependent Quinone Reductase with Nitroreductase Activity
3. Discussion
4. Materials and Methods
4.1. Biochemicals
4.2. Axenic Culture, Harvest, and Storage of G. lamblia Trophozoites
4.3. Size Exclusion Chromatography
4.4. Proteomic Analysis of the Fractions by Mass Spectrometry
4.5. Cloning, Expression, and Affinity Purification of the Protein Encoded by ORF 17150
4.6. Functional Assays
4.7. Drug Susceptibility Tests in E. coli
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, N.; Müller, J. Giardia. In Molecular Parasitology; Walochnik, J., Duchêne, M., Eds.; Springer-Verlag: Vienna, Austria, 2016; pp. 93–114. [Google Scholar]
- Cernikova, L.; Faso, C.; Hehl, A.B. Five facts about Giardia lamblia. PLoS Pathog. 2018, 14, e1007250. [Google Scholar] [CrossRef]
- Hemphill, A.; Müller, N.; Müller, J. Comparative pathobiology of the intestinal protozoan parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum. Pathogens 2019, 8, 116. [Google Scholar] [CrossRef]
- Van Voorhis, W. Protozoan infections. Sci. Am. Med. 2014. [Google Scholar] [CrossRef]
- Gardner, T.B.; Hill, D.R. Treatment of giardiasis. Clin. Microbiol. Rev. 2001, 14, 114–128. [Google Scholar] [CrossRef]
- Nash, T.E. Treatment of Giardia lamblia infections. Pediatr. Infect. Dis. J. 2001, 20, 193–195. [Google Scholar]
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2017, 146, 1167–1178. [Google Scholar] [CrossRef]
- Hemphill, A.; Müller, N.; Müller, J. Thiazolides, a novel class of anti-infective drugs, effective against viruses, bacteria, intracellular and extracellular protozoan parasites and proliferating mammalian cells. Antiinfect. Agents 2013, 11, 22–30. [Google Scholar]
- Brown, D.M.; Upcroft, J.A.; Edwards, M.R.; Upcroft, P. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int. J. Parasitol. 1998, 28, 149–164. [Google Scholar]
- Leitsch, D.; Burgess, A.G.; Dunn, L.A.; Krauer, K.G.; Tan, K.; Duchêne, M.; Upcroft, P.; Eckmann, L.; Upcroft, J.A. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 2011, 66, 1756–1765. [Google Scholar] [CrossRef]
- Müller, J.; Hemphill, A. New approaches for the identification of drug targets in protozoan parasites. Int. Rev. Cell Mol. Biol. 2013, 301, 359–401. [Google Scholar] [CrossRef]
- Müller, J.; Wastling, J.; Sanderson, S.; Müller, N.; Hemphill, A. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 2007, 51, 1979–1986. [Google Scholar] [CrossRef]
- Nillius, D.; Müller, J.; Müller, N. Nitroreductase (GlNR1) increases susceptibility of Giardia lamblia and Escherichia coli to nitro drugs. J. Antimicrob. Chemother. 2011, 66, 1029–1035. [Google Scholar] [CrossRef]
- Müller, J.; Schildknecht, P.; Müller, N. Metabolism of nitro drugs metronidazole and nitazoxanide in Giardia lamblia: Characterization of a novel nitroreductase (GlNR2). J. Antimicrob. Chemother. 2013, 68, 1781–1789. [Google Scholar] [CrossRef]
