Allergen Immunotherapy in Asthma
Abstract
1. Introduction
2. Effect of Subcutaneous Immunotherapy (SCIT) on Bronchial Asthma
3. Effect of Allergen Immunotherapy (AIT) on Asthma with Allergic Rhinitis
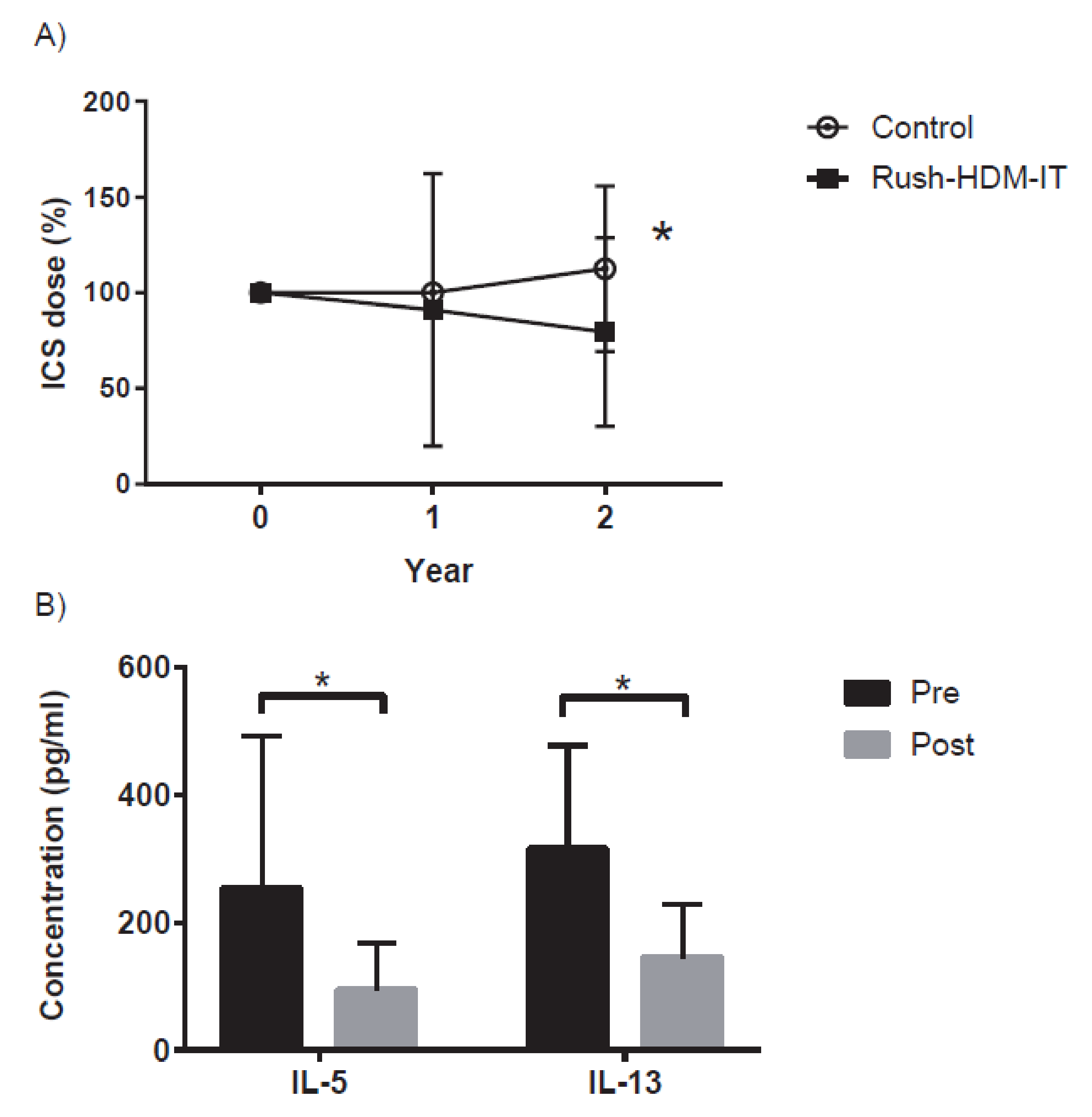
4. Introduction of House Dust Mite (HDM)-SCIT for Asthma
5. Safety of HDM-SCIT
6. Mechanisms and Biomarkers of AIT
7. Effects of AIT on Natural Course of Allergic Disease
8. Effect of Sublingual Immunotherapy (SLIT) on Bronchial Asthma
9. AIT in Japan
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Nagata, M.; Nakagome, K. Allergen immunotherapy in asthma: Current status and future perspectives. Allergol. Int. 2010, 59, 15–19. [Google Scholar] [CrossRef]
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese Society of Allergology. Japanese guidelines for allergic rhinitis 2020. Allergol. Int. 2020, 69, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T.; Järvinen, M.; Kava, T.; Kiviranta, K.; Koskinen, S.; Lehtonen, K.; Nikander, K.; Persson, T.; Selroos, O.; Sovijärvi, A.; et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N. Engl. J. Med. 1994, 331, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Takaku, Y.; Nakagome, K.; Kobayashi, T.; Yamaguchi, T.; Nishihara, F.; Soma, T.; Hagiwara, K.; Kanazawa, M.; Nagata, M. Changes in airway inflammation and hyperresponsiveness after inhaled corticosteroid cessation in allergic asthma. Int. Arch. Allergy Immunol. 2010, 152 (Suppl. 1), 41–46. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.J.; Puy, R.M.; Weiner, J.M. Injection allergen immunotherapy for asthma. Cochrane Database Syst. Rev. 2010, 4, CD001186. [Google Scholar] [CrossRef] [PubMed]
- Dhami, S.; Kakourou, A.; Asamoah, F.; Agache, I.; Lau, S.; Jutel, M.; Muraro, A.; Roberts, G.; Akdis, C.A.; Bonini, M.; et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy 2017, 72, 1825–1848. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, P.; Zanolla, L.; Pozzan, M.; Fabbri, L.M. Regione Veneto Study Group on the “Effect of immunotherapy in allergic asthma”. Effect of specific immunotherapy added to pharmacologic treatment and allergen avoidance in asthmatic patients allergic to house dust mite. J. Allergy Clin. Immunol. 2004, 113, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Nakagome, K.; Iemura, H.; Naito, E.; Miyauchi, S.; Uchida, Y.; Soma, T.; Nagata, M. Clinical evaluation of rush immunotherapy using house dust mite allergen in Japanese asthmatics. Asia Pac. Allergy 2021, 11, e32. [Google Scholar] [CrossRef] [PubMed]
- Zielen, S.; Kardos, P.; Madonini, E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: A randomized controlled trial. J. Allergy Clin. Immunol. 2010, 126, 942–949. [Google Scholar] [CrossRef]
- Ameal, A.; Vega-Chicote, J.M.; Fernández, S.; Miranda, A.; Carmona, M.J.; Rondón, M.C.; Reina, E.; García-González, J.J. Double-blind and placebo-controlled study to assess efficacy and safety of a modified allergen extract of Dermatophagoides pteronyssinus in allergic asthma. Allergy 2005, 60, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- García-Robaina, J.C.; Sánchez, I.; de la Torre, F.; Fernández-Caldas, E.; Casanovas, M. Successful management of mite-allergic asthma with modified extracts of Dermatophagoides pteronyssinus and Dermatophagoides farinae in a double-blind, placebo-controlled study. J. Allergy Clin. Immunol. 2006, 118, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- García Robaina, J.C.; Polanco Sánchez, C.; Estella Pérez, E. Savings associated with high-dose hypoallergenic house dust mite immunotherapy in rhinitis and/or asthma patients in Spain. Clinicoecon. Outcomes Res. 2016, 8, 235–241. [Google Scholar][Green Version]
- U.S. Department of Health and Human Services: National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma; NHLBI: Bethesda, MD, USA, 2007.
- U.S. Department of Health and Human Services: National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group; NHLBI: Bethesda, MD, USA, 2020.
