Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology
Abstract
1. Introduction
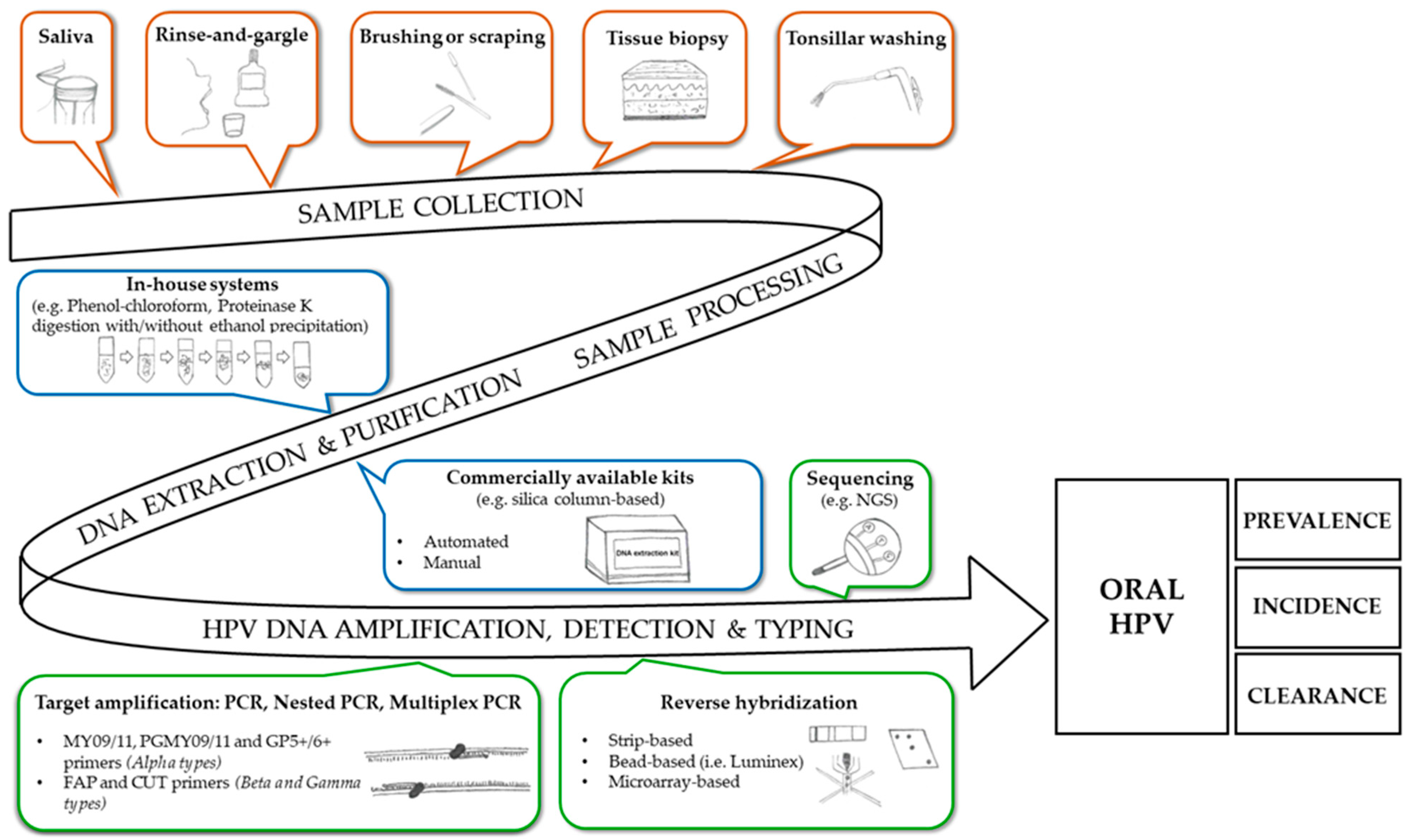
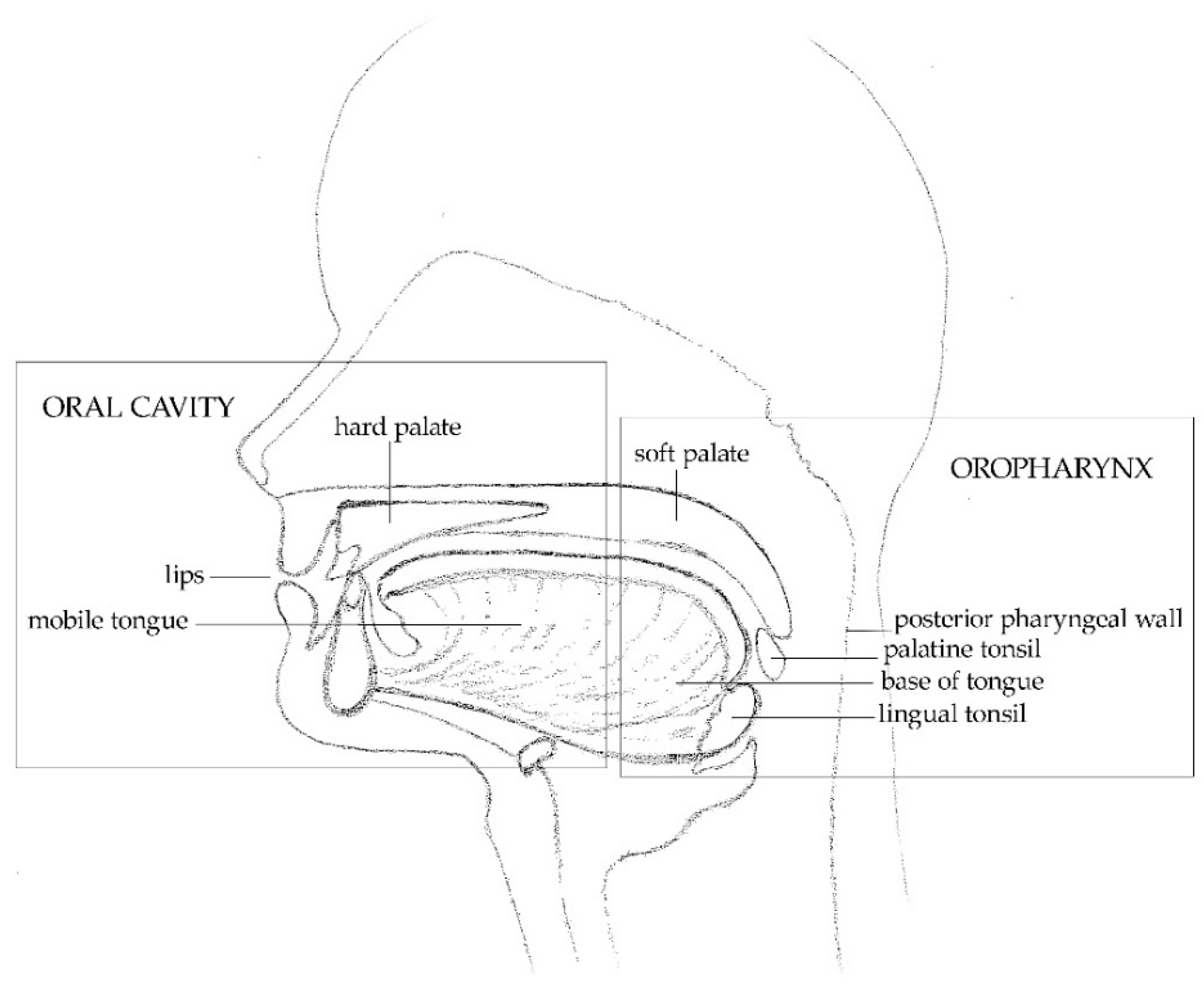
2. Oral Infection by Alpha, Beta and Gamma HPVs: The Methodological Aspects
2.1. Sample Collection, Nucleic Acid Extraction and Purification
2.2. HPV-DNA Detection and Typing
2.2.1. Alpha-HPVs
2.2.2. Beta and Gamma HPVs
2.2.3. Known and Novel HPVs
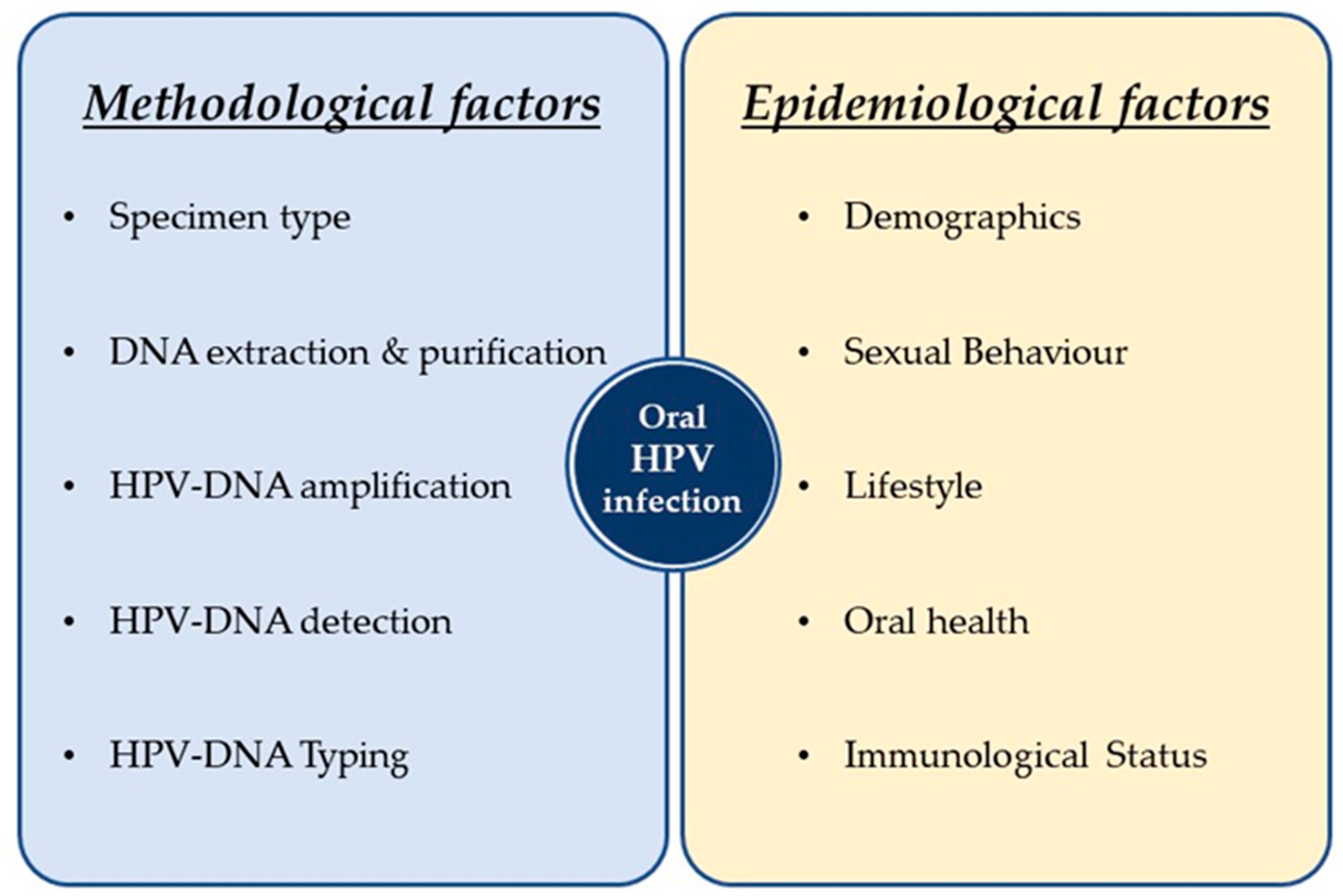
3. Oral Infection by Alpha, Beta and Gamma HPVs: The Epidemiological Aspects
3.1. General Population
3.1.1. Prevalence
3.1.2. Predictors
3.1.3. Natural History
3.2. MSM and HIV-Infected Individuals
3.2.1. Prevalence
3.2.2. Predictors
3.2.3. Natural History
4. Oral Infection by Alpha, Beta and Gamma HPVs: The Clinical Aspects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef]
- De Villiers, E.M. Cross-roads in the classification of papillomaviruses. Virology 2013, 445, 2–10. [Google Scholar] [CrossRef]
- Donà, M.G.; Gheit, T.; Latini, A.; Benevolo, M.; Torres, M.; Smelov, V.; McKay-Chopin, S.; Giglio, A.; Cristaudo, A.; Zaccarelli, M.; et al. Alpha, beta and gamma human papillomaviruses in the anal canal of HIV-infected and uninfected men who have sex with men. J. Infect. 2015, 71, 74–84. [Google Scholar] [CrossRef]
