Coffee Pulp, a By-Product of Coffee Production, Modulates Gut Microbiota and Improves Metabolic Syndrome in High-Carbohydrate, High-Fat Diet-Fed Rats
Abstract
:1. Introduction
2. Results
2.1. Intake of Coffee Pulp Components
2.2. Dietary Intake, Body Composition, and Plasma Biochemistry
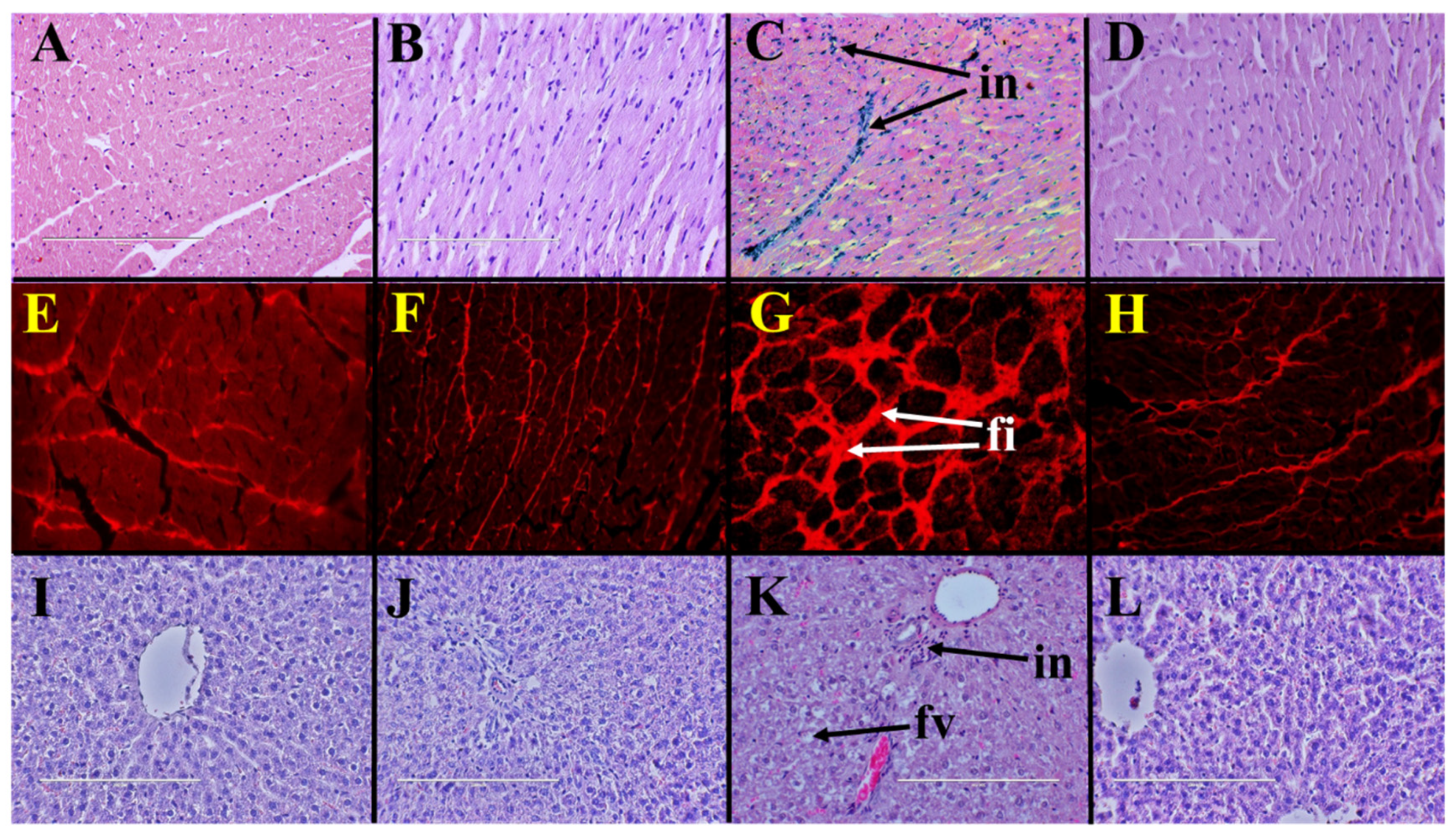
2.3. Cardiovascular Structure and Function
2.4. Liver Structure and Function
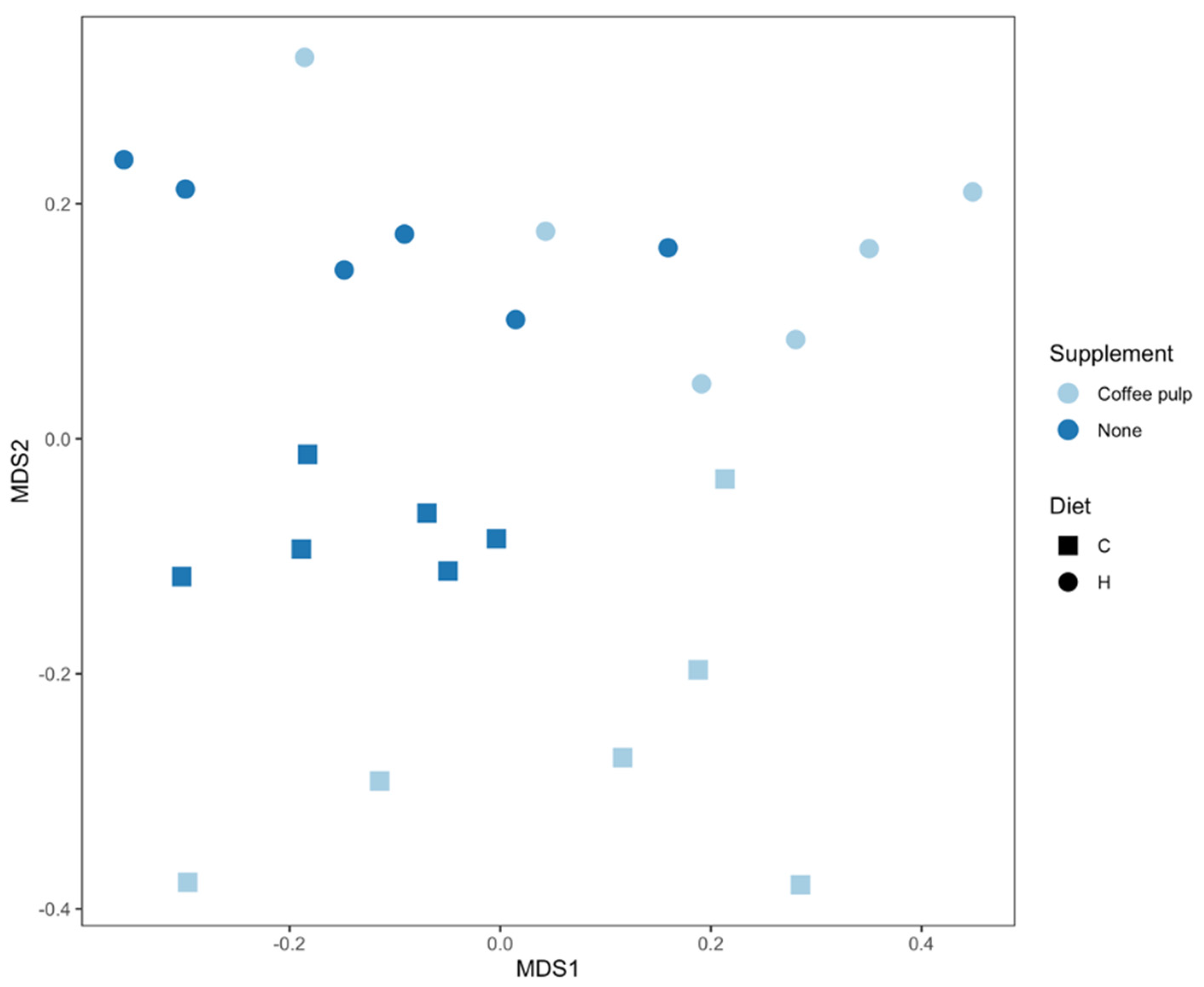
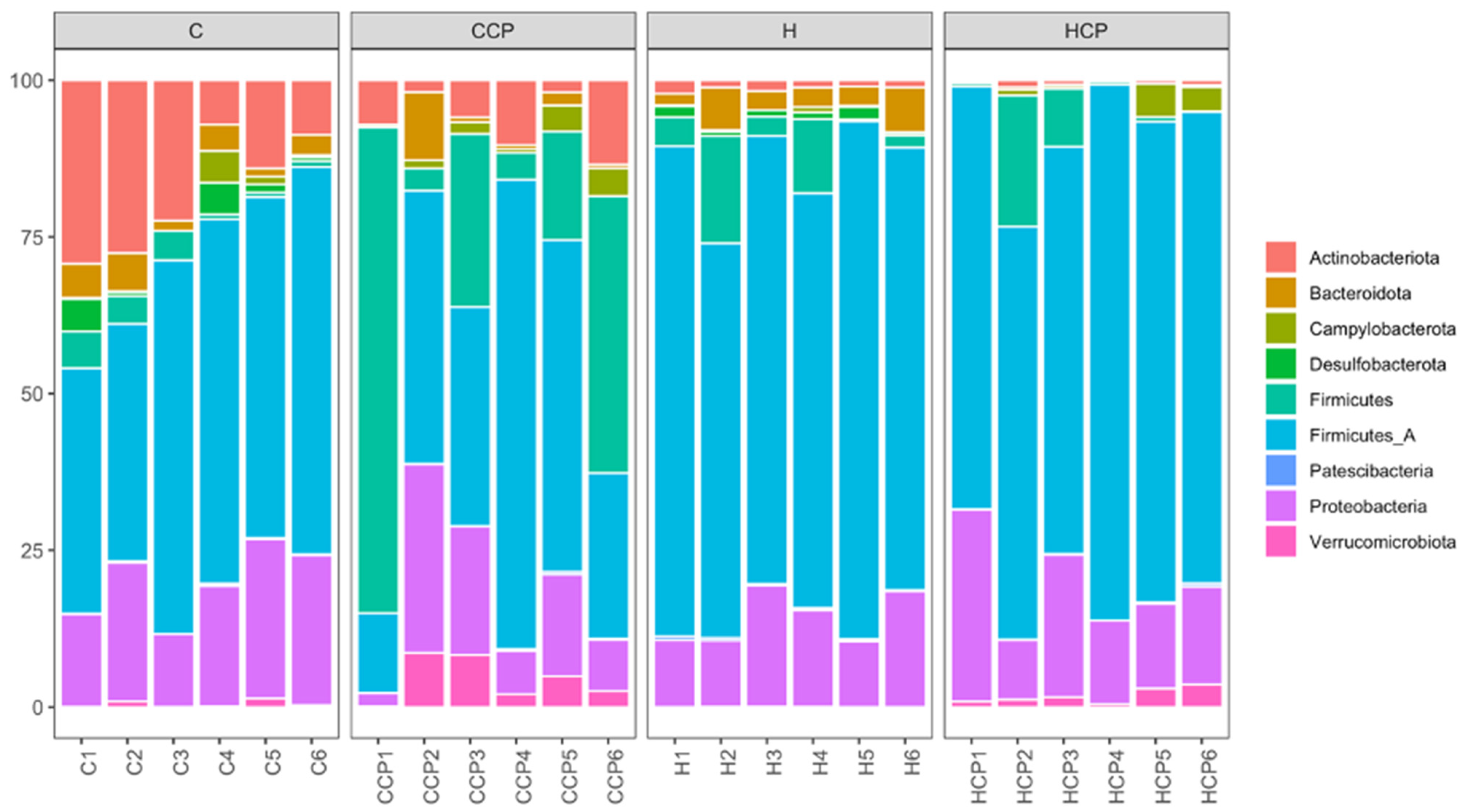
2.5. Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Preparation of Coffee Pulp Powder
4.2. Characterisation of Coffee Pulp Powder
4.3. Rats, Diets and Treatments
4.4. Physiological Parameters in Live Rats
4.5. Measurements after Euthanasia
4.6. Stastical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICO. Monthly Coffee Market Report (June 2021); International Coffee Organization: London, UK, 2021; Available online: https://www.ico.org/documents/cy2020-21/cmr-0621-e.pdf (accessed on 12 August 2021).
- Potts, J.; Lynch, M.; Wilkings, A.; Huppé, G.A.; Cunningham, M.; Voora, V.A. Chapter 8. Coffee Market. In The State of Sustainability Initiatives Review: Standards and the Green Economy; Ilnyckyj, R., Holmes, D., Rickert, E., Eds.; International Institute for Sustainable Development: Winnipeg, MB, Canada, 2014; pp. 155–186. Available online: https://www.iisd.org/system/files/pdf/2014/ssi_2014.pdf (accessed on 12 August 2021).