- Müller, J.; Müller, N. Nitroreductases of bacterial origin in Giardia lamblia: Potential role in detoxification of xenobiotics. Microbiologyopen 2019, e904. [Google Scholar] [CrossRef]
- Müller, J.; Rout, S.; Leitsch, D.; Vaithilingam, J.; Hehl, A.; Müller, N. Comparative characterisation of two nitroreductases from Giardia lamblia as potential activators of nitro compounds. Int. J. Parasitol. Drugs Drug. Resist. 2015, 5, 37–43. [Google Scholar] [CrossRef]
- Leitsch, D.; Müller, J.; Müller, N. Evaluation of Giardia lamblia thioredoxin reductase as drug activating enzyme and as drug target. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 148–153. [Google Scholar] [CrossRef]
- Lalle, M.; Camerini, S.; Cecchetti, S.; Finelli, R.; Sferra, G.; Müller, J.; Ricci, G.; Pozio, E. The FAD-dependent glycerol-3-phosphate dehydrogenase of Giardia duodenalis: An unconventional enzyme that interacts with the g14-3-3 and it is a target of the antitumoral compound NBDHEX. Front. Microbiol. 2015, 6, 544. [Google Scholar] [CrossRef]
- Mastronicola, D.; Testa, F.; Forte, E.; Bordi, E.; Pucillo, L.P.; Sarti, P.; Giuffrè, A. Flavohemoglobin and nitric oxide detoxification in the human protozoan parasite Giardia intestinalis. Biochem. Biophys. Res. Commun. 2010, 399, 654–658. [Google Scholar] [CrossRef]
- Rafferty, S.; Luu, B.; March, R.E.; Yee, J. Giardia lamblia encodes a functional flavohemoglobin. Biochem. Biophys. Res. Commun. 2010, 399, 347–351. [Google Scholar] [CrossRef]
- Di Matteo, A.; Scandurra, F.M.; Testa, F.; Forte, E.; Sarti, P.; Brunori, M.; Giuffrè, A. The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. J. Biol. Chem. 2008, 283, 4061–4068. [Google Scholar] [CrossRef]
- Vicente, J.B.; Testa, F.; Mastronicola, D.; Forte, E.; Sarti, P.; Teixeira, M.; Giuffrè, A. Redox properties of the oxygen-detoxifying flavodiiron protein from the human parasite Giardia intestinalis. Arch. Biochem. Biophys. 2009, 488, 9–13. [Google Scholar] [CrossRef]
- Arguello-Garcia, R.; Cruz-Soto, M.; Gonzalez-Trejo, R.; Paz-Maldonado, L.M.; Bazan-Tejeda, M.L.; Mendoza-Hernandez, G.; Ortega-Pierres, G. An antioxidant response is involved in resistance of Giardia duodenalis to albendazole. Front. Microbiol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Ma’ayeh, S.Y.; Knorr, L.; Svard, S.G. Transcriptional profiling of Giardia intestinalis in response to oxidative stress. Int. J. Parasitol. 2015, 45, 925–938. [Google Scholar] [CrossRef]
- Mastronicola, D.; Falabella, M.; Forte, E.; Testa, F.; Sarti, P.; Giuffre, A. Antioxidant defence systems in the protozoan pathogen Giardia intestinalis. Mol. Biochem. Parasitol. 2016, 206, 56–66. [Google Scholar] [CrossRef]
- Emery, S.J.; Baker, L.; Ansell, B.R.E.; Mirzaei, M.; Haynes, P.A.; McConville, M.J.; Svard, S.G.; Jex, A.R. Differential protein expression and post-translational modifications in metronidazole-resistant Giardia duodenalis. Gigascience 2018, 7. [Google Scholar] [CrossRef]