- Forslund, O.; Johansson, H.; Madsen, K.G.; Kofoed, K. The nasal mucosa contains a large spectrum of human papillomavirus types from the betapapillomavirus and gammapapillomavirus genera. J. Infect. Dis. 2013, 208, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, B.; Kocjan, B.J.; Hošnjak, L.; Fujs Komloš, K.; Milošević, M.; Poljak, M. Betapapillomaviruses in the anal canal of HIV positive and HIV negative men who have sex with men. J. Clin. Virol. 2014, 61, 237–241. [Google Scholar] [CrossRef]
- Nunes, E.M.; Sudenga, S.L.; Gheit, T.; Tommasino, M.; Baggio, M.L.; Ferreira, S.; Galan, L.; Silva, R.C.; Pierce Campbell, C.M.; Lazcano-Ponce, E.; et al. Diversity of beta-papillomavirus at anogenital and oral anatomic sites of men: The HIM study. Virology 2016, 495, 33–41. [Google Scholar] [CrossRef]
- Smelov, V.; Muwonge, R.; Sokolova, O.; McKay-Chopin, S.; Eklund, C.; Komyakov, B.; Gheit, T. Beta and gamma human papillomaviruses in anal and genital sites among men: Prevalence and determinants. Sci. Rep. 2018, 8, 8241. [Google Scholar] [CrossRef]
- Altamura, G.; Tommasino, M.; Borzacchiello, G. Cutaneous vs. mucosal tropism: The papillomavirus paradigm comes to an “and”. Front. Microbiol. 2020, 11, 588663. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans—Volume 100B—A Review of Human Carcinogens: Biological Agents; IARC: Lyon, France, 2012.