- Banerjee, S.; Meghashree, H.M.; Haldar, S. Coffee cultivation, processing and aroma: A detailed review. Acta Sci. Nutr. Health 2019, 3, 14–18. [Google Scholar]
- Brando, C.H.J. Harvesting and Green Coffee Processing. In Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers; Wintgens, J.N., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 604–715. [Google Scholar] [CrossRef]
- Blinová, L.; Sirotiak, M.; Bartošová, A.; Soldán, M. Review: Utilization of waste from coffee production. Res. Pap. Fac. Mat. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, M.C.; Pellegrino, E.; Nuti, M. Chapter 9. The solid wastes of coffee production and of olive oil extraction: Management perspectives in rural areas. In Solid Waste Management in Rural Areas; Mihai, F.-C., Ed.; IntechOpen: London, UK, 2017; pp. 165–189. [Google Scholar] [CrossRef] [Green Version]
- Rathinavelu, R.; Graziosi, G. Potential Alternative Use of Coffee Wastes and By-Products; International Coffee Organization: London, UK, 2005; pp. 1–4. Available online: https://www.ico.org/documents/ed1967e.pdf (accessed on 12 August 2021).
- Figueroa, G.A.; Homann, T.; Rawel, H.M. Coffee production wastes: Potentials and perspectives. Austin Food Sci. 2016, 1, 1014. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Braham, J.E.; Bressani, R. Coffee Pulp: Composition, Technology, and Utilization; International Development Research Centre: Ottawa, ON, Canada, 1979. [Google Scholar]
- Korikanthimath, V.S.; Hosmani, M.M. Organic recycling of coffee pulp in coffee based cropping systems. Indian Coffee 2000, 64, 4–6. [Google Scholar]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandarkar, N.S.; Mouatt, P.; Goncalves, P.; Thomas, T.; Brown, L.; Panchal, S.K. Modulation of gut microbiota by spent coffee grounds attenuates diet-induced metabolic syndrome in rats. FASEB J. 2020, 34, 4783–4797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameca, G.M.; Cerrilla, M.E.O.; Córdoba, P.Z.; Cruz, A.D.; Hernández, M.S.; Haro, J.H. Chemical composition and antioxidant capacity of coffee pulp. Ciênc. Agrotec. 2018, 42, 307–313. [Google Scholar] [CrossRef]
- Magoni, C.; Bruni, I.; Guzzetti, L.; Dell’Agli, M.; Sangiovanni, E.; Piazza, S.; Regonesi, M.E.; Maldini, M.; Spezzano, R.; Caruso, D.; et al. Valorizing coffee pulp by-products as anti-inflammatory ingredient of food supplements acting on IL-8 release. Food Res. Int. 2018, 112, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ontawong, A.; Duangjai, A.; Muanprasat, C.; Pasachan, T.; Pongchaidecha, A.; Amornlerdpison, D.; Srimaroeng, C. Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine 2019, 52, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Ontawong, A.; Boonphang, O.; Pasachan, T.; Duangjai, A.; Pongchaidecha, A.; Phatsara, M.; Jinakote, M.; Amornlerdpison, D.; Srimaroeng, C. Hepatoprotective effect of coffee pulp aqueous extract combined with simvastatin against hepatic steatosis in high-fat diet-induced obese rats. J. Funct. Foods 2019, 54, 568–577. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Garralda-Del-Villar, M.; Carlos-Chillerón, S.; Diaz-Gutierrez, J.; Ruiz-Canela, M.; Gea, A.; Martínez-González, M.A.; Bes-Rastrollo, M.; Ruiz-Estigarribia, L.; Kales, S.N.; Fernández-Montero, A. Healthy lifestyle and incidence of metabolic syndrome in the SUN cohort. Nutrients 2019, 11, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. North Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- John, O.D.; du Preez, R.; Panchal, S.K.; Brown, L. Tropical foods as functional foods for metabolic syndrome. Food Funct. 2020, 11, 6946–6960. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Wang, Y.F.; Fan, G.W.; Wang, X.Y.; Xu, S.Y.; Zhu, Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front. Microbiol. 2017, 8, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Heeger, A.; Kosinska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.; Al-Shannag, M.; Knapp, S.; Das, T.; Singsaas, E.; Alkasrawi, M. Technical possibilities of bioethanol production from coffee pulp: A renewable feedstock. Clean Technol. Environ. Policy 2016, 18, 269–278. [Google Scholar] [CrossRef]