- Müller, J.; Braga, S.; Heller, M.; Müller, N. Resistance formation to nitro drugs in Giardia lamblia: No common markers identified by comparative proteomics. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 112–119. [Google Scholar]
- Brown, D.M.; Upcroft, J.A.; Upcroft, P. A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur. J. Biochem. 1996, 241, 155–161. [Google Scholar]
- Müller, J.; Hemphill, A.; Müller, N. Physiological aspects of nitro drug resistance in Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 271–277. [Google Scholar] [CrossRef]
- Müller, J.; Braga, S.; Uldry, A.C.; Heller, M.; Müller, N. Comparative proteomics of three Giardia lamblia strains: Investigation of antigenic variation in the post-genomic era. Parasitology 2020, 147, 1008–1018. [Google Scholar] [CrossRef]
- Vermathen, M.; Müller, J.; Furrer, J.; Müller, N.; Vermathen, P. 1H HR-MAS NMR spectroscopy to study the metabolome of the protozoan parasite Giardia lamblia. Talanta 2018, 188, 429–441. [Google Scholar] [CrossRef]
- Saghaug, C.S.; Klotz, C.; Kallio, J.P.; Brattbakk, H.R.; Stokowy, T.; Aebischer, T.; Kursula, I.; Langeland, N.; Hanevik, K. Genetic variation in metronidazole metabolism and oxidative stress pathways in clinical Giardia lamblia assemblage A and B isolates. Infect. Drug Resist. 2019, 12, 1221–1235. [Google Scholar] [CrossRef]
- Jain, V.; Yogavel, M.; Sharma, A. Dimerization of arginyl-tRNA synthetase by free heme drives Its inactivation in Plasmodium falciparum. Structure 2016, 24, 1476–1487. [Google Scholar] [CrossRef]
- Nixon, J.E.; Wang, A.; Field, J.; Morrison, H.G.; McArthur, A.G.; Sogin, M.L.; Loftus, B.J.; Samuelson, J. Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica. Eukaryot. Cell 2002, 1, 181–190. [Google Scholar]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef]
- Anlezark, G.M.; Vaughan, T.; Fashola-Stone, E.; Michael, N.P.; Murdoch, H.; Sims, M.A.; Stubbs, S.; Wigley, S.; Minton, N.P. Bacillus amyloliquefaciens orthologue of Bacillus subtilis ywrO encodes a nitroreductase enzyme which activates the prodrug CB 1954. Microbiology 2002, 148, 297–306. [Google Scholar]
- Halling-Sorensen, B.; Sengelov, G.; Tjornelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Pham, J.K.; Nosala, C.; Scott, E.Y.; Nguyen, K.F.; Hagen, K.D.; Starcevich, H.N.; Dawson, S.C. Transcriptomic profiling of high-density Giardia foci encysting in the murine proximal intestine. Front. Cell. Infect. Microbiol. 2017, 7, 227. [Google Scholar] [CrossRef]
- Zenno, S.; Koike, H.; Kumar, A.N.; Jayaraman, R.; Tanokura, M.; Saigo, K. Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J. Bacteriol. 1996, 178, 4508–4514. [Google Scholar]