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, S0140-6736(21)01550-6. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Partricia, S.; Mathan, G. Overview of high-risk HPV’s 16 and 18 infected cervical cancer: Pathogenesis to prevention. Biomed. Pharmacother. 2015, 70, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; Kleter, B.; Zhou, M.; Ayala, G.; Cubilla, A.L.; Quint, W.G.; Pirog, E.C. Detection and typing of human papillomavirus DNA in penile carcinoma: Evidence for multiple independent pathways of penile carcinogenesis. Am. J. Pathol. 2001, 159, 1211–1218. [Google Scholar] [CrossRef]
- De Vuyst, H.; Clifford, G.M.; Nascimento, M.C.; Madeleine, M.M.; Franceschi, S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. Int. J. Cancer 2009, 124, 1626–1636. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguière, A.; Manuguerra, J.C.; et al. Human skin microbiota: High diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS ONE 2012, 7, e38499. [Google Scholar] [CrossRef]
- Hampras, S.S.; Reed, R.A.; Bezalel, S.; Cameron, M.; Cherpelis, B.; Fenske, N.; Sondak, V.K.; Messina, J.; Tommasino, M.; Gheit, T.; et al. Cutaneous human papillomavirus infection and development of subsequent squamous cell carcinoma of the skin. J. Skin Cancer 2016, 2016, 1368103. [Google Scholar] [CrossRef]
- Bottalico, D.; Chen, Z.; Dunne, A.; Ostoloza, J.; McKinney, S.; Sun, C.; Schlecht, N.F.; Fatahzadeh, M.; Herrero, R.; Schiffman, M.; et al. The oral cavity contains abundant known and novel human papillomaviruses from the betapapillomavirus and gammapapillomavirus genera. J. Infect. Dis. 2011, 204, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Muhr, L.S.A.; Eklund, C.; Dillner, J. Towards quality and order in human papillomavirus research. Virology 2018, 519, 74–76. [Google Scholar] [CrossRef]
- Kellokoski, J.K.; Syrjanen, S.M.; Chang, F.; Yliskoski, M.; Syrjänen, K.J. Southern blot hybridization and PCR in detection of oral human papillomavirus (HPV) infections in women with genital HPV infections. J. Oral Pathol. Med. 1992, 21, 459–464. [Google Scholar] [CrossRef]
- Broccolo, F.; Cocuzza, C.E. Automated extraction and quantitation of oncogenic HPV genotypes from cervical samples by a real-time PCR-based system. J. Virol. Methods 2008, 148, 48–57. [Google Scholar] [CrossRef]
- Seaman, W.T.; Andrews, E.; Couch, M.; Kojic, E.M.; Cu-Uvin, S.; Palefsky, J.; Deal, A.M.; Webster-Cyriaque, J. Detection and quantitation of HPV in genital and oral tissues and fluids by real time PCR. Virol. J. 2010, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; de Souza, M.; Wood, Z.C.; Carroll, A.; Van, K.; Paterson, L.; Pandeya, N.; Whiteman, D.C. Natural history of oral HPV infection: Longitudinal analyses in prospective cohorts from australia. Int. J. Cancer 2021, 148, 1964–1972. [Google Scholar] [CrossRef]
- Antonsson, A.; Neale, R.E.; Boros, S.; Lampe, G.; Coman, W.B.; Pryor, D.I.; Porceddu, S.V.; Whiteman, D.C. Human papillomavirus status and p16(INK4A) expression in patients with mucosal squamous cell carcinoma of the head and neck in queensland, australia. Cancer Epidemiol. 2015, 39, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Emmett, S.; Boros, S.; Whiteman, D.C.; Porceddu, S.V.; Panizza, B.J.; Antonsson, A. Sexual behaviour, HPV status and p16(INK4a) expression in oropharyngeal and oral cavity squamous cell carcinomas: A case-case comparison study. J. Gen. Virol. 2018, 99, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Emmett, S.; Jenkins, G.; Boros, S.; Whiteman, D.C.; Panizza, B.; Antonsson, A. Low prevalence of human papillomavirus in oral cavity squamous cell carcinoma in queensland, australia. ANZ J. Surg. 2017, 87, 714–719. [Google Scholar] [CrossRef]
- Wendland, E.M.; Kops, N.L.; Comerlato, J.; Horvath, J.D.C.; Bessel, M.; Sperb, D.; Pimenta, C.; de Souza, F.M.A.; Mendes Pereira, G.F.; Falcetta, F.S. STOP HPV study protocol: A nationwide case-control study of the association between oropharyngeal cancer and human papillomavirus (HPV) infection in brazil. BMJ Open 2020, 10, e031602. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.M.A.; Hartel, G.; Whiteman, D.C.; Antonsson, A. Detection of oral HPV infection—Comparison of two different specimen collection methods and two HPV detection methods. Diagn Microbiol. Infect. Dis. 2018, 90, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Fuessel Haws, A.L.; He, Q.; Rady, P.L.; Zhang, L.; Grady, J.; Hughes, T.K.; Stisser, K.; Konig, R.; Tyring, S.K. Nested PCR with the PGMY09/11 and GP5(+)/6(+) primer sets improves detection of HPV-DNA in cervical samples. J. Virol. Methods 2004, 122, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kishikawa, T.; Tokita, Y.; Suzuki, M.; Takemoto, N.; Hanamoto, A.; Fukusumi, T.; Yamamoto, M.; Fujii, M.; Ohno, Y.; et al. Prevalence of human papillomavirus in oral gargles and tonsillar washings. Oral Oncol. 2020, 105, 104669. [Google Scholar] [CrossRef] [PubMed]
- Donà, M.G.; Pichi, B.; Rollo, F.; Benevolo, M.; Latini, A.; Laquintana, V.; Pellini, R.; Colafigli, M.; Frasca, M.; Giuliani, M.; et al. Human papillomavirus detection in matched oral rinses, oropharyngeal and oral brushings of cancer-free high-risk individuals. Oral Oncol. 2019, 91, 1–6. [Google Scholar] [CrossRef]
- D’Souza, G.; Sugar, E.; Ruby, W.; Gravitt, P.; Gillison, M. Analysis of the effect of DNA purification on detection of human papillomavirus in oral rinse samples by PCR. J. Clin. Microbiol. 2005, 43, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Poljak, M.; Ostrbenk Valencak, A.; Gimpelj Domjanic, G.; Xu, L.; Arbyn, M. Commercially available molecular tests for human papillomaviruses: A global overview. Clin. Microbiol. Infect. 2020, 26, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Manos, M.M.; Ting Shin, Y.; Wright, D.K.; Lewis, A.I.; Broker, T.R.; Wolinsky, S.M.; Manos, M.; Ting, Y.C. The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells 1989, 7, 209–214. [Google Scholar]