- KC, Y.; Subba, R.; Shiwakoti, L.D.; Dhungana, P.K.; Bajagain, R.; Chaudhary, D.K.; Pant, B.R.; Bajgai, T.R.; Lamichhane, J.; Timilsina, S.; et al. Utilizing coffee pulp and mucilage for producing alcohol-based beverage. Fermentation 2021, 7, 53. [Google Scholar] [CrossRef]
- Dos Santos, É.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Delgado, S.R.; Arbelaez, A.F.A.; Rojano, B. Antioxidant capacity, bioactive compounds in coffee pulp and implementation in the production of infusions. Acta Sci. Pol. Technol. Aliment. 2019, 18, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Geremu, M.; Tola, Y.B.; Sualeh, A. Extraction and determination of total polyphenols and antioxidant capacity of red coffee (Coffea arabica L.) pulp of wet processing plants. Chem. Biol. Technol. Agric. 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.-P.; Liu, C.; Chan, L.-P.; Liang, C.-H. Coffee pulp supplement affects antioxidant status and favors anti-aging of skin in healthy subjects. J. Cosmet. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Martinez, J.R. Phenolic compounds in coffee pulp: Quantitative determination by HPLC. J. Sci. Food Agric. 1988, 43, 135–144. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; Del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2016, 35, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Li, X.; Jiang, X. Coffee consumption and risk of the metabolic syndrome: A meta-analysis. Diabetes Metab. 2016, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Maki, C.; Funakoshi-Tago, M.; Aoyagi, R.; Ueda, F.; Kimura, M.; Kobata, K.; Tago, K.; Tamura, H. Coffee extract inhibits adipogenesis in 3T3-L1 preadipocyes by interrupting insulin signaling through the downregulation of IRS1. PLoS ONE 2017, 12, e0173264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, S.K.; Poudyal, H.; Waanders, J.; Brown, L. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high-carbohydrate, high-fat diet-fed male rats. J. Nutr. 2012, 142, 690–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, S.K.; Wong, W.Y.; Kauter, K.; Ward, L.C.; Brown, L. Caffeine attenuates metabolic syndrome in diet-induced obese rats. Nutrition 2012, 28, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, N.S.; Mouatt, P.; Brown, L.; Panchal, S.K. Green coffee ameliorates components of diet-induced metabolic syndrome in rats. J. Funct. Foods 2019, 57, 141–149. [Google Scholar] [CrossRef]
- De Sotillo, D.V.R.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L.; Menon, V. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem. Biol. Interact. 2010, 188, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Meng, J.; Shen, Q.; Zhang, Y.; Pan, S.; Chen, Z.; Zhu, L.-Q.; Lu, Y.; Huang, Y.; Zhang, G. Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat. Commun. 2017, 8, 15904. [Google Scholar] [CrossRef] [PubMed]