- Agache, I.; Lau, S.; Akdis, C.A.; Smolinska, S.; Bonini, M.; Cavkaytar, O.; Flood, B.; Gajdanowicz, P.; Izuhara, K.; Kalayci, O.; et al. EAACI Guidelines on Allergen Immunotherapy: House dust mite-driven allergic asthma. Allergy 2019, 74, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Bousquet, P.J.; Aizawa, H.; Akiyama, K.; Adachi, M.; Ichinose, M.; Ebisawa, M.; Tamura, G.; Nagai, A.; Nishima, S.; et al. Prevalence and impact of rhinitis in asthma. SACRA, a cross-sectional nation-wide study in Japan. Allergy 2011, 66, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Adinoff, A.D.; Irvin, C.G. Changes in bronchial responsiveness following nasal provocation with allergen. J. Allergy Clin. Immunol. 1992, 89, 611–618. [Google Scholar] [CrossRef]
- Braunstahl, G.J.; Kleinjan, A.; Overbeek, S.E.; Prins, J.B.; Hoogsteden, H.C.; Fokkens, W.J. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am. J. Respir Crit. Care Med. 2000, 161, 2051–2057. [Google Scholar] [CrossRef]
- Crystal-Peters, J.; Neslusan, C.; Crown, W.H.; Torres, A. Treating allergic rhinitis in patients with comorbid asthma: The risk of asthma-related hospitalizations and emergency department visits. J. Allergy Clin. Immunol. 2002, 109, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. World Health Organization; GA(2)LEN.; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy 2008, 63 (Suppl. 86), 8–160. [Google Scholar] [CrossRef]
- Nakada, H.; Nakagome, K.; Takaku, Y.; Nishihara, F.; Yamaguchi, T.; Soma, T.; Hagiwara, K.; Kanazawa, M.; Kase, Y.; Nagata, M. Questionnaire for determining relationship between nasal and asthma symptoms. Arerugi 2010, 59, 688–698. [Google Scholar] [PubMed]
- Nagata, M.; Yamamoto, H.; Tabe, K.; Tanaka, K.; Kimura, I.; Sakamoto, K.; Sakamoto, Y.; Yamamoto, K.; Dohi, Y. A clinical evaluation of rush immunotherapy in adult patients with severe bronchial asthma. Arerugi 1999, 48, 1316–1321. [Google Scholar] [PubMed]
- Cox, L. Accelerated immunotherapy schedules: Review of efficacy and safety. Ann. Allergy Asthma Immunol. 2006, 97, 126–137. [Google Scholar] [CrossRef]
- Cox, L. Allergen immunotherapy: Immunomodulatory treatment for allergic diseases. Expert Rev. Clin. Immunol. 2006, 2, 533–546. [Google Scholar] [CrossRef]
- Cox, L.; Larenas-Linnemann, D.; Lockey, R.F.; Passalacqua, G. Speaking the same language: The World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J. Allergy Clin. Immunol. 2010, 125, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Epstein, T.G.; Liss, G.M.; Murphy-Berendts, K.; Bernstein, D.I. AAAAI/ACAAI Surveillance Study of Subcutaneous Immunotherapy, Years 2008-2012: An Update on Fatal and Nonfatal Systemic Allergic Reactions. J. Allergy Clin. Immunol. Pract. 2014, 2, 161–167. [Google Scholar] [CrossRef]
- Eifan, A.O.; Calderon, M.A.; Durham, S.R. Allergen immunotherapy for house dust mite: Clinical efficacy and immunological mechanisms in allergic rhinitis and asthma. Expert Opin. Biol. Ther. 2013, 13, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Nakai, Y.; Tanaka, A.; Kakinoki, Y.; Washio, Y.; Ohno, Y.; Yamada, K.; Nasako, Y. Risk factors for adverse systemic reactions occurring during immunotherapy with standardized Dermatophagoides farinae extracts. Acta Otolaryngol. Suppl. 1998, 538, 113–117. [Google Scholar] [PubMed]
- James, L.K.; Shamji, M.H.; Walker, S.M.; Wilson, D.R.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Kimber, I.; Till, S.J.; Durham, S.R. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J. Allergy Clin. Immunol. 2011, 127, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.N.; James, L.K.; Paraskevopoulos, G.; Wong, C.; Calderon, M.A.; Durham, S.R.; Till, S.J. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J. Allergy Clin. Immunol. 2008, 121, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Ljorring, C.; Francis, J.N.; Calderon, M.A.; Larche, M.; Kimber, I.; Frew, A.J.; Ipsen, H.; Lund, K.; Würtzen, P.A.; et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy 2012, 67, 217–226. [Google Scholar] [CrossRef]