- Jacobs, M.V.; de Roda Husman, A.M.; van den Brule, A.J.; Snijders, P.J.; Meijer, C.J.; Walboomers, J.M. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J. Clin. Microbiol. 1995, 33, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Gravitt, P.E.; Peyton, C.L.; Alessi, T.Q.; Wheeler, C.M.; Coutlée, F.; Hildesheim, A.; Schiffman, M.H.; Scott, D.R.; Apple, R.J. Improved amplification of genital human papillomaviruses. J. Clin. Microbiol. 2000, 38, 357–361. [Google Scholar] [CrossRef]
- Kleter, B.; van Doorn, L.J.; ter Schegget, J.; Schrauwen, L.; van Krimpen, K.; Burger, M.; ter Harmsel, B.; Quint, W. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am. J. Pathol. 1998, 153, 1731–1739. [Google Scholar] [CrossRef]
- Kleter, B.; van Doorn, L.J.; Schrauwen, L.; Molijn, A.; Sastrowijoto, S.; ter Schegget, J.; Lindeman, J.; ter Harmsel, B.; Burger, M.; Quint, W. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J. Clin. Microbiol. 1999, 37, 2508–2517. [Google Scholar] [CrossRef]
- Morbini, P.; Dal Bello, B.; Alberizzi, P.; Mannarini, L.; Mevio, N.; Bertino, G.; Benazzo, M. Exfoliated cells of the oral mucosa for HPV typing by SPF10 in head and neck cancer. J. Virol. Methods 2012, 186, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Beachler, D.C.; Lang Kuhs, K.A.; Struijk, L.; Schussler, J.; Herrero, R.; Porras, C.; Hildesheim, A.; Cortes, B.; Sampson, J.; Quint, W.; et al. The natural history of oral human papillomavirus in young Costa Rican women. Sex Transm. Dis. 2017, 44, 442–449. [Google Scholar] [CrossRef]
- Bettampadi, D.; Sirak, B.A.; Fulp, W.J.; Abrahamsen, M.; Villa, L.L.; Lazcano-Ponce, E.; Salmeron, J.; Isaacs-Soriano, K.A.; Baggio, M.L.; Trenado, M.Q.; et al. Oral HPV prevalence assessment by linear array vs. SPF10 PCR-DEIA-LiPA25 system in the HPV infection in men (HIM) study. Papillomavirus Res. 2020, 9, 100199. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R. Human papillomavirus in head and neck cancer. Cancers 2014, 6, 1705–1726. [Google Scholar] [CrossRef]
- Forslund, O.; Antonsson, A.; Nordin, P.; Stenquist, B.; Hansson, B.G. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999, 80, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Forslund, O.; Ly, H.; Reid, C.; Higgins, G. A broad spectrum of human papillomavirus types is present in the skin of australian patients with non-melanoma skin cancers and solar keratosis. Br. J. Dermatol. 2003, 149, 64–73. [Google Scholar] [CrossRef]
- Forslund, O. Genetic diversity of cutaneous human papillomaviruses. J. Gen. Virol. 2007, 88, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Schiffman, M.; Herrero, R.; Burk, R.D. Identification and characterization of two novel human papillomaviruses (HPVs) by overlapping PCR: HPV102 and HPV106. J. Gen. Virol. 2007, 88, 2952–2955. [Google Scholar] [CrossRef]
- Vasiljevic, N.; Hazard, K.; Dillner, J.; Forslund, O. Four novel human betapapillomaviruses of species 2 preferentially found in actinic keratosis. J. Gen. Virol. 2008, 89, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Chouhy, D.; Gorosito, M.; Sanchez, A.; Serra, E.C.; Bergero, A.; Fernandez Bussy, R.; Giri, A.A. New generic primer system targeting mucosal/genital and cutaneous human papillomaviruses leads to the characterization of HPV 115, a novel beta-papillomavirus species 3. Virology 2010, 397, 205–216. [Google Scholar] [CrossRef]
- Chouhy, D.; Bolatti, E.M.; Perez, G.R.; Giri, A.A. Analysis of the genetic diversity and phylogenetic relationships of putative human papillomavirus types. J. Gen. Virol. 2013, 94, 2480–2488. [Google Scholar] [CrossRef]
- Rector, A.; Tachezy, R.; Van Ranst, M. A sequence-independent strategy for detection and cloning of circular DNA virus genomes by using multiply primed rolling-circle amplification. J. Virol. 2004, 78, 4993–4998. [Google Scholar] [CrossRef]
- Martin, E.; Dang, J.; Bzhalava, D.; Stern, J.; Edelstein, Z.R.; Koutsky, L.A.; Kiviat, N.B.; Feng, Q. Characterization of three novel human papillomavirus types isolated from oral rinse samples of healthy individuals. J. Clin. Virol. 2014, 59, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Gottschling, M.; Forster, J.; Rowert-Huber, J.; Stockfleth, E.; Nindl, I. Genomic characterization of a novel human papillomavirus (HPV-117) with a high viral load in a persisting wart. Virology 2010, 399, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Gottschling, M.; Manning, K.; Lehmann, M.D.; Schulz, E.; Kruger-Corcoran, D.; Stockfleth, E.; Nindl, I. Genomic characterization of ten novel cutaneous human papillomaviruses from keratotic lesions of immunosuppressed patients. J. Gen. Virol. 2011, 92, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Gheit, T.; Landi, S.; Gemignani, F.; Snijders, P.J.; Vaccarella, S.; Franceschi, S.; Canzian, F.; Tommasino, M. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J. Clin. Microbiol. 2006, 44, 2025–2031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gheit, T.; Billoud, G.; de Koning, M.N.; Gemignani, F.; Forslund, O.; Sylla, B.S.; Vaccarella, S.; Franceschi, S.; Landi, S.; Quint, W.G.; et al. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect betapapillomavirus types. J. Clin. Microbiol. 2007, 45, 2537–2544. [Google Scholar] [CrossRef]
- Nilyanimit, P.; Chansaenroj, J.; Poomipak, W.; Praianantathavorn, K.; Payungporn, S.; Poovorawan, Y. Comparison of four human papillomavirus genotyping methods: Next-generation sequencing, INNO-LiPA, electrochemical DNA chip, and nested-PCR. Ann. Lab. Med. 2018, 38, 139–146. [Google Scholar] [CrossRef]
