First Evidence of Chelonid Herpesvirus 5 (ChHV5) Infection in Green Turtles (Chelonia mydas) from Sabah, Borneo
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Animals and Ethics Statement
3.2. Field Sampling
3.3. Histopathological Examinations
3.4. Molecular Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaro-Núñez, A.; Bertelsen, M.F.; Bojesen, A.M.; Rasmussen, I.; Zepeda-Mendoza, L.; Olsen, M.T.; Gilbert, M.T.P. Global distribution of Chelonid fibropapilloma-associated herpesvirus among clinically healthy sea turtles. BMC Evol. Biol. 2014, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Page-Karjian, A.; Norton, T.M.; Krimer, P.; Groner, M.; Nelson, S.E.; Gottdenker, N.L. Factors Influencing Survivorship of Rehabilitating Green Sea Turtles (Chelonia mydas) with Fibropapillomatosis. J. Zoo Wild. Med. 2014, 45, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Coates, C.W. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus). Zool. Sci. Contrib. N. Y. Zool. Soc. 1938, 23, 93–98. [Google Scholar] [CrossRef]
- Patrício, A.; Diez, C.; Van Dam, R.; Godley, B. Novel insights into the dynamics of green turtle fibropapillomatosis. Mar. Ecol. Prog. Ser. 2016, 547, 247–255. [Google Scholar] [CrossRef]
- Li, T.-H.; Balazs, G.-H.; Work, T.M.; Tseng, C.-T.; Chang, C.-C.; Hsu, W.-L.; Lan, Y.-C. Identification of Chelonid herpesvirus 5 (ChHV5) in endangered green turtles (Chelonia mydas) with fibropapillomatosis in Asia. Bull. Mar. Sci. 2017, 93, 1011–1022. [Google Scholar] [CrossRef]
- Adnyana, W.; Ladds, P.W.; Blair, D. Observations of fibropapillomatosis in green turtles (Chelonia mydas) in Indonesia. Aust. Veter- J. 1997, 75, 737–742. [Google Scholar] [CrossRef]
- Work, T.; Dagenais, J.; Balazs, G.H.; Schumacher, J.; Lewis, T.D.; Leong, J.-A.C.; Casey, R.N.; Casey, J.W. In vitro biology of fibropapilloma-associated turtle herpesvirus and host cells in Hawaiian green turtles (Chelonia mydas). J. Gen. Virol. 2009, 90, 1943–1950. [Google Scholar] [CrossRef]
- Work, T.; Balazs, G.; Rameyer, R.; Morris, R. Retrospective pathology survey of green turtles Chelonia mydas with fibropapillomatosis in the Hawaiian Islands, 1993–2003. Dis. Aquat. Org. 2004, 62, 163–176. [Google Scholar] [CrossRef]
- Reséndiz, E.; Flores-Ramírez, S.; Koch, V.; Cordero-Tapia, A. First Record of Fibropapillomatosis in a Green Turtle Chelonia mydas from the Baja California Peninsula. J. Aquat. Anim. Health 2016, 28, 252–257. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Torres, F.; Zhang, J.; Rivera, S.; Diez, C.; Moore, A.P.; Moore, D.; Brown, C. Presence of chelonid fibropapilloma-associated herpesvirus in tumored and non-tumored green turtles, as detected by polymerase chain reaction, in endemic and non-endemic aggregations, Puerto Rico. SpringerPlus 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in Green turtles (Chelonia mydas). Veter-J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Klein, P.A.; Curry, S.; Brown, D.R.; Homer, B.L.; Garber, R.L.; Mader, D.R.; Moretti, R.H.; Patterson, A.D.; Herbst, L.H.; Oros, J.; et al. Prevalence and Cultivation of a Chelonid herpesvirus Associated with Fibropapillomas of the Green Turtle, Chelonia mydas, and the Loggerhead Turtle, Caretta caretta, in Florida. Research Work Order no. 161, Florida Cooperative Fish and Wildlife Re-search Unit, University of Florida, Gainesville. 1998. Available online: http://aquaticcommons.org/id/eprint/1092 (accessed on 7 December 2018).
- Quackenbush, S.L.; Casey, R.N.; Murcek, R.J.; Paul, T.A.; Work, T.; Limpus, C.J.; Chaves, A.; Dutoit, L.; Perez, J.V.; Aguirre, A.; et al. Quantitative Analysis of Herpesvirus Sequences from Normal Tissue and Fibropapillomas of Marine Turtles with Real-Time PCR. Virology 2001, 287, 105–111. [Google Scholar] [CrossRef][Green Version]
- Alfaro-Núñez, A.; Bojesen, A.M.; Bertelsen, M.F.; Wales, N.; Balazs, G.H.; Gilbert, M.T.P. Further evidence of Chelonid herpesvirus 5 (ChHV5) latency: High levels of ChHV5 DNA detected in clinically healthy marine turtles. PeerJ 2016, 4, e2274. [Google Scholar] [CrossRef]
- Alfaro-Núñez, A.; Gilbert, M. Validation of a sensitive PCR assay for the detection of Chelonid fibropapilloma-associated herpesvirus in latent turtle infections. J. Virol. Methods 2014, 206, 38–41. [Google Scholar] [CrossRef]
- Herbst, L.H.; Klein, A.P. Green turtle fibropapillomatosis: Challenges to assessing the role of environmental cofactors. Environ. Health Perspect. 1995, 103, 27–30. [Google Scholar] [CrossRef]
- Ackermann, M.; Koriabine, M.; Hartmann-Fritsch, F.; De Jong, P.J.; Lewis, T.D.; Schetle, N.; Work, T.M.; Dagenais, J.; Balazs, G.H.; Leong, J.-A.C. The Genome of Chelonid Herpesvirus 5 Harbors Atypical Genes. PLoS ONE 2012, 7, e46623. [Google Scholar] [CrossRef]
- Hargrove, S.A.; Work, T.M.; Brunson, S.; Foley, A.M.; Balazs, G.H. Proceedings of the 2015 International Summit on Fibropapil-Lomatosis: Global Status, Trends, and Population Impacts. NOAA Technical Memorandum NMFS-PIFSC-054. Available online: https://pubs.er.usgs.gov/publication/70186653 (accessed on 25 January 2021).