- Zenno, S.; Koike, H.; Tanokura, M.; Saigo, K. Conversion of NfsB, a minor Escherichia coli nitroreductase, to a flavin reductase similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri, by a single amino acid substitution. J. Bacteriol. 1996, 178, 4731–4733. [Google Scholar]
- Mowday, A.M.; Copp, J.N.; Syddall, S.P.; Dubois, L.J.; Wang, J.; Lieuwes, N.G.; Biemans, R.; Ashoorzadeh, A.; Abbattista, M.R.; Williams, E.M.; et al. E. coli nitroreductase NfsA is a reporter gene for non-invasive PET imaging in cancer gene therapy applications. Theranostics 2020, 10, 10548–10562. [Google Scholar] [CrossRef]
- Clark, C.G.; Diamond, L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar]
- Müller, J.; Rühle, G.; Müller, N.; Rossignol, J.F.; Hemphill, A. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 2006, 50, 162–170. [Google Scholar] [CrossRef][Green Version]
- Eng, J.K.; Hoopmann, M.R.; Jahan, T.A.; Egertson, J.D.; Noble, W.S.; MacCoss, M.J. A deeper look into Comet--implementation and features. J. Am. Soc. Mass Spectrom. 2015, 26, 1865–1874. [Google Scholar] [CrossRef]
- Craig, R.; Beavis, R.C. A method for reducing the time required to match protein sequences with tandem mass spectra. Rapid Commun. Mass Spectrom. 2003, 17, 2310–2316. [Google Scholar] [CrossRef]
- Kim, S.; Pevzner, P.A. MS-GF+ makes progress towards a universal database search tool for proteomics. Nat. Commun. 2014, 5, 5277. [Google Scholar] [CrossRef]
- Tabb, D.L.; Fernando, C.G.; Chambers, M.C. MyriMatch: Highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J. Proteome Res. 2007, 6, 654–661. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Mendoza, L.; Shteynberg, D.; Farrah, T.; Lam, H.; Tasman, N.; Sun, Z.; Nilsson, E.; Pratt, B.; Prazen, B.; et al. A guided tour of the Trans-Proteomic Pipeline. Proteomics 2010, 10, 1150–1159. [Google Scholar] [CrossRef]
- Choi, H.; Ghosh, D.; Nesvizhskii, A.I. Statistical validation of peptide identifications in large-scale proteomics using the target-decoy database search strategy and flexible mixture modeling. J. Proteome Res. 2008, 7, 286–292. [Google Scholar] [CrossRef]
- Shteynberg, D.; Deutsch, E.W.; Lam, H.; Eng, J.K.; Sun, Z.; Tasman, N.; Mendoza, L.; Moritz, R.L.; Aebersold, R.; Nesvizhskii, A.I. iProphet: Multi-level integrative analysis of shotgun proteomic data improves peptide and protein identification rates and error estimates. Mol. Cell Proteom. 2011, 10, M111-007690. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Aebersold, R. Interpretation of shotgun proteomic data: The protein inference problem. Mol. Cell Proteom. 2005, 4, 1419–1440. [Google Scholar] [CrossRef]
- Zybailov, B.L.; Florens, L.; Washburn, M.P. Quantitative shotgun proteomics using a protease with broad specificity and normalized spectral abundance factors. Mol. Biosyst. 2007, 3, 354–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Z.; Washburn, M.P.; Florens, L. Refinements to label free proteome quantitation: How to deal with peptides shared by multiple proteins. Anal. Chem. 2010, 82, 2272–2281. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation: Vienna, Austria, 2012. [Google Scholar]