- Flores-Miramontes, M.G.; Torres-Reyes, L.A.; Alvarado-Ruiz, L.; Romero-Martinez, S.A.; Ramirez-Rodriguez, V.; Balderas-Pena, L.M.; Vallejo-Ruiz, V.; Pina-Sanchez, P.; Cortes-Gutierrez, E.I.; Jave-Suarez, L.F.; et al. Human papillomavirus genotyping by linear array and next-generation sequencing in cervical samples from western mexico. Virol. J. 2015, 12, 161–164. [Google Scholar] [CrossRef]
- Flores-Miramontes, M.G.; Olszewski, D.; Artaza-Irigaray, C.; Willemsen, A.; Bravo, I.G.; Vallejo-Ruiz, V.; Leal-Herrera, Y.A.; Pina-Sanchez, P.; Molina-Pineda, A.; Canton-Romero, J.C.; et al. Detection of alpha, beta, gamma, and unclassified human papillomaviruses in cervical cancer samples from mexican women. Front. Cell Infect. Microbiol. 2020, 10, 234. [Google Scholar] [CrossRef]
- Wagner, S.; Roberson, D.; Boland, J.; Yeager, M.; Cullen, M.; Mirabello, L.; Dunn, S.T.; Walker, J.; Zuna, R.; Schiffman, M.; et al. Development of the TypeSeq assay for detection of 51 human papillomavirus genotypes by next-generation sequencing. J. Clin. Microbiol. 2019, 57, e01794-18. [Google Scholar] [CrossRef]
- Schmitt, M.; Dondog, B.; Waterboer, T.; Pawlita, M.; Tommasino, M.; Gheit, T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010, 48, 143–149. [Google Scholar] [CrossRef]
- Pastrana, D.V.; Peretti, A.; Welch, N.L.; Borgogna, C.; Olivero, C.; Badolato, R.; Notarangelo, L.D.; Gariglio, M.; FitzGerald, P.C.; McIntosh, C.E.; et al. Metagenomic discovery of 83 new human papillomavirus types in patients with immunodeficiency. mSphere 2018, 3, e00645-18. [Google Scholar] [CrossRef]
- Ganly, I.; Pei, Z.; Hao, Y.; Ma, Y.; Rosenthal, M.; Wu, Z.; Migliacci, J.; Huang, B.; Katabi, N.; Tseng, W.; et al. Case control study comparing the HPV genome in patients with oral cavity squamous cell carcinoma to normal patients using metagenomic shotgun sequencing. Sci. Rep. 2021, 11, 3867. [Google Scholar] [CrossRef] [PubMed]
- Carlander, A.F.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Jensen, J.S.; Gronhoj, C.; Buchwald, C.V. A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses 2021, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Nogues, J.C.; Fassas, S.; Mulcahy, C.; Zapanta, P.E. Human papillomavirus-associated head and neck cancer. J. Am. Board. Fam. Med. 2021, 34, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Bunge, E.; Bakker, M.; Castellsague, X. The incidence, clearance and persistence of non-cervical human papillomavirus infections: A systematic review of the literature. BMC Infect. Dis. 2016, 16, 293–299. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Bhatia, R.K.; Messeguer, A.L.; GonzÃlez, P.; Herrero, R.; Giuliano, A.R. Oral human papillomavirus in healthy individuals: A systematic review of the literature. Sex Transm. Dis. 2010, 37, 386–391. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Villa, A.; Nyitray, A.G.; Abrahamsen, M.; Papenfuss, M.; Smith, D.; Hildesheim, A.; Villa, L.L.; Lazcano-Ponce, E.; Giuliano, A.R. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol. Biomark. Prev. 2011, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Vlantis, A.C.; Liang, M.; Wong, P.Y.; Ho, W.C.S.; Boon, S.S.; Sze, R.K.H.; Leung, C.; Chan, P.K.S.; Chen, Z. Prevalence and epidemiologic profile of oral infection with alpha, beta, and gamma papillomaviruses in an Asian Chinese population. J. Infect. Dis. 2018, 218, 388–397. [Google Scholar] [CrossRef]
- Hang, D.; Liu, F.; .Liu, M.; He, Z.; Sun, M.; Liu, Y.; Li, J.; Pan, Y.; Ning, T.; Guo, C.; et al. Oral human papillomavirus infection and its risk factors among 5, 410 healthy adults in China, 2009–2011. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2101–2110. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Pickard, R.K.; Xiao, W.; Gillison, M.L. High-risk oral human papillomavirus load in the US population, national health and nutrition examination survey 2009–2010. J. Infect. Dis. 2014, 210, 441–447. [Google Scholar] [CrossRef]
- Castle, P.E.; Schiffman, M.; Herrero, R.; Hildesheim, A.; Rodriguez, A.C.; Bratti, M.C.; Sherman, M.E.; Wacholder, S.; Tarone, R.; Burk, R.D. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in guanacaste, costa rica. J. Infect. Dis. 2005, 191, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pineres, A.J.; Hildesheim, A.; Herrero, R.; Trivett, M.; Williams, M.; Atmetlla, I.; Ramirez, M.; Villegas, M.; Schiffman, M.; Rodriguez, A.C.; et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006, 66, 11070–11076. [Google Scholar] [CrossRef]
- Pierce Campbell, C.M.; Gheit, T.; Tommasino, M.; Lin, H.Y.; Torres, B.N.; Messina, J.L.; Stoler, M.H.; Rollison, D.E.; Sirak, B.A.; Abrahamsen, M.; et al. Cutaneous beta human papillomaviruses and the development of male external genital lesions: A case-control study nested within the HIM study. Virology 2016, 497, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. NHANES 2009–2012 findings: Association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res. 2015, 75, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; McNeel, T.S.; Fakhry, C. Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann. Oncol. 2017, 28, 3065–3069. [Google Scholar] [CrossRef]
- Beachler, D.C.; Jenkins, G.; Safaeian, M.; Kreimer, A.R.; Wentzensen, N. Natural acquired immunity against subsequent genital human papillomavirus infection: A systematic review and meta-analysis. J. Infect. Dis. 2016, 213, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Agrawal, Y.; Halpern, J.; Bodison, S.; Gillison, M.L. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J. Infect. Dis. 2009, 199, 1263–1269. [Google Scholar] [CrossRef]