- Work, T.M.; Balazs, G.H. Relating Tumor Score to Hematology in Green Turtles with Fibropapillomatosis in Hawaii. J. Wildl. Dis. 1999, 35, 804–807. [Google Scholar] [CrossRef]
- Greenblatt, R.J.; Quackenbush, S.L.; Casey, R.N.; Rovnak, J.; Balazs, G.H.; Work, T.M.; Casey, J.W.; Sutton, C.A. Genomic Variation of the Fibropapilloma-Associated Marine Turtle Herpesvirus across Seven Geographic Areas and Three Host Species. J. Virol. 2005, 79, 1125–1132. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Xu, S.; Chen, C.; Tong, X.; Liang, Y.; Dong, X.; Lei, Y.; Zheng, X.; Yu, J.; et al. Detection of HPV-2 and identification of novel mutations by whole genome sequencing from biopsies of two patients with multiple cutaneous horns. J. Clin. Virol. 2007, 39, 34–42. [Google Scholar] [CrossRef]
- Work, T.M.; Dagenais, J.; Balazs, G.H.; Schettle, N.; Ackermann, M. Dynamics of Virus Shedding and In Situ Confirmation of Chelonid Herpesvirus 5 in Hawaiian Green Turtles with Fibropapillomatosis. Veter-Pathol. 2014, 52, 1195–1201. [Google Scholar] [CrossRef]
- Chaves, L.B.; Berrocal, A.; Meneses, A.I.; Sánchez, C.J.; Vásquez, C.M.O. Study on the etiology of fibropapillomatosis of olive ridley sea turtles (Lepidochelys olivacea) nesting in the National Wildlife Refuge at Ostional, Guanacaste, Costa Rica. Rev. Cienc. Mar. Y Costeras 2013, 5, 119–134. [Google Scholar] [CrossRef][Green Version]
- Monezi, T.A.; Mehnert, D.U.; de Moura, E.M.; Müller, N.M.; Garrafa, P.; Matushima, E.R.; Werneck, M.R.; Borella, M.I. Chelonid herpesvirus 5 in secretions and tumor tissues from green turtles (Chelonia mydas) from Southeastern Brazil: A ten-year study. Veter-Microbiol. 2016, 186, 150–156. [Google Scholar] [CrossRef]
- Jacobson, E.; Mansell, J.; Sundberg, J.; Hajjar, A.; Reichmann, M.; Ehrhart, L.; Walsh, M.; Murru, F. Cutaneous fibropapillomas of green turtles (Chelonia mydas). J. Comp. Pathol. 1989, 101, 39–52. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Yu, Q.; Aguirre, A.A.; Balazs, G.H.; Nerurkar, V.R.; Yanagihara, R. Detection of herpesviral sequences in tissues of green turtles with fibropapilloma by polymerase chain reaction. Arch. Virol. 2000, 145, 1885–1893. [Google Scholar] [CrossRef]
- Morrison, C.L.; Iwanowicz, L.; Work, T.M.; Fahsbender, E.; Breitbart, M.; Adams, C.; Iwanowicz, D.; Sanders, L.; Ackermann, M.; Cornman, R.S. Genomic evolution, recombination, and inter-strain diversity of chelonid alphaherpesvirus 5 from Florida and Hawaii green sea turtles with fibropapillomatosis. PeerJ 2018, 6, e4386. [Google Scholar] [CrossRef]
- Tristan, T.; Shaver, D.J.; Kimbro, J.; Demaar, T.; Metz, T.; George, J.; Amos, A. Identification of Fibropapillomatosis in Green Sea Turtles (Chelonia mydas) on the Texas Coast. J. Herpetol. Med. Surg. 2010, 20, 109–112. [Google Scholar] [CrossRef]
- Rittenburg, L.T.; Kelley, J.R.; Mansfield, K.L.; Savage, E.A. Marine leech parasitism of sea turtles varies across host species, seasons, and the tumor disease fibropapillomatosis. Dis. Aquat. Org. 2021, 143, 1–12. [Google Scholar] [CrossRef]
- Kane, R.A.; Christodoulides, N.; Jensen, I.M.; Becker, D.J.; Mansfield, K.L.; Savage, A.E. Gene expression changes with tumor disease and leech parasitism in the juvenile green sea turtle skin transcriptome. Gene 2021, 800, 145800. [Google Scholar] [CrossRef]
- Work, T.M.; Dagenais, J.; Willimann, A.; Balazs, G.; Mansfield, K.; Ackermann, M. Differences in Antibody Responses against Chelonid Alphaherpesvirus 5 (ChHV5) Suggest Differences in Virus Biology in ChHV5-Seropositive Green Turtles from Hawaii and ChHV5-Seropositive Green Turtles from Florida. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Ene, A.; Su, M.; Lemaire, S.; Rose, C.; Schaff, S.; Moretti, R.; Lenz, J.; Herbst, L.H. Distribution of Chelonid Fibropapillomatosis-Associated Herpesvirus Variants in Florida: Molecular Genetic Evidence for Infection of Turtles Following Recruitment to Neritic Developmental Habitats. J. Wildl. Dis. 2005, 41, 489–497. [Google Scholar] [CrossRef]
- Cheng-Tsung, T.; I-Jiunn, C. Two New Records of Marine Ozobranchid Leeches (Oligochaete: Ozobranchidae) in Taiwan. Comp. Parasitol. 2013, 80, 105–109. [Google Scholar] [CrossRef]
- Mashkour, N.; Jones, K.; Kophamel, S.; Hipolito, T.; Ahasan, S.; Walker, G.; Jakob-Hoff, R.; Whittaker, M.; Hamann, M.; Bell, I.; et al. Disease risk analysis in sea turtles: A baseline study to inform conservation efforts. PLoS ONE 2020, 15, e0230760. [Google Scholar] [CrossRef] [PubMed]
- Perrault, J.; Levin, M.; Mott, C.; Bovery, C.; Bresette, M.; Chabot, R.; Gregory, C.; Guertin, J.; Hirsch, S.; Ritchie, B.; et al. Insights on Immune Function in Free-Ranging Green Sea Turtles (Chelonia mydas) with and without Fibropapillomatosis. Animals 2021, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Dujon, A.M.; Schofield, G.; Venegas, R.M.; Thomas, F.; Ujvari, B. Sea Turtles in the Cancer Risk Landscape: A Global Meta-Analysis of Fibropapillomatosis Prevalence and Associated Risk Factors. Pathogens 2021, 10, 1295. [Google Scholar] [CrossRef]