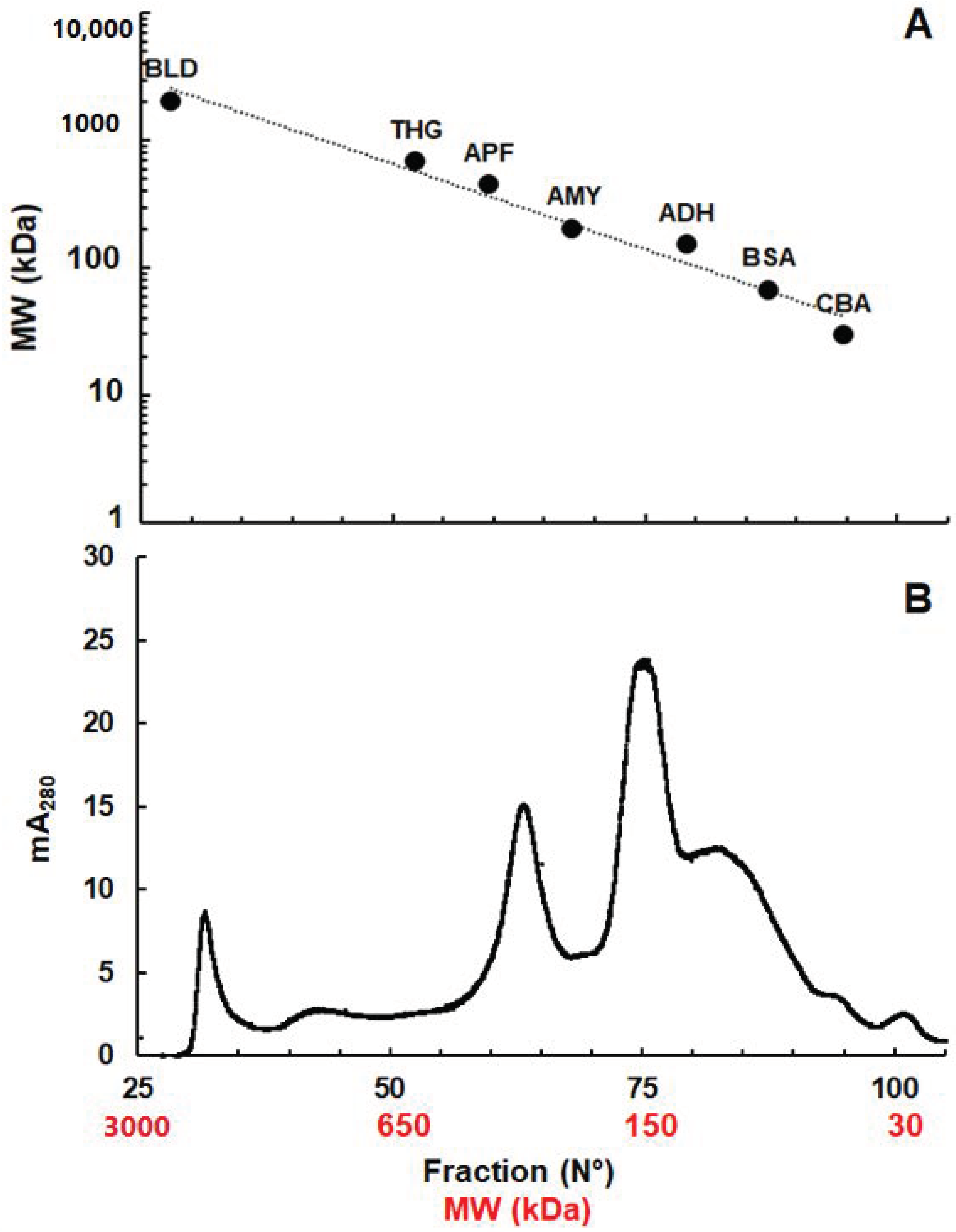



| Pool | Fractions | MW Approx. (kDa) | Identified Proteins (Number) | NR Activity | Confirmed Nitroreductases (GiardiaDB ORF - MW) | |
|---|---|---|---|---|---|---|
| 1 | 32–34 | 2000–1600 | 15 | Yes | No confirmed nitroreductases | |
| 2 | 44–46 | 950–840 | 104 | No | A-type FP 10358 | 45 |
| 3 | 61–63 | 330–280 | 405 | Yes | A-type FP 10358 | 45 |
| G3PDH 16125 | 119 | |||||
| NO 9719 | 47 | |||||
| NOLT 33769 | 53 | |||||
| PFOR 114609 | 138 | |||||
| PFOR 17063 | 132 | |||||
| TrxR 9827 | 35 | |||||
| 4 | 64–66 | 277–245 | 426 | Yes | A-type FP 10358 | 45 |
| G3PDH 16125 | 119 | |||||
| NO 9719 | 47 | |||||
| NOLT 33769 | 53 | |||||
| PFOR 114609 | 138 | |||||
| PFOR 17063 | 132 | |||||
| TrxR 9827 | 35 | |||||
| 5 | 70–72 | 190–170 | 985 | Yes | A-type FP 10358 | 45 |
| G3PDH 16125 | 119 | |||||
| NO 9719 | 47 | |||||
| NOLT 33769 | 53 | |||||
| PFOR 114609 | 138 | |||||
| PFOR 17063 | 132 | |||||
| TrxR 9827 | 35 | |||||
| NR1 22677 | 29 | |||||
| NR3 15307 | 19 | |||||
| 6 | 93–95 | 46–41 | 22 | Yes | No confirmed nitroreductases | |
| Giardia DB ORF. | Protein Annotation | Pools |
|---|---|---|
| 10521 | Arginyl-tRNA-synthetase | 5 |
| 11043 | Fructose-bisphosphate aldolase | 3,4,5 |
| 137716 | Axoneme-associated protein GASP-180 | 2,5 |
| 17411 | TCP1-chaperon-subunit gamma | 3,4,5 |
| 3331 | Malate dehydrogenase | 3,4,5 |
| 6175 | Nitroreductase family protein fused to ferredoxin domain Fd-NR1 (“NR2”) | nd |
| 7532 | Vacuolar ATP-synthase catalytic subunit A | 3,4,5 |
| 90872 | Phosphoglycerate kinase | 3,4,5 |
| 9183 | Hypothetical protein | 2,3,4,5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, J.; Heller, M.; Uldry, A.-C.; Braga, S.; Müller, N. Nitroreductase Activites in Giardia lamblia: ORF 17150 Encodes a Quinone Reductase with Nitroreductase Activity. Pathogens 2021, 10, 129. https://doi.org/10.3390/pathogens10020129
Müller J, Heller M, Uldry A-C, Braga S, Müller N. Nitroreductase Activites in Giardia lamblia: ORF 17150 Encodes a Quinone Reductase with Nitroreductase Activity. Pathogens. 2021; 10(2):129. https://doi.org/10.3390/pathogens10020129
Chicago/Turabian StyleMüller, Joachim, Manfred Heller, Anne-Christine Uldry, Sophie Braga, and Norbert Müller. 2021. "Nitroreductase Activites in Giardia lamblia: ORF 17150 Encodes a Quinone Reductase with Nitroreductase Activity" Pathogens 10, no. 2: 129. https://doi.org/10.3390/pathogens10020129
APA StyleMüller, J., Heller, M., Uldry, A.-C., Braga, S., & Müller, N. (2021). Nitroreductase Activites in Giardia lamblia: ORF 17150 Encodes a Quinone Reductase with Nitroreductase Activity. Pathogens, 10(2), 129. https://doi.org/10.3390/pathogens10020129