- Windon, M.J.; Waterboer, T.; Hillel, A.T.; Chien, W.; Best, S.; Stewart, C.; Akst, L.; Troy, T.; Bender, N.; Miles, B.; et al. Sex differences in HPV immunity among adults without cancer. Hum. Vaccines Immunother. 2019, 15, 1935–1941. [Google Scholar] [CrossRef]
- Tobian, A.A.; Kong, X.; Gravitt, P.E.; Eaton, K.P.; Kigozi, G.; Serwadda, D.; Oliver, A.E.; Nalugoda, F.; Makumbi, F.; Chen, M.Z.; et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda. Int. J. Cancer 2011, 129, 2970–2975. [Google Scholar] [CrossRef][Green Version]
- D’Souza, G.; Cullen, K.; Bowie, J.; Thorpe, R.; Fakhry, C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS ONE 2014, 9, e86023. [Google Scholar] [CrossRef]
- D’Souza, G.; Wentz, A.; Kluz, N.; Zhang, Y.; Sugar, E.; Youngfellow, R.M.; Guo, Y.; Xiao, W.; Gillison, M.L. Sex differences in risk factors and natural history of oral human papillomavirus infection. J. Infect. Dis. 2016, 213, 1893–1896. [Google Scholar] [CrossRef]
- Lang Kuhs, K.A.; Gonzalez, P.; Struijk, L.; Castro, F.; Hildesheim, A.; van Doorn, L.J.; Rodriguez, A.C.; Schiffman, M.; Quint, W.; Lowy, D.R.; et al. Prevalence of and risk factors for oral human papillomavirus among young women in Costa Rica. J. Infect. Dis. 2013, 208, 1643–1652. [Google Scholar] [CrossRef]
- Rintala, M.A.; Grenman, S.E.; Jarvenkyla, M.E.; Syrjanen, K.J.; Syrjanen, S.M. High-risk types of human papillomavirus (HPV) DNA in oral and genital mucosa of infants during their first 3 years of life: Experience from the finnish HPV family study. Clin. Infect. Dis. 2005, 41, 1728–1733. [Google Scholar] [CrossRef]
- Martinelli, M.; Zappa, A.; Bianchi, S.; Frati, E.; Colzani, D.; Amendola, A.; Tanzi, E. Human papillomavirus (HPV) infection and genotype frequency in the oral mucosa of newborns in milan, italy. Clin. Microbiol. Infect. 2012, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Termine, N.; Giovannelli, L.; Matranga, D.; Caleca, M.P.; Bellavia, C.; Perino, A.; Campisi, G. Oral human papillomavirus infection in women with cervical HPV infection: New data from an italian cohort and a metanalysis of the literature. Oral Oncol. 2011, 47, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Pickard, R.K.; Xiao, W.; Broutian, T.R.; He, X.; Gillison, M.L. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sex Transm. Dis. 2012, 39, 559–566. [Google Scholar] [CrossRef]
- Gupta, A.; Perkins, R.B.; Ortega, G.; Feldman, S.; Villa, A. Barrier use during oro-genital sex and oral human papillomavirus prevalence: Analysis of NHANES 2009–2014. Oral Dis. 2019, 25, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Read, T.R.; Hocking, J.S.; Vodstrcil, L.A.; Tabrizi, S.N.; McCullough, M.J.; Grulich, A.E.; Garland, S.M.; Bradshaw, C.S.; Chen, M.Y.; Fairley, C.K. Oral human papillomavirus in men having sex with men: Risk-factors and sampling. PLoS ONE 2012, 7, e49324. [Google Scholar] [CrossRef]
- Moscicki, A.B.; Ma, Y.; Gheit, T.; McKay-Chopin, S.; Farhat, S.; Widdice, L.E.; Tommasino, M. Prevalence and transmission of beta and gamma human papillomavirus in heterosexual couples. Open Forum Infect. Dis. 2017, 4, ofw216. [Google Scholar] [CrossRef]
- Winer, R.L.; Gheit, T.; Feng, Q.; Stern, J.E.; Lin, J.; Cherne, S.; Tommasino, M. Prevalence and correlates of beta- and gamma-human papillomavirus detection in oral samples from mid-adult women. J. Infect. Dis. 2019, 219, 1067–1075. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Pierce Campbell, C.M.; Lin, H.Y.; Fulp, W.; Papenfuss, M.R.; Abrahamsen, M.; Hildesheim, A.; Villa, L.L.; Salmeron, J.J.; Lazcano-Ponce, E.; et al. Incidence and clearance of oral human papillomavirus infection in men: The HIM cohort study. Lancet 2013, 382, 877–887. [Google Scholar] [CrossRef]
- Shigeishi, H.; Sugiyama, M. Risk factors for oral human papillomavirus infection in healthy individuals: A systematic review and meta-analysis. J. Clin. Med. Res. 2016, 8, 721–729. [Google Scholar] [CrossRef]
- Kero, K.; Rautava, J.; Syrjanen, K.; Willberg, J.; Grenman, S.; Syrjanen, S. Smoking increases oral HPV persistence among men: 7-year follow-up study. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Vianna, L.M.S.; Carneiro, F.P.; Amorim, R.; Guerra, E.N.D.S.; Cavalcanti Neto, F.F.; Tiziani, V.; Motoyama, A.B.; Bocca, A.L. Oropharynx HPV status and its relation to HIV infection. PeerJ 2018, 6, e4407. [Google Scholar] [CrossRef]
- Quabius, E.S.; Gorogh, T.; Fischer, G.S.; Hoffmann, A.S.; Gebhard, M.; Evert, M.; Beule, A.; Maune, S.; Knecht, R.; Ovari, A.; et al. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett. 2015, 357, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.C.; Markham, C.M.; Ross, M.W.; Mullen, P.D. Examining the association between oral health and oral HPV infection. Cancer Prev. Res. 2013, 6, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Dalla Torre, D.; Burtscher, D.; Solder, E.; Rasse, M.; Puelacher, W. The correlation between the quality of oral hygiene and oral HPV infection in adults: A prospective cross-sectional study. Clin. Oral Investig. 2019, 23, 179–185. [Google Scholar] [CrossRef]
- Pierce Campbell, C.M.; Kreimer, A.R.; Lin, H.Y.; Fulp, W.; O’Keefe, M.T.; Ingles, D.J.; Abrahamsen, M.; Villa, L.L.; Lazcano-Ponce, E.; Giuliano, A.R. Long-term persistence of oral human papillomavirus type 16: The HPV infection in men (HIM) study. Cancer Prev. Res. 2015, 8, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Bettampadi, D.; Sirak, B.A.; Abrahamsen, M.E.; Reich, R.R.; Villa, L.L.; Ponce, E.L.; Giuliano, A.R. Factors associated with persistence and clearance of high-risk oral HPV among participants in the HPV infection in men (HIM) study. Clin. Infect. Dis. 2020, ciaa1701. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Vlantis, A.C.; Liang, M.; Wong, P.Y.; Ho, W.C.S.; Boon, S.S.; Leung, C.; Chan, P.K.S.; Chen, Z. Persistence and clearance of oral human papillomavirus infections: A prospective population-based cohort study. J. Med. Virol. 2020, 92, 3807–3814. [Google Scholar] [CrossRef]