- Page-Karjian, A. Fibropapillomatosis in marine turtles. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Miller, R.E., Lamberski, N., Calle, P.P., Eds.; Elsevier Inc.: Berkeley, CA, USA, 2019; Volume 9, pp. 398–403. [Google Scholar]
- Seminoff, J.A.; Shigueto, J.A.; Amorocho, D.; Arauz, R.; Gallegos, A.B.; Chaverri, D.C.; Gaos, A.; Kelez, S.; Mangel, J.C.; Urteaga, J.; et al. Biology and conservation of sea turtles in the Eastern Pacific ocean. In The Sea Turtles of the Eastern Pacific: Advances in Re-search and Conservation, 2nd ed.; Seminoff, J.A., Wallace, B.P., Eds.; The University of Arizona Press: Tucson, AZ, USA, 2012; pp. 1–38. [Google Scholar]
- Putman, N.F.; Naro-Maciel, E. Finding the ‘lost years’ in green turtles: Insights from ocean circulation models and genetic analysis. Proc. R. Soc. B Boil. Sci. 2013, 280, 20131468. [Google Scholar] [CrossRef]
- Jensen, M.; Pilcher, N.; Fitzsimmons, N. Genetic markers provide insight on origins of immature green turtles Chelonia mydas with biased sex ratios at foraging grounds in Sabah, Malaysia. Endanger. Species Res. 2016, 31, 191–201. [Google Scholar] [CrossRef]
- Joseph, J.; Nishizawa, H.; Arshaad, W.M.; Kadir, S.A.S.; Jaaman, S.A.; Bali, J.; Jamaludin, N.A.; Katoh, M. Genetic stock compositions and natal origin of green turtle (Chelonia mydas) foraging at Brunei Bay. Glob. Ecol. Conserv. 2016, 6, 16–24. [Google Scholar] [CrossRef]
- Snover, M.L. Ontogenetic habitat shifts in marine organisms: Influencing factors and the impact of climate variability. Bull. Mar. Sci. 2008, 83, 53–67. [Google Scholar]
- Hayashi, R.; Nishizawa, H. Body size distribution demonstrates flexible habitat shift of green turtle (Chelonia mydas). Glob. Ecol. Conserv. 2015, 3, 115–120. [Google Scholar] [CrossRef]
- Bourjea, J.; Lapegue, S.; Gagnevin, L.; Broderick, D.; Mortimer, J.A.; Ciccione, S.; Roos, D.; Taquet, C.; Grizel, H. Phylogeography of the green turtle, Chelonia mydas, in the Southwest Indian Ocean. Mol. Ecol. 2006, 16, 175–186. [Google Scholar] [CrossRef]
- Roberts, A.M.; Schwartz, T.S.; Karl, A.S. Global Population Genetic Structure and Male-Mediated Gene Flow in the Green Sea Turtle (Chelonia mydas): Analysis of Microsatellite Loci. Genetics 2004, 166, 1857–1870. [Google Scholar] [CrossRef]
- Dethmers, K.E.M.; Jensen, M.P.; Fitzsimmons, N.N.; Broderick, D.; Limpus, C.J.; Moritz, C. And Migration of green turtles (Chelonia mydas) from Australasian feeding grounds inferred from genetic analyses. Mar. Freshw. Res. 2010, 61, 1376–1387. [Google Scholar] [CrossRef]
- Palaniappan, P.; Hamid, H.H.A. Spatial site fidelity of sea turtles at a foraging ground in Mabul Island, Sabah, Malaysia. Inter. J. Fish. Aqua. Stud. 2017, 5, 140–144. [Google Scholar]
- Limpus, C. The hawksbill turtle, Eretmochelys imbricata, in Queensland: Population structure within a southern Great Barrier Reef feeding ground. Wildl. Res. 1992, 19, 489–505. [Google Scholar] [CrossRef]
- Limpus, C. Estimation of tag loss in marine turtle research. Wildl. Res. 1992, 19, 457–469. [Google Scholar] [CrossRef]
- Limpus, C.J.; Reed, P.C. The Green turtle, Chelonia mydas, in Queensland: A preliminary description of the population structure in a coral reef feeding ground. In Biology of Australasian Frogs and Reptiles; Grigg, G., Shine, R., Ehmann, H., Eds.; Surrey Beatty: New South Wales, Australia, 1985; pp. 47–52. [Google Scholar]
- Bolten, A.B. Techniques for measuring sea turtles. In Research and Management Techniques for the Conservation of Sea Turtle; Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, A., Donnelly, M., Eds.; IUCN/SSC Marine Turtle Specialist Group Publication No. 4: Washington, DC, USA, 1999; pp. 110–114. [Google Scholar]
- Dutton, P.H. Methods for collection and preservation of samples for sea turtle genetic studies. In Proceedings of the International Symposium on Sea Turtle Conservation Genetics; Bowen, B.W., Witzel, W.N., Eds.; NOOA Technical Memorandum NMFS-SEFSC-396: Seattle, WA, USA, 1996; pp. 17–24. [Google Scholar]
- Bancroft, J.D.; Suvarna, K.; Layton, C. Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingston: Nottingham, UK; Sheffield, UK, 2012. [Google Scholar]
- Hunter, E. Practical Electron Microscopy: A Beginner’s Illustrated Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Bruford, M.W.; Hanotte, O.; Brookfield, J.F.Y.; Burke, T. Single locus and multilocus DNA fingerprinting. In Molecular Genetic Analysis of Populations–A Practical Approach; Hoelzel, A.R., Ed.; IRL Press: Oxford, UK, 1992; pp. 227–229. [Google Scholar]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, A Graphical Viewer of Phylogenetic Trees. 2016. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 9 November 2020).