- Tharaheswari, M.; Jayachandra Reddy, N.; Kumar, R.; Varshney, K.C.; Kannan, M.; Sudha Rani, S. Trigonelline and diosgenin attenuate ER stress, oxidative stress-mediated damage in pancreas and enhance adipose tissue PPARγ activity in type 2 diabetic rats. Mol. Cell. Biochem. 2014, 396, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-F.; Zhang, F.; Zhang, J.; Zhang, R.-M.; Li, R. Protection effect of trigonelline on liver of rats with non-alcoholic fatty liver diseases. Asian Pac. J. Trop. Med. 2015, 8, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilavenil, S.; Arasu, M.V.; Lee, J.-C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, G.J.; Mayakrishnan, V.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food. Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L.M.; Van Gent, T.; Buytenhek, R.; Princen, H.M.; Katan, M.B. Consumption of French-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J. Intern. Med. 2000, 248, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urgert, R.; Weusten-van der Wouw, M.P.; Hovenier, R.; Meyboom, S.; Beynen, A.C.; Katan, M.B. Diterpenes from coffee beans decrease serum levels of lipoprotein(a) in humans: Results from four randomised controlled trials. Eur. J. Clin. Nutr. 1997, 51, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Kim, D.H.; Jeong, H.G. Inhibitory effect of the coffee diterpene kahweol on carrageenan-induced inflammation in rats. Biofactors 2006, 26, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Cafestol, a bioactive substance in coffee, stimulates insulin secretion and increases glucose uptake in muscle cells: Studies in vitro. J. Nat. Prod. 2015, 78, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F.B.; Jeppesen, P.B.; Shokouh, P.; Laustsen, C.; Hermansen, K.; Gregersen, S. Cafestol, a bioactive substance in coffee, has antidiabetic properties in KKAy mice. J. Nat. Prod. 2017, 80, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, S.G.; Kang, Y.J.; Kwon, T.K.; Nam, J.O. Kahweol inhibits adipogenesis of 3T3-L1 adipocytes through downregulation of PPARγ. Nat. Prod. Res. 2018, 32, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-H.; Kim, N.-J.; Song, J.-K.; Chun, K.-H. Kahweol inhibits lipid accumulation and induces glucose-uptake through activation of AMP-activated protein kinase (AMPK). BMB Rep. 2017, 50, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Melo, M.; Schwan, R.; Silva, C. A new alternative use for coffee pulp from semi-dry process to β-glucosidase production by Bacillus subtilis. Lett. Appl. Microbiol. 2015, 61, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Raman, M.; Rioux, K.P.; Reimer, R.A. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012, 32, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Núñez, A.C.; Rentería, I.D.C.D.; Villagómez, M.Á.L.; Elorza, P.; Martínez, C.E.M.S.; Pulido, S.A.A.; Rojas-Ronquillo, R. The use of coffee pulp as a potential alternative supplement in ruminant diets. J. Agric. Sci. Technol. A 2015, 5, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Adam, C.L.; Williams, P.A.; Dalby, M.J.; Garden, K.; Thomson, L.M.; Richardson, A.J.; Gratz, S.W.; Ross, A.W. Different types of soluble fermentable dietary fibre decrease food intake, body weight gain and adiposity in young adult male rats. Nutr. Metab. 2014, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, J.; Hasegawa, S.; Kasubuchi, M.; Ichimura, A.; Nakajima, A.; Kimura, I. Nutritional signaling via free fatty acid receptors. Int. J. Mol. Sci. 2016, 17, 450. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.; Pierre, F.; Patry, Y.; Champ, M.; Berreur, M.; Pradal, G.; Bornet, F.; Meflah, K.; Menanteau, J. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 2001, 48, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Louis, S.; Rings, A.; Messner, S.; Camarinha-Silva, A.; Seifert, J.; Bischoff, S.C.; et al. Intestinal microbiota and microbial metabolites are changed in a pig model fed a high-fat/low-fiber or a low-lat/high-fiber diet. PLoS ONE 2016, 11, e0154329. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; An, Y.; Hao, F.; Wang, Y.; Tang, H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Sci. Rep. 2016, 6, 21618. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, J.; Gao, J.; Wu, X.; Cui, C.; Wei, H.; Zheng, R.; Peng, J. Combined soluble fiber-mediated intestinal microbiota improve insulin sensitivity of obese mice. Nutrients 2020, 12, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Carmen, M.-T.; Lorena, Z.-C.; Alexander, V.-A.; Amandio, V.; Raúl, S. Coffee pulp: An industrial by-product with uses in agriculture, nutrition and biotechnology. Rev. Agric. Sci. 2020, 8, 323–342. [Google Scholar] [CrossRef]
- Muzaifa, M.; Rahmi, F.; Syarifudin. Utilization of coffee by-products as profitable foods—A mini review. IOP Conf. Ser. Earth Environ. Sci. 2021, 672, 012077. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; del Castillo, M.D. Applications of compounds from coffee processing by-products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Cozzano, S.; Pérez, A.M.; Arcia, P.; Curutchet, A. Coffee pulp waste as a functional ingredient: Effect on salty cookies quality. J. Food Nutr. Res. 2019, 7, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Kurzrock, T.; Speer, K. Identification of kahweol fatty acid esters in Arabica coffee by means of LC/MS. J. Sep. Sci. 2001, 24, 843–848. [Google Scholar] [CrossRef]
- Kolling-Speer, I.; Strohschneider, S.; Speer, K. Determination of free diterpenes in green and roasted coffees. J. High Resol. Chromatogr. 1999, 22, 43–46. [Google Scholar] [CrossRef]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.Y.; Li, Z.; Zhang, N.; Yu, H.T.; Wang, S.R.; Liu, J.R. Effects of gastrointestinal motility on obesity. Nutr. Metab. 2014, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prodan, A.; Tremaroli, V.; Brolin, H.; Zwinderman, A.H.; Nieuwdorp, M.; Levin, E. Comparing bioinformatic pipelines for microbial 16S rRNA amplicon sequencing. PLoS ONE 2020, 15, e0227434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Lin, H.; Revanna, K.; Dong, Q. A Bayesian taxonomic classification method for 16S rRNA gene sequences with improved species-level accuracy. BMC Bioinform. 2017, 18, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.A.; Hugenholtz, P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018, 36, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. mvabund—An R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
| Components | CCP | HCP |
|---|---|---|
| Caffeine, mg/kg/day | 24.7 ± 0.5 | 17.1 ± 0.9 |
| Chlorogenic acid, mg/kg/day | 4.31 ± 0.08 | 2.98 ± 0.16 |
| Phenolic acids, mg/kg/day | 7.97 ± 0.15 | 5.51 ± 0.29 |
| Trigonelline, mg/kg/day | 14.6 ± 0.3 | 10.1 ± 0.5 |
| Diterpenes, mg/kg/day | 106.8 ± 2.0 | 73.9 ± 3.9 |
| Variables | C | CCP | H | HCP | p Values | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Diet × Treatment | |||||
| Initial body weight, g | 339 ± 1 | 338 ± 1 | 340 ± 1 | 339 ± 1 | 1.00 | 0.32 | 0.32 |
| Final body weight, g | 380 ± 3 c | 386 ± 6 c | 551 ± 11 a | 494 ± 9 b | <0.0001 | 0.0022 | 0.0002 |
| Food intake, g/day | 35.2 ± 1.3 a | 34.2 ± 0.6 a | 27.2 ± 0.7 b | 28.0 ± 1.5 b | <0.0001 | 0.93 | 0.42 |
| Water intake, g/day | 31.0 ± 2.0 a,b | 36.8 ± 2.1 a | 26.6 ± 1.6 b | 32.2 ± 1.7 a,b | 0.02 | 0.004 | 0.96 |
| Energy intake, kJ/d | 405 ± 15 c | 384 ± 10 c | 556 ± 19 b | 616 ± 17 a | <0.0001 | 0.22 | 0.013 |
| Feed efficiency, g/kJ | 0.10 ± 0.01 c | 0.12 ± 0.02 c | 0.36 ± 0.02 a | 0.25 ± 0.02 b | <0.0001 | 0.016 | 0.0008 |
| Heat, kcal/h | 3.66 ± 0.25 b,c | 3.14 ± 0.31 c | 4.27 ± 0.16 a,b | 4.51 ± 0.16 a | <0.0001 | 0.54 | 0.10 |