- Torres, M.; Gheit, T.; McKay-Chopin, S.; Rodriguez, C.; Romero, J.D.; Filotico, R.; Donà, M.G.; Ortiz, M.; Tommasino, M. Prevalence of beta and gamma human papillomaviruses in the anal canal of men who have sex with men is influenced by HIV status. J. Clin. Virol. 2015, 67, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schlecht, N.F.; Chen, Z.; Bottalico, D.; McKinney, S.; Ostoloza, J.; Dunne, A.; Burk, R.D. Oral human papillomavirus detection in older adults who have human immunodeficiency virus infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 505–514. [Google Scholar] [CrossRef]
- King, E.M.; Oomeer, S.; Gilson, R.; Copas, A.; Beddows, S.; Soldan, K.; Jit, M.; Edmunds, W.J.; Sonnenberg, P. Oral human papillomavirus infection in men who have sex with men: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0157976. [Google Scholar] [CrossRef] [PubMed]
- Beachler, D.C.; Weber, K.M.; Margolick, J.B.; Strickler, H.D.; Cranston, R.D.; Burk, R.D.; Wiley, D.J.; Minkoff, H.; Reddy, S.; Stammer, E.E.; et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol. Biomark. Prev. 2012, 21, 122–133. [Google Scholar] [CrossRef]
- Rollo, F.; Latini, A.; Pichi, B.; Colafigli, M.; Benevolo, M.; Sinopoli, I.; Sperduti, I.; Laquintana, V.; Fabbri, G.; Frasca, M.; et al. Prevalence and determinants of oral infection by human papillomavirus in HIV-infected and uninfected men who have sex with men. PLoS ONE 2017, 12, e0184623. [Google Scholar] [CrossRef]
- Rollo, F.; Pichi, B.; Benevolo, M.; Giuliani, M.; Latini, A.; Lorenzon, L.; Colafigli, M.; Frasca, M.; Pellini, R.; Cristaudo, A.; et al. Oral testing for high-risk human papillomavirus DNA and E6/E7 messenger RNA in healthy individuals at risk for oral infection. Cancer 2019, 125, 2587–2593. [Google Scholar] [CrossRef]
- Beachler, D.; D’Souza, G. Oral human papillomavirus infection and head and neck cancers in HIV-infected individuals. Curr. Opin. Oncol. 2013, 25, 503–510. [Google Scholar] [CrossRef]
- Gheit, T.; Rollo, F.; Brancaccio, R.N.; Robitaille, A.; Galati, L.; Giuliani, M.; Latini, A.; Pichi, B.; Benevolo, M.; Cuenin, C.; et al. Oral infection by mucosal and cutaneous human papillomaviruses in the men who have sex with men from the OHMAR study. Viruses 2020, 12, 899. [Google Scholar] [CrossRef]
- Mooij, S.H.; Boot, H.J.; Speksnijder, A.G.; Stolte, I.G.; Meijer, C.J.; Snijders, P.J.; Verhagen, D.W.; King, A.J.; de Vries, H.J.; Quint, W.G.; et al. Oral human papillomavirus infection in HIV-negative and HIV-infected MSM. AIDS 2013, 27, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Gheit, T.; Rollo, F.; Tommasino, M.; Latini, A.; Benevolo, M.; Pichi, B.; Pellini, R.; McKay-Chopin, S.; Cristaudo, A.; et al. Predictors of oral infection by mucosal and cutaneous human papillomaviruses in HIV-infected and uninfected men who have sex with men of the OHMAR study. J. Clin. Med. 2021, 10, 2804. [Google Scholar] [CrossRef] [PubMed]
- Poynten, I.M.; Machalek, D.; Templeton, D.; Jin, F.; Hillman, R.; Zablotzska, I.; Prestage, G.; Holt, M.; Grulich, A. Comparison of age-specific patterns of sexual behaviour and anal HPV prevalence in homosexual men with patterns in women. Sex Transm. Infect. 2016, 92, 228–231. [Google Scholar] [CrossRef]
- Gaester, K.; Fonseca, L.A.; Luiz, O.; Assone, T.; Fontes, A.S.; Costa, F.; Duarte, A.J.; Casseb, J. Human papillomavirus infection in oral fluids of HIV-1-positive men: Prevalence and risk factors. Sci. Rep. 2014, 4, 6592. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Rollo, F.; Vescio, M.F.; Pichi, B.; Latini, A.; Benevolo, M.; Pellini, R.; Cristaudo, A.; Donà, M.G. Oral human papillomavirus infection in HIV-infected and HIV-uninfected MSM: The OHMAR prospective cohort study. Sex Transm. Infect. 2020, 96, 528–536. [Google Scholar] [CrossRef]
- Looker, K.J.; Ronn, M.M.; Brock, P.M.; Brisson, M.; Drolet, M.; Mayaud, P.; Boily, M.C. Evidence of synergistic relationships between HIV and human papillomavirus (HPV): Systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J. Int. AIDS Soc. 2018, 21, e25110. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Alberg, A.J.; Daniel, R.; Gravitt, P.E.; Viscidi, R.; Garrett, E.S.; Shah, K.V.; Gillison, M.L. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J. Infect. Dis. 2004, 189, 686–698. [Google Scholar] [CrossRef]
- Robbins, H.A.; Fennell, C.E.; Gillison, M.; Xiao, W.; Guo, Y.; Wentz, A.; Kirk, G.D.; Mehta, S.H.; D’Souza, G. Prevalence of and risk factors for oral human papillomavirus infection among HIV-positive and HIV-negative people who inject drugs. PLoS ONE 2015, 10, e0143698. [Google Scholar] [CrossRef]
- Beachler, D.C.; Sugar, E.A.; Margolick, J.B.; Weber, K.M.; Strickler, H.D.; Wiley, D.J.; Cranston, R.D.; Burk, R.D.; Minkoff, H.; Reddy, S.; et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am. J. Epidemiol. 2015, 181, 40–53. [Google Scholar] [CrossRef]
- Darwich, L.; Canadas, M.P.; Videla, S.; Coll, J.; Molina-Lopez, R.A.; Cobarsi, P.; Sirera, G.; Clotet, B.; Can Ruti HIV-HPV Team. Oral human papillomavirus type-specific infection in HIV-infected men: A prospective cohort study among men who have sex with men and heterosexual men. Clin. Microbiol. Infect. 2014, 20, 585. [Google Scholar] [CrossRef]