- Pilette, C.; Nouri-Aria, K.T.; Jacobson, M.R.; Wilcock, L.K.; Detry, B.; Walker, S.M.; Francis, J.N.; Durham, S.R. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J. Immunol. 2007, 178, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Ooijevaar-de Heer, P.; Bende, O.; Aalberse, R.C. Mechanism of immunoglobulin G4 fab-arm exchange. J. Am. Chem. Soc. 2011, 133, 10302–10311. [Google Scholar] [CrossRef]
- Van Neerven, R.J.; Wikborg, T.; Lund, G.; Jacobsen, B.; Brinch-Nielsen, A.; Arnved, J.; Ipsenet, H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J. Immunol. 1999, 163, 2944–2952. [Google Scholar]
- Hakanson, L.; Heinrich, C.; Rak, S.; Venge, P. Priming of eosinophil adhesion in patients with birch pollen allergy during pollen season: Effect of immunotherapy. J. Allergy Clin. Immunol. 1997, 99, 551–562. [Google Scholar] [CrossRef]
- Scadding, G.W.; Eifan, A.O.; Lao-Araya, M.; Penagos, M.; Poon, S.Y.; Steveling, E.; Yan, R.; Switzer, A.; Phippard, D.; Togias, A.; et al. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy 2015, 70, 689–696. [Google Scholar] [CrossRef]
- Tulic, M.K.; Fiset, P.O.; Christodoulopoulos, P.; Vaillancourt, P.; Desrosiers, M.; Lavigne, F.; Eiden, J.; Hamid, Q. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J. Allergy Clin. Immunol. 2004, 113, 235–241. [Google Scholar] [CrossRef]
- Radulovic, S.; Jacobson, M.R.; Durham, S.R.; Nouri-Aria, K.T. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J. Allergy Clin. Immunol. 2008, 121, 1467–1472. [Google Scholar] [CrossRef]
- Scadding, G.W.; Shamji, M.H.; Jacobson, M.R.; Lee, D.I.; Wilson, D.; Lima, M.T.; Pitkin, L.; Pilette, C.; Nouri-Aria, K.; Durham, S.R. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin. Exp. Allergy 2010, 40, 598–606. [Google Scholar]
- Nouri-Aria, K.T.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Walker, S.M.; Wilcock, L.K.; Staple, S.Q.; Aalberse, R.C.; Till, S.J.; Durham, S.R. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J. Immunol. 2004, 172, 3252–3259. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Van de Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Söllner, S.; Akdis, D.G.; Rückert, B.; Akdis, C.A.; Akdis, M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef]
- Hamid, Q.A.; Schitman, E.; Jacobson, M.R.; Walker, S.M.; Durham, S.R. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J. Allergy Clin. Immunol. 1997, 99, 254–260. [Google Scholar] [CrossRef]
- Lao-Araya, M.; Steveling, E.; Scadding, G.W.; Durham, S.R.; Shamji, M.H. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J. Allergy Clin. Immunol. 2014, 134, 1193–1195. [Google Scholar] [CrossRef]
- Lombardi, V.; Beuraud, C.; Neukirch, C.; Moussu, H.; Morizur, L.; Horiot, S.; Luce, S.; Wambre, E.; Linsley, P.; Chollet-Martin, S.; et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J. Allergy Clin. Immunol. 2016, 138, 305–308. [Google Scholar] [CrossRef]
- Shamji, M.H.; Kappen, J.H.; Akdis, M.; Jensen-Jarolim, E.; Knol, E.F.; Kleine-Tebbe, J.; Bohle, B.; Chaker, A.M.; Till, S.J.; Valenta, R.; et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI position paper. Allergy 2017, 72, 1156–1173. [Google Scholar] [CrossRef]
- Shamji, M.H.; Durham, S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J. Allergy Clin. Immunol. 2017, 140, 1485–1498. [Google Scholar] [CrossRef]
- Dahl, R.; Kapp, A.; Colombo, G.; de Monchy, J.G.; Rak, S.; Emminger, W.; Riis, B.; Grønager, P.M.; Durham, S.R. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J. Allergy Clin. Immunol. 2008, 121, 512–518. [Google Scholar] [CrossRef]