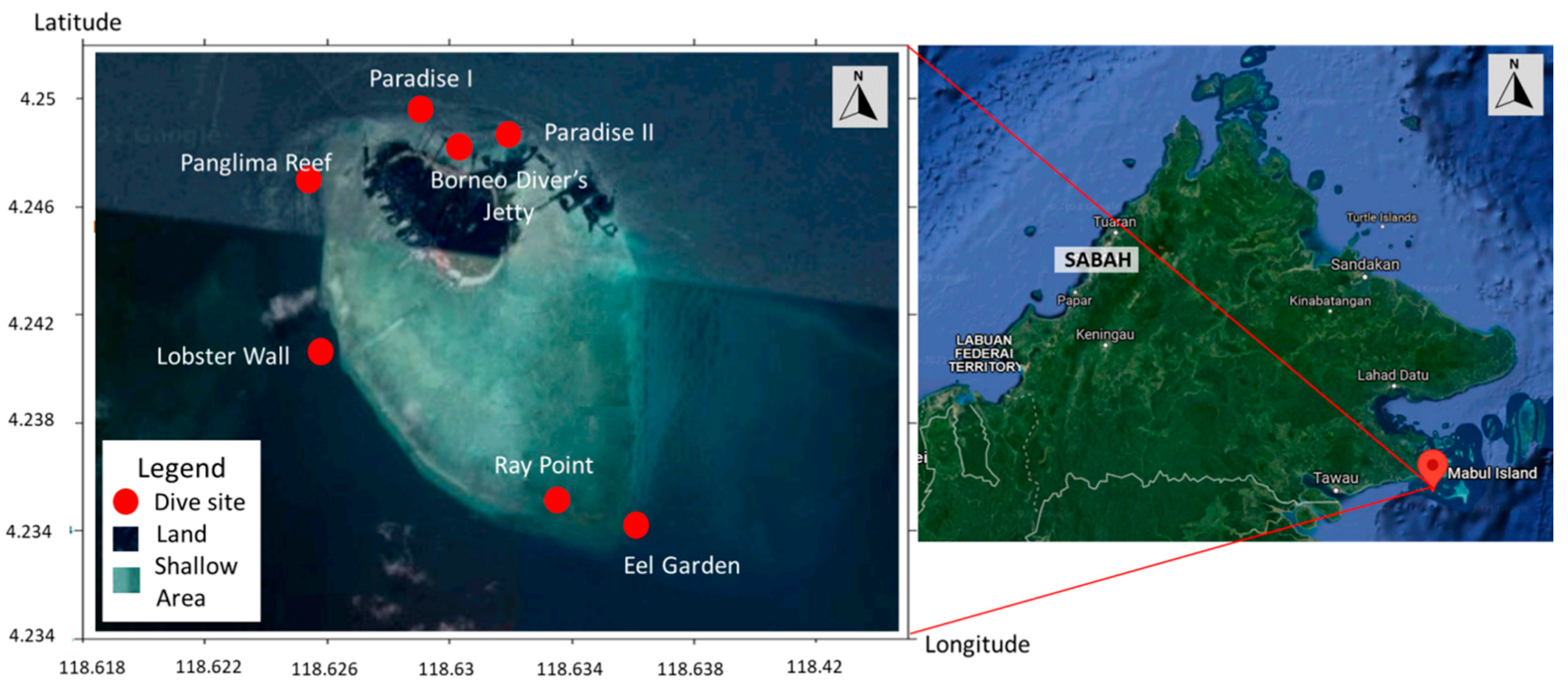
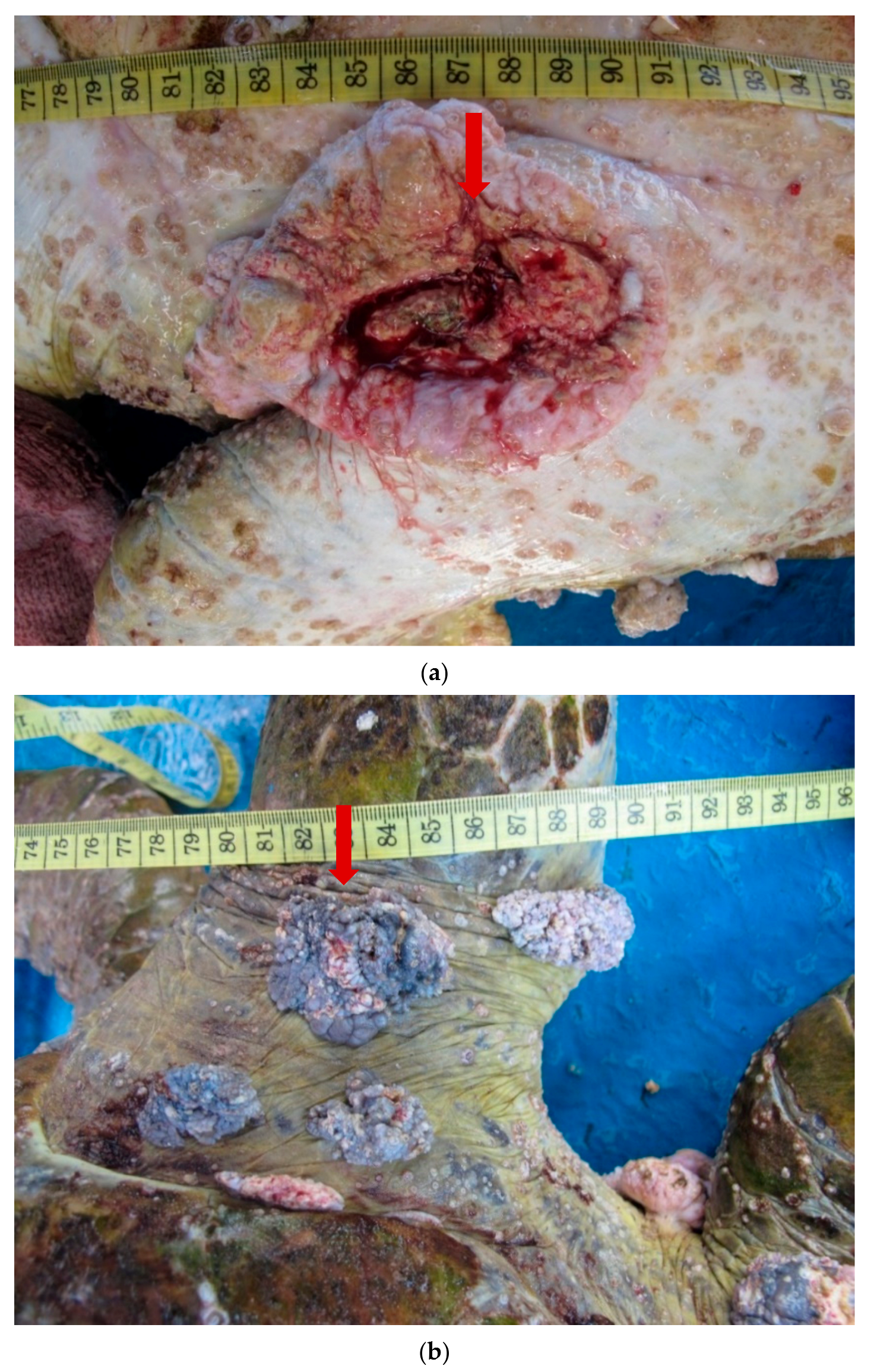
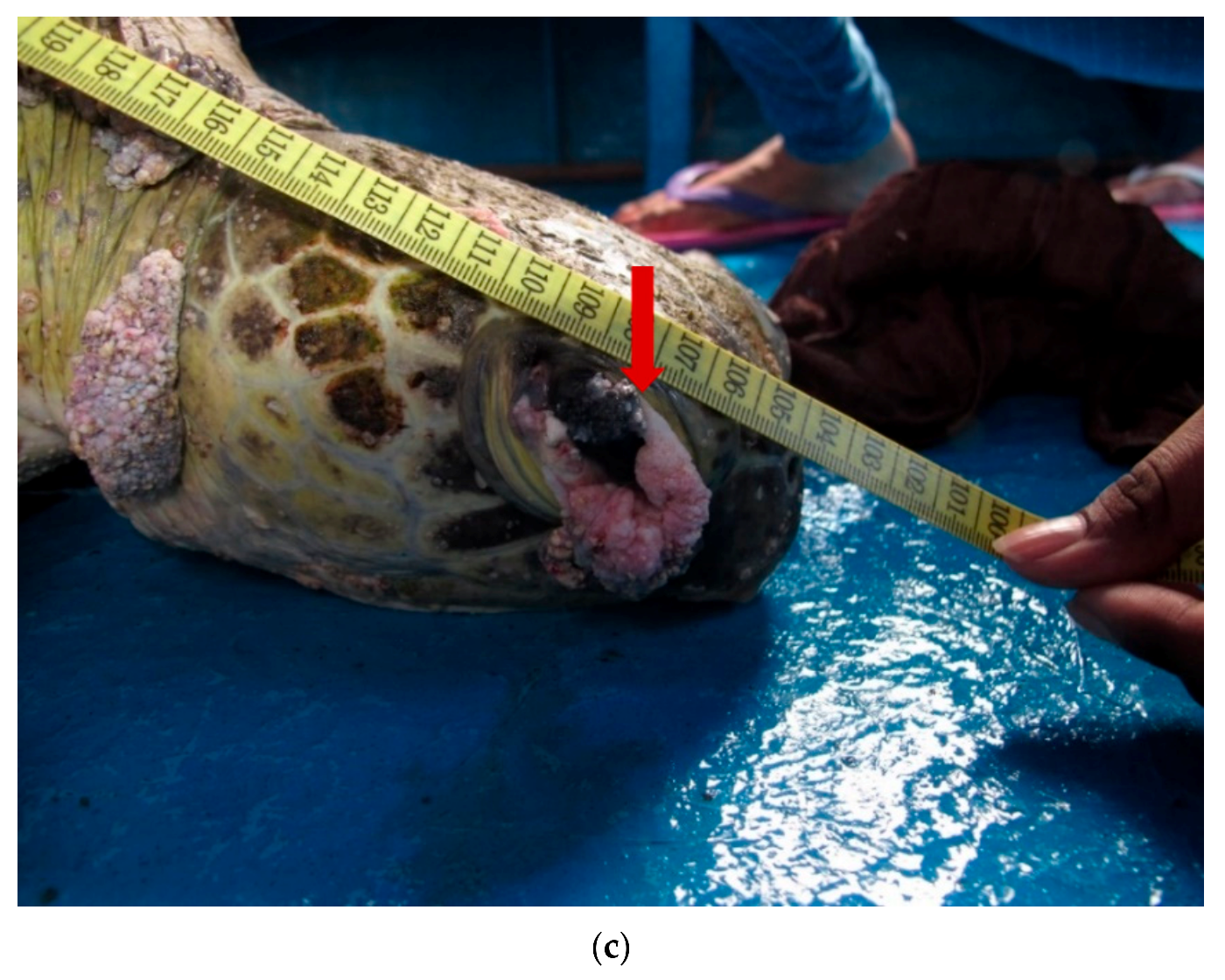
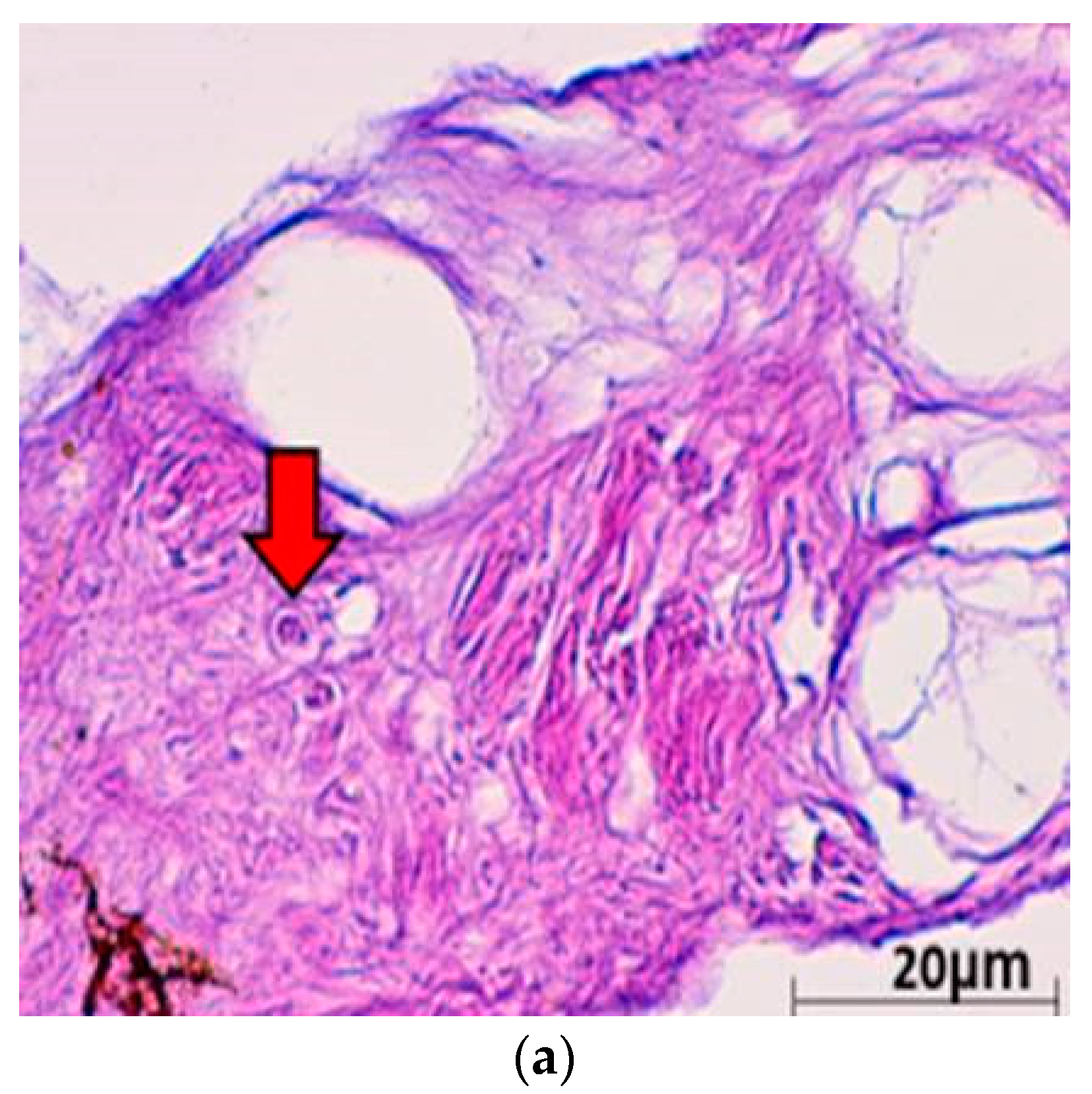
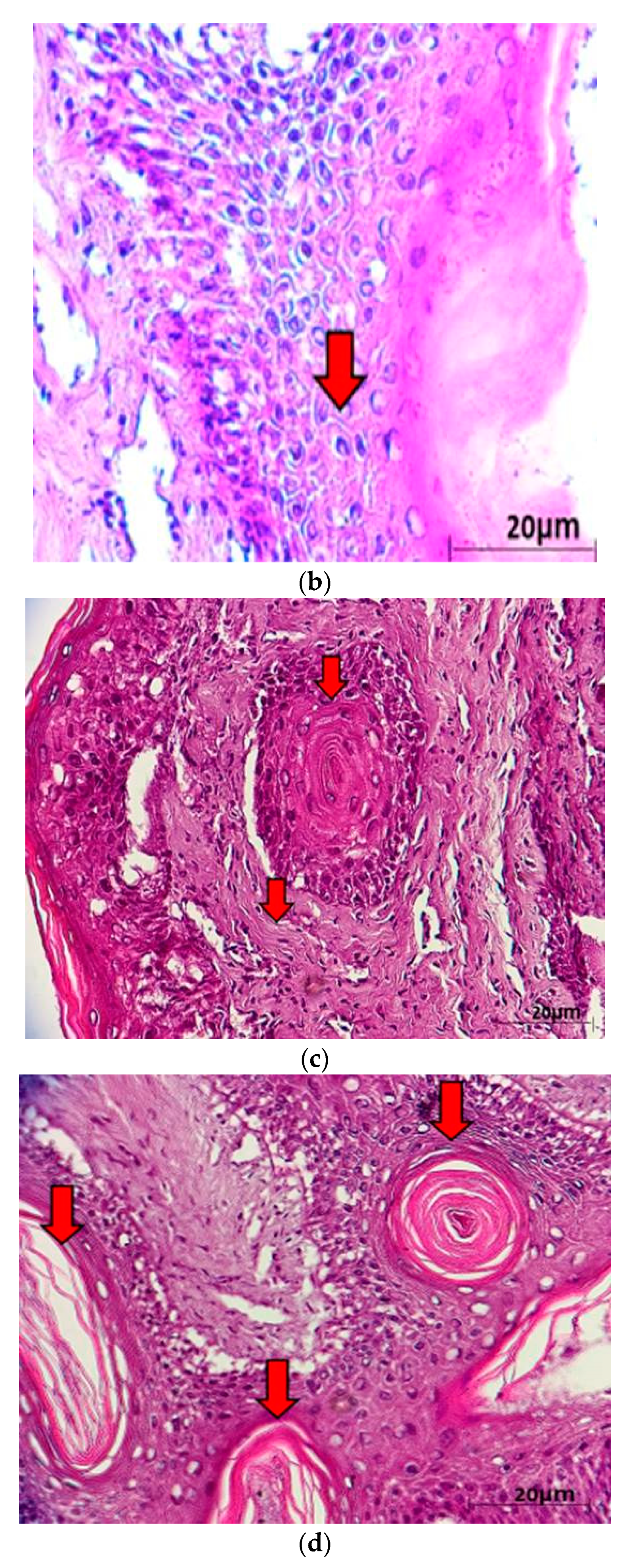

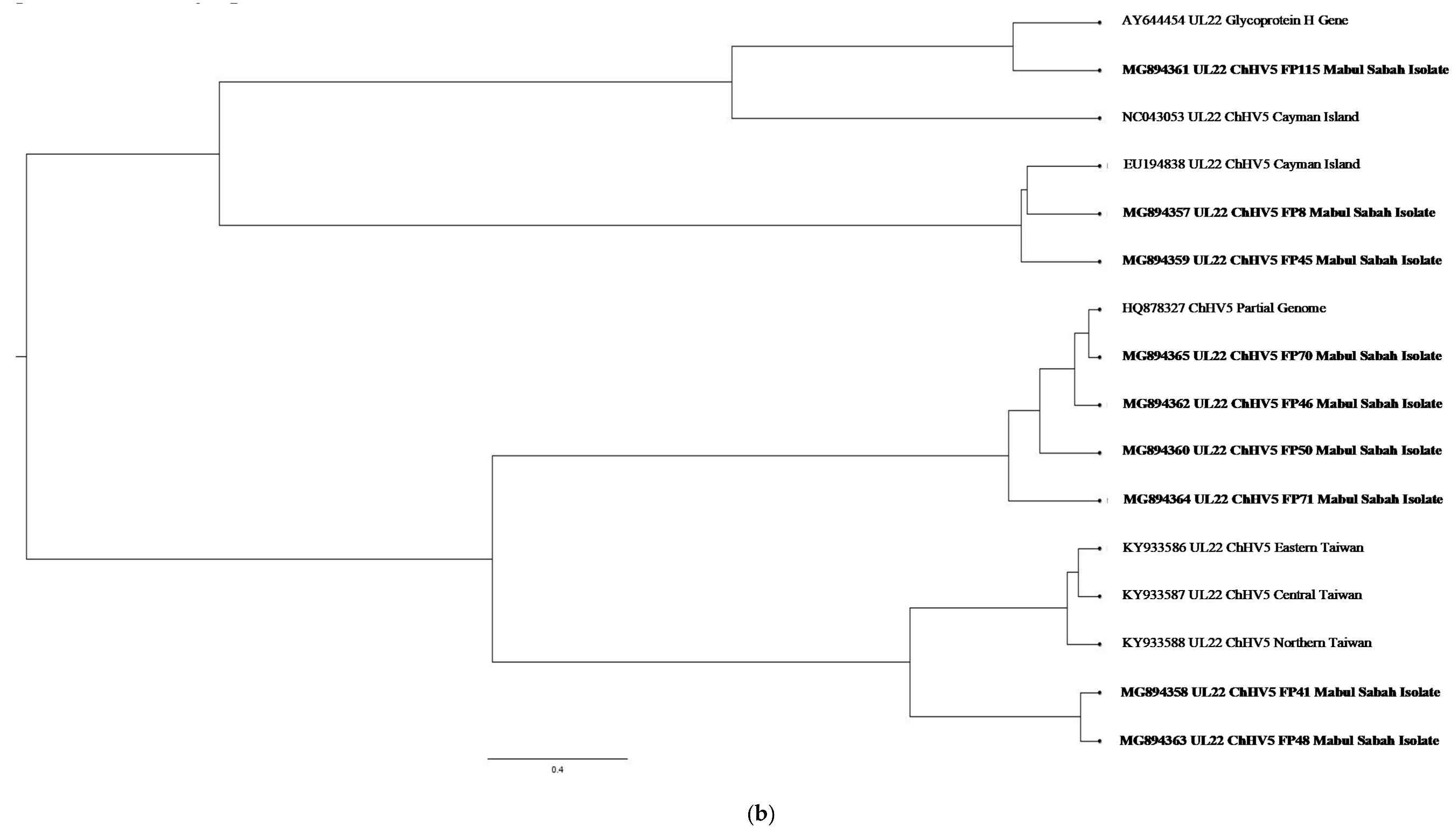
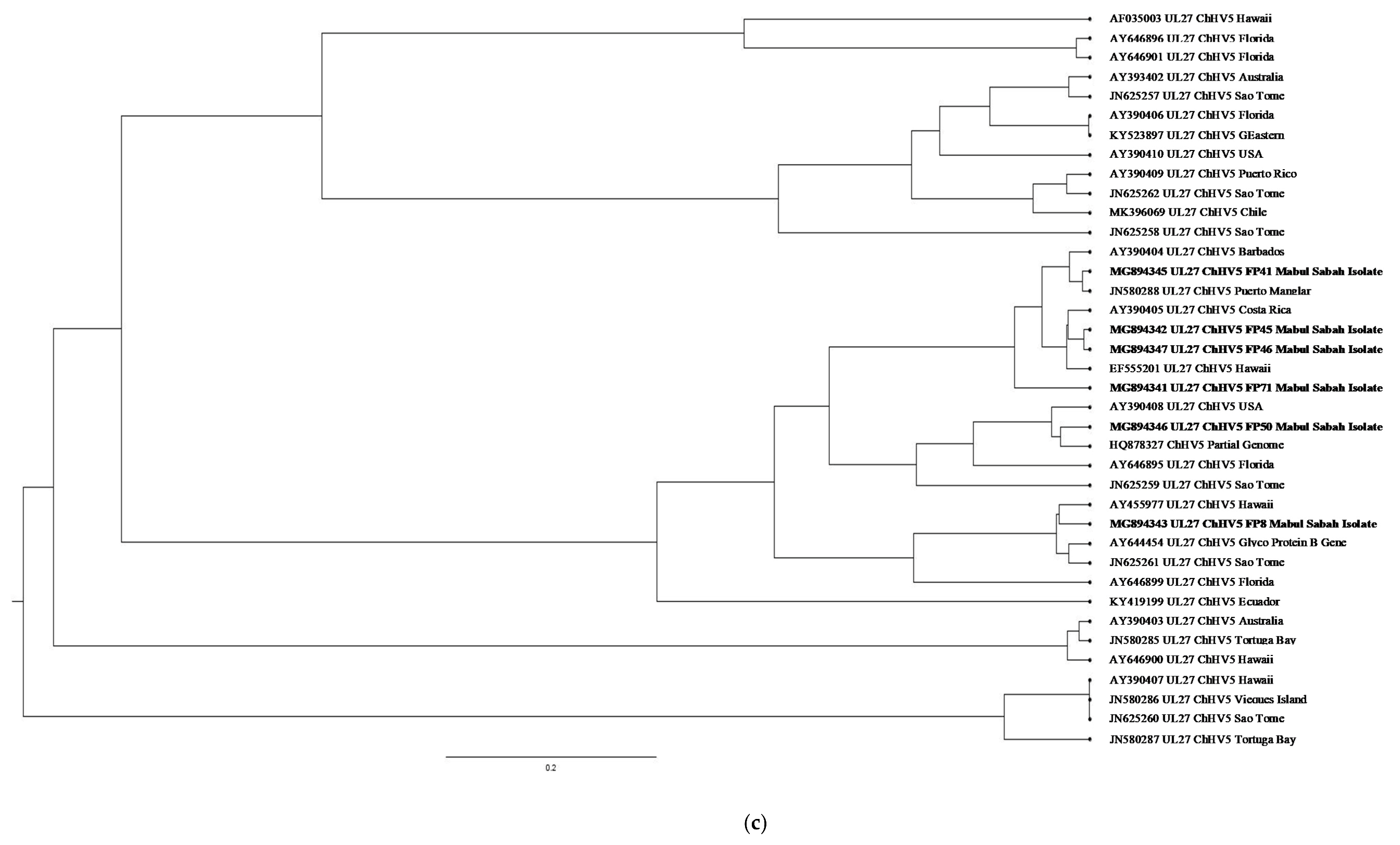
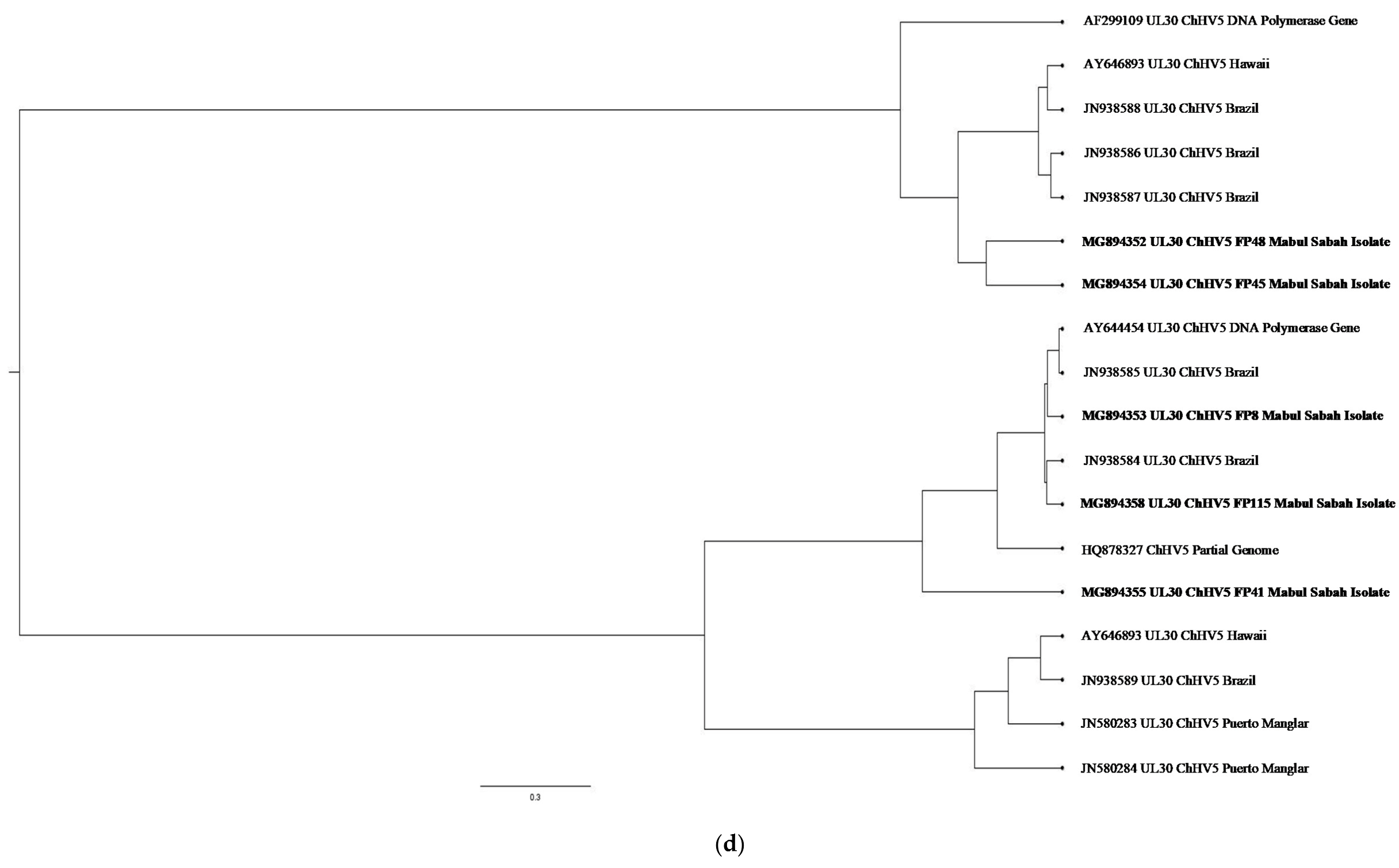

| (a). Detection of ChHV5 in Tissues with and without Tumours in the Green and Hawksbill Turtles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Turtle Species | Turtle Captured | Turtles with Tumours (Visual Inspection and Positive Identification Using PCR) | Turtle without Tumours (Positive Identification Using PCR) | ||||||