- Van Aar, F.; Mooij, S.H.; van der Sande, M.A.; Meijer, C.J.; King, A.J.; Verhagen, D.W.; Heijman, T.; Coutinho, R.A.; Schim van der Loeff, M.F. Twelve-month incidence and clearance of oral HPV infection in HIV-negative and HIV-infected men who have sex with men: The H2M cohort study. BMC Infect. Dis. 2014, 14, 668. [Google Scholar] [CrossRef]
- Ong, J.J.; Read, T.R.; Vodstrcil, L.A.; Walker, S.; Chen, M.; Bradshaw, C.S.; Garland, S.M.; Tabrizi, S.N.; Cornall, A.; Grulich, A.; et al. Detection of oral human papillomavirus in HIV-positive men who have sex with men 3 years after baseline: A follow up cross-sectional study. PLoS ONE 2014, 9, e102138. [Google Scholar] [CrossRef] [PubMed]
- Mooij, S.H.; Boot, H.J.; Speksnijder, A.G.; Meijer, C.J.; King, A.J.; Verhagen, D.W.; de Vries, H.J.; Quint, W.G.; Molijn, A.; de Koning, M.N.; et al. Six-month incidence and persistence of oral HPV infection in HIV-negative and HIV-infected men who have sex with men. PLoS ONE 2014, 9, e98955. [Google Scholar] [CrossRef]
- Tam, S.; Fu, S.; Xu, L.; Krause, K.J.; Lairson, D.R.; Miao, H.; Sturgis, E.M.; Dahlstrom, K.R. The epidemiology of oral human papillomavirus infection in healthy populations: A systematic review and meta-analysis. Oral Oncol. 2018, 82, 91–99. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Clemens, G.; Strickler, H.D.; Wiley, D.J.; Troy, T.; Struijk, L.; Gillison, M.; Fakhry, C. Long-term persistence of oral HPV over 7 years of follow-up. JNCI Cancer Spectr. 2020, 4, pkaa047. [Google Scholar] [CrossRef]
- Beachler, D.C.; D’Souza, G.; Sugar, E.A.; Xiao, W.; Gillison, M.L. Natural history of anal vs oral HPV infection in HIV-infected men and women. J. Infect. Dis. 2013, 208, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Haeggblom, L.; Ramqvist, T.; Tommasino, M.; Dalianis, T.; Nasman, A. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. 2017, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef]
- Castellsague, X.; Alemany, L.; Quer, M.; Halec, G.; Quiros, B.; Tous, S.; Clavero, O.; Alos, L.; Biegner, T.; Szafarowski, T.; et al. HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef] [PubMed]
- Stjernstrom, K.D.; Jensen, J.S.; Jakobsen, K.K.; Gronhoj, C.; von Buchwald, C. Current status of human papillomavirus positivity in oropharyngeal squamous cell carcinoma in Europe: A systematic review. Acta Otolaryngol. 2019, 139, 1112–1116. [Google Scholar] [CrossRef]
- Viens, L.J.; Henley, S.J.; Watson, M.; Markowitz, L.E.; Thomas, C.C.; Thompson, T.D.; Razzaghi, H.; Saraiya, M. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 661–666. [Google Scholar] [CrossRef]
- Agalliu, I.; Gapstur, S.; Chen, Z.; Wang, T.; Anderson, R.L.; Teras, L.; Kreimer, A.R.; Hayes, R.B.; Freedman, N.D.; Burk, R.D. Associations of oral alpha-, beta-, and gamma-human papillomavirus types with risk of incident head and neck cancer. JAMA Oncol. 2016, 2, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Carlander, A.F.; Gronhoj Larsen, C.; Jensen, D.H.; Garnaes, E.; Kiss, K.; Andersen, L.; Olsen, C.H.; Franzmann, M.; Hogdall, E.; Kjaer, S.K.; et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: A danish population-based study from 2011 to 2014. Eur. J. Cancer 2017, 70, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Fung, N.; Tewari, S.R.; D’Souza, G. Unique role of HPV16 in predicting oropharyngeal cancer risk more than other oncogenic oral HPV infections. Oral Oncol. 2020, 111, 104981. [Google Scholar] [CrossRef]
- Rivera, G.A.; Morell, F. Laryngeal papillomas. In Anonymous StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Vera-Iglesias, E.; Garcia-Arpa, M.; Sanchez-Caminero, P.; Romero-Aguilera, G.; Cortina de la Calle, P. Focal epithelial hyperplasia. Actas Dermosifiliogr. 2007, 98, 621–623. [Google Scholar] [CrossRef]
- Padayachee, A. Human papillomavirus (HPV) types 2 and 57 in oral verrucae demonstrated by in situ hybridization. J. Oral Pathol. Med. 1994, 23, 413–417. [Google Scholar] [CrossRef]
- Donà, M.G.; Pichi, B.; Rollo, F.; Gheit, T.; Laquintana, V.; Covello, R.; Pescarmona, E.; Spriano, G.; Pellini, R.; Giuliani, M.; et al. Mucosal and cutaneous human papillomaviruses in head and neck squamous cell papillomas. Head Neck. 2017, 39, 254–259. [Google Scholar] [CrossRef]
- Ambulos, N.P.; Schumaker, L.M.; Mathias, T.J.; White, R.; Troyer, J.; Wells, D.; Cullen, K.J. Next-generation sequencing-based HPV genotyping assay validated in formalin-fixed, paraffin-embedded oropharyngeal and cervical cancer specimens. J. Biomol. Tech. 2016, 27, 46–52. [Google Scholar] [CrossRef][Green Version]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Mattox, A.K.; Roelands, J.; Saal, T.M.; Cheng, Y.; Rinchai, D.; Hendrickx, W.; Young, G.D.; Diefenbach, T.J.; Berger, A.E.; Westra, W.H.; et al. Myeloid cells are enriched in tonsillar crypts, providing insight into the host tropism of human papillomavirus. Am. J. Pathol. 2021, 191, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliani, E.; Rollo, F.; Donà, M.G.; Garbuglia, A.R. Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology. Pathogens 2021, 10, 1411. https://doi.org/10.3390/pathogens10111411
Giuliani E, Rollo F, Donà MG, Garbuglia AR. Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology. Pathogens. 2021; 10(11):1411. https://doi.org/10.3390/pathogens10111411
Chicago/Turabian StyleGiuliani, Eugenia, Francesca Rollo, Maria Gabriella Donà, and Anna Rosa Garbuglia. 2021. "Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology" Pathogens 10, no. 11: 1411. https://doi.org/10.3390/pathogens10111411
APA StyleGiuliani, E., Rollo, F., Donà, M. G., & Garbuglia, A. R. (2021). Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology. Pathogens, 10(11), 1411. https://doi.org/10.3390/pathogens10111411