- Gleich, G.J.; Zimmermann, E.M.; Henderson, L.L.; Yunginger, J.W. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: A six-year prospective study. J. Allergy Clin. Immunol. 1982, 70, 261–271. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Mansueto, P.; Pacor, M.L.; Rizzo, M.; Castello, F.; Martinelli, N.; Ditta, V.; Lo Bianco, C.; Leto-Barone, M.S.; D’Alcamo, A.; et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2009, 123, 1103–1110. [Google Scholar] [CrossRef]
- Fujimura, T.; Yonekura, S.; Horiguchi, S.; Taniguchi, Y.; Saito, A.; Yasueda, H.; Inamine, A.; Nakayama, T.; Takemori, T.; Taniguchi, M.; et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin. Immunol. 2011, 139, 65–74. [Google Scholar] [CrossRef]
- Bohle, B.; Kinaciyan, T.; Gerstmayr, M.; Radakovics, A.; Jahn-Schmid, B.; Ebner, C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J. Allergy Clin. Immunol. 2007, 120, 707–713. [Google Scholar] [CrossRef]
- Schulten, V.; Tripple, V.; Seumois, G.; Qian, Y.; Scheuermann, R.H.; Fu, Z.; Locci, M.; Rosales, S.; Vijayanand, P.; Sette, A.; et al. Allergen-specific immunotherapy modulates the balance of circulating Tfh and Tfr cells. J. Allergy Clin. Immunol. 2018, 141, 775–777. [Google Scholar] [CrossRef]
- Ciepiela, O.; Zawadzka-Krajewska, A.; Kotula, I.; van Overveld, F.; Kulus, M.; Demkow, U. Sublingual immunotherapy for asthma: Affects T-cells but does not impact basophil activation. Pediatr. Allergy Immunol. Pulmonol. 2014, 27, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Wilcock, L.K.; Wachholz, P.A.; Dearman, R.J.; Kimber, I.; Wurtzen, P.A.; Larché, M.; Durham, S.R.; Francis, J.N. The IgE-facilitated allergen binding (FAB) assay: Validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J. Immunol. Methods 2006, 317, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Francis, J.N.; Wurtzen, P.A.; Lund, K.; Durham, S.R.; Till, S.J. Cell-free detection of allergen-IgE cross-linking with immobilized phase CD23: Inhibition by blocking antibody responses after immunotherapy. J. Allergy Clin. Immunol. 2013, 132, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Walker, S.M.; Varga, E.M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999, 341, 468–475. [Google Scholar] [CrossRef]
- Jacobsen, L.; Niggemann, B.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Koivikko, A.; Norberg, L.A.; Valovirta, E.; Wahn, U.; et al. The PAT investigator group. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy 2007, 62, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, V.; Marcucci, F.; Puccinelli, P.; Parmiani, S.; Frati, F.; Sensi, L.; Canonica, G.W.; Passalacqua, G. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: A 10-year prospective study. Clin. Exp. Allergy 2003, 33, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Marogna, M.; Spadolini, I.; Massolo, A.; Canonica, G.W.; Passalacqua, G. Long-lasting effects of sublingual immunotherapy according to its duration: A 15-year prospective study. J. Allergy Clin. Immunol. 2010, 126, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Möller, C.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Jacobsen, L.; Koivikko, A.; Koller, D.Y.; Niggemann, B.; Norberg, L.A.; et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J. Allergy Clin. Immunol. 2002, 109, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, D.; Plaia, A.; Leto-Barone, M.S.; La Piana, S.; Macchia, L.; Di Lorenzo, G. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: A systematic review. Allergy 2017, 72, 691–704. [Google Scholar] [CrossRef]
- Inal, A.; Altintas, D.U.; Yilmaz, M.; Karakoc, G.B.; Kendirli, S.G.; Sertdemir, Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J. Investig. Allergol. Clin. Immunol. 2007, 17, 85–91. [Google Scholar] [PubMed]
- Gulen, F.; Zeyrek, D.; Can, D.; Altinoz, S.; Koksoy, H.; Demir, E.; Tanac, R. Development of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. Asian Pac. J. Allergy Immunol. 2007, 25, 7–11. [Google Scholar] [PubMed]