| Green | 115 | 3 | 6 | ||||||
| Hawksbill | 16 | 0 | 0 | ||||||
| Total | 131 | 9 1 | |||||||
| (b). Green Turtle ID | |||||||||
| Gene | FP8 2 | FP41 | FP45 | FP46 | FP48 | FP50 | FP70 2 | FP71 2 | FP115 |
| Size class | Adult Male | Juvenile | Juvenile | Juvenile | Juvenile | Juvenile | Sub-Adult | Sub-Adult | Juvenile |
| Capsid Protein (UL18) | √ | √ | √ | √ | |||||
| Glycoprotein H (UL22) | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Glycoprotein B (UL27) | √ | √ | √ | √ | √ | √ | |||
| DNA Polymerase (UL30) | √ | √ | √ | √ | √ | ||||
| Region | Primer Name | Primer Sequences 5′–3′ | Size (bp) | Reference |
|---|---|---|---|---|
| Capsid protein gene | UL18 1′ | F: CACCACGAGGGGGAAAATGA R: TCAAATCCCCCGTTCACTCG | 717 | Alfaro-Núñez et al., 2014 |
| UL18 2′ | F: GTGGAACCCCGCCGGGTAAT R: TGATCCGGGCCGAGTAGCGG | 140 | ||
| Glyco-protein H gene | UL22 1′ | F: ACGGCGTTGGCTAGTGAATC R: GCAGTTCGGTACACACCTCT | 386 | Alfaro-Núñez et al., 2014 |
| UL22 2′ | F: AACGCCCTTTCCTCCGACCCATATT R: GCTGGGGGAGCATCGTGCAAA | 179 | ||
| Glyco-protein B gene | UL27 1′ | F: TAACAAGAAAGAACCGCGCG R: ATTTTCCCGGTCAGTGCCAA | 352 | Alfaro-Núñez et al., 2014 |
| UL27 2′ | F: CTAGATACATACTGGCCRTGCTCGTC R: GCCAGCGACCATCCGGAG | 143 | ||