- Bousquet, J.; Scheinmann, P.; Guinnepain, M.T.; Perrin-Fayolle, M.; Sauvaget, J.; Tonnel, A.B.; Pauli, G.; Caillaud, D.; Dubost, R.; Leynadier, F.; et al. Sublingual-swallow immunotherapy (SLIT) in patients with asthma due to house-dust mites: A double-blind, placebo-controlled study. Allergy 1999, 54, 249–260. [Google Scholar] [CrossRef]
- Marogna, M.; Spadolini, I.; Massolo, A.; Berra, D.; Zanon, P.; Chiodini, E.; Canonica, G.W.; Passalacqua, G. Long-term comparison of sublingual immunotherapy vs inhaled budesonide in patients with mild persistent asthma due to grass pollen. Ann. Allergy Asthma Immunol. 2009, 102, 69–75. [Google Scholar] [CrossRef]
- Chelladurai, Y.; Suarez-Cuervo, C.; Erekosima, N.; Kim, J.M.; Ramanathan, M.; Segal, J.B.; Lin, S.Y. Effectiveness of subcutaneous versus sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: A systematic review. J. Allergy Clin. Immunol. Pract. 2013, 1, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Mosbech, H.; Deckelmann, R.; de Blay, F.; Pastorello, E.A.; Trebas-Pietras, E.; Andres, L.P.; Malcus, I.; Ljørring, C.; Canonica, G.W. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2014, 134, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Virchow, J.C.; Backer, V.; Kuna, P.; Prieto, L.; Nolte, H.; Villesen, H.H.; Ljørring, C.; Riis, B.; de Blay, F. Efficacy of a House Dust Mite Sublingual Allergen Immunotherapy Tablet in Adults with Allergic Asthma: A Randomized Clinical Trial. JAMA 2016, 315, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global strategy for the diagnosis and prevention. Available online: http://www.ginasthma.org (accessed on 30 August 2021).
- Senna, G.; Lombardi, C.; Canonica, G.W.; Passalacqua, G. How adherent to sublingual immunotherapy prescriptions are patients? The manufacturers’ viewpoint. J. Allergy Clin. Immunol. 2010, 126, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Akitsu, K.; Kubota, K. Effect of Sublingual Immunotherapy on Airway Inflammation and Airway Wall Thickness in Allergic Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 2804–2811. [Google Scholar] [CrossRef]
- Tanaka, A.; Tohda, Y.; Okamiya, K.; Azuma, R.; Terada, I.; Adachi, M. Efficacy and Safety of HDM SLIT Tablet in Japanese Adults with Allergic Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Ohta, K.; Iikura, M.; Hirashima, J.; Sugiyama, H.; Takahashi, K. The impact of co-existing seasonal allergic rhinitis caused by Japanese Cedar Pollinosis (SAR-JCP) upon asthma control status. Allergol. Int. 2015, 64, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, S.; Nakagome, K.; Kobayashi, T.; Soma, T.; Kamijo, A.; Nagata, M. Sublingual Immunotherapy for Japanese Cedar Pollinosis Attenuates Asthma Exacerbation. Allergy Asthma Immunol. Res. 2019, 11, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Yuta, A.; Ogawa, Y.; Suzuki, Y.; Arikata, M.; Kozaki, H.; Shimizu, T.; Ohta, N. Adherence and Clinical Outcome of Sublingual Immunotherapy for Japanese Cedar Pollinosis. Nihon Jibiinkoka Gakkai Kaiho 2016, 119, 1504–1510. [Google Scholar] [CrossRef]
- Kikkawa, S.; Kamijo, A.; Nakagome, K.; Soma, T.; Kobayashi, T.; Uchida, Y.; Kase, Y.; Nagata, M. Predictors of adherence to sublingual immunotherapy for Japanese cedar pollinosis: A prospective analysis. Asian Pac. J. Allergy Immunol. 2019, in press. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagome, K.; Nagata, M. Allergen Immunotherapy in Asthma. Pathogens 2021, 10, 1406. https://doi.org/10.3390/pathogens10111406
Nakagome K, Nagata M. Allergen Immunotherapy in Asthma. Pathogens. 2021; 10(11):1406. https://doi.org/10.3390/pathogens10111406
Chicago/Turabian StyleNakagome, Kazuyuki, and Makoto Nagata. 2021. "Allergen Immunotherapy in Asthma" Pathogens 10, no. 11: 1406. https://doi.org/10.3390/pathogens10111406
APA StyleNakagome, K., & Nagata, M. (2021). Allergen Immunotherapy in Asthma. Pathogens, 10(11), 1406. https://doi.org/10.3390/pathogens10111406