| DNA Polymerase Subunit Pol gene | UL30 1′ | F: AGCATCATCCAGGCCCACAATCT R: CGGCCAGTTCCGGCGCGTCGACCA | 445 | Lu et al., 2000 |
| UL30 2′ | F: AGCATGTCGCGCCCTACGGTGGTGAC R: CTGCTGACCGACTGGCTGGC | 206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loganathan, A.L.; Palaniappan, P.; Subbiah, V.K. First Evidence of Chelonid Herpesvirus 5 (ChHV5) Infection in Green Turtles (Chelonia mydas) from Sabah, Borneo. Pathogens 2021, 10, 1404. https://doi.org/10.3390/pathogens10111404
Loganathan AL, Palaniappan P, Subbiah VK. First Evidence of Chelonid Herpesvirus 5 (ChHV5) Infection in Green Turtles (Chelonia mydas) from Sabah, Borneo. Pathogens. 2021; 10(11):1404. https://doi.org/10.3390/pathogens10111404
Chicago/Turabian StyleLoganathan, Aswini Leela, Pushpa Palaniappan, and Vijay Kumar Subbiah. 2021. "First Evidence of Chelonid Herpesvirus 5 (ChHV5) Infection in Green Turtles (Chelonia mydas) from Sabah, Borneo" Pathogens 10, no. 11: 1404. https://doi.org/10.3390/pathogens10111404
APA StyleLoganathan, A. L., Palaniappan, P., & Subbiah, V. K. (2021). First Evidence of Chelonid Herpesvirus 5 (ChHV5) Infection in Green Turtles (Chelonia mydas) from Sabah, Borneo. Pathogens, 10(11), 1404. https://doi.org/10.3390/pathogens10111404