| Respiratory exchange ratio | 0.99 ± 0.02 a | 0.95 ± 0.04 a,b,c | 0.88 ± 0.02 c | 0.89 ± 0.01 b,c | 0.0014 | 0.55 | 0.32 |
| Body mass index, g/cm2 | 0.61 ± 0.01 d | 0.66 ± 0.01 c | 0.83 ± 0.02 a | 0.75 ± 0.01 b | <0.0001 | 0.26 | <0.0001 |
| Abdominal circumference, cm | 18.8 ± 0.1 c | 17.8 ± 0.2 d | 23.7 ± 0.3 a | 20.2 ± 0.2 b | <0.0001 | <0.0001 | <0.0001 |
| Basal blood glucose, mmol/L | 4.0 ± 0.2 b | 2.9 ± 0.1 c | 4.7 ± 0.3 a | 3.2 ± 0.1 c | 0.013 | <0.0001 | 0.31 |
| Area under the curve for glucose tolerance, mmol/L × min | 695 ± 15 b | 421 ± 13 d | 797 ± 21 a | 571 ± 13 c | <0.0001 | <0.0001 | 0.14 |
| Retroperitoneal fat, mg/mm | 155 ± 14 b | 196 ± 14 b | 518 ± 42 a | 458 ± 22 a | <0.0001 | 0.71 | 0.06 |
| Epididymal fat, mg/mm | 85 ± 5 c | 86 ± 10 c | 234 ± 17 a | 183 ± 10 b | <0.0001 | 0.033 | 0.027 |
| Omental fat, mg/mm | 124 ± 7 c | 117 ± 11 c | 262 ± 12 a | 213 ± 12 b | <0.0001 | 0.012 | 0.06 |
| Total abdominal fat, mg/mm | 364 ± 18 c | 399 ± 28 c | 1014 ± 65 a | 855 ± 33 b | <0.0001 | 0.13 | 0.019 |
| Whole-body lean mass, g | 297 ± 4 a,b | 295 ± 4 a,b | 285 ± 8 b | 314 ± 7 a | 0.56 | 0.03 | 0.014 |
| Whole-body fat mass, g | 88 ± 6 c | 77 ± 7 c | 239 ± 12 a | 168 ± 11 b | <0.0001 | <0.0001 | 0.003 |
| Bone mineral content, g | 11.8 ± 0.4 b | 12.2 ± 0.5 b | 17.4 ± 0.6 a | 15.8 ± 0.5 a | <0.0001 | 0.24 | 0.06 |
| Bone mineral density, g/cm2 | 0.172 ± 0.002 c | 0.188 ± 0.002 b | 0.185 ± 0.002 b | 0.197 ± 0.005 a | 0.0002 | <0.0001 | 0.47 |
| Intestinal transit, % | 74.1 ± 8.1 a,b | 52.2 ± 7.5 b | 90.2 ± 6.2 a | 58.2 ± 8.0 b | 0.15 | 0.0008 | 0.50 |
| Plasma total cholesterol, mmol/L | 1.79 ± 0.09 a | 0.56 ± 0.06 b | 1.56 ± 0.08 a | 1.84 ± 0.13 a | <0.0001 | <0.0001 | <0.0001 |
| Plasma triglycerides, mmol/L | 0.57 ± 0.06 b | 0.33 ± 0.04 b | 1.28 ± 0.14 a | 0.61 ± 0.07 b | <0.0001 | <0.0001 | 0.016 |
| Plasma non-esterified fatty acids, mmol/L | 1.39 ± 0.18 b | 0.76 ± 0.14 c | 3.46 ± 0.22 a | 1.53 ± 0.20 b | <0.0001 | <0.0001 | 0.001 |
| Systolic blood pressure, mmHg | 127 ± 1 b | 125 ± 1 b | 143 ± 2 a | 129 ± 1 b | <0.0001 | <0.0001 | <0.0001 |
| Diastolic stiffness constant (κ) | 21.9 ± 0.5 b | 20.5 ± 0.4 b | 29.3 ± 1.9 a | 21.0 ± 0.3 b | <0.0001 | 0.0004 | 0.002 |
| Left ventricle + septum wet weight, mg/mm | 21.4 ± 0.8 b | 20.4 ± 0.7 b | 25.4 ± 1.5 a | 23.5 ± 0.8 a,b | 0.001 | 0.16 | 0.66 |
| Right ventricle wet weight, mg/mm | 4.82 ± 0.33 | 4.44 ± 0.20 | 5.42 ± 0.49 | 5.15 ± 0.20 | 0.051 | 0.33 | 0.87 |
| Liver wet weight, mg/mm | 227 ± 6 c | 245 ± 10 c | 349 ± 7 a | 293 ± 10 b | <0.0001 | 0.029 | <0.0001 |
| Plasma aspartate transaminase activity, U/L | 70.5 ± 3.3 c | 94.7 ± 3.9 a,b | 81.0 ± 5.4 b,c | 103.1 ± 6.7 a | 0.07 | <0.0001 | 0.83 |
| Plasma alanine transaminase activity, U/L | 29.4 ± 4.2 | 37.4 ± 2.8 | 36.6 ± 3.6 | 42.1 ± 3.5 | 0.10 | 0.06 | 0.73 |
| Physiological Variable | R2 | p Value |
|---|---|---|
| Epididymal fat | 0.74 | 0.001 |
| Liver wet weight | 0.71 | 0.001 |
| Omental fat | 0.64 | 0.001 |
| Total abdominal fat | 0.64 | 0.001 |
| Retroperitoneal fat | 0.57 | 0.001 |
| Kidneys wet weight | 0.49 | 0.001 |
| Water intake | 0.48 | 0.002 |
| Alanine transaminase activity | 0.47 | 0.002 |
| Systolic blood pressure | 0.40 | 0.005 |
| Spleen wet weight | 0.37 | 0.002 |
| Feed efficiency | 0.33 | 0.013 |
| Non-esterified fatty acids | 0.28 | 0.032 |
| Body weight gain | 0.28 | 0.030 |
| Aspartate transaminase activity | 0.26 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandarkar, N.S.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Brown, L.; Panchal, S.K. Coffee Pulp, a By-Product of Coffee Production, Modulates Gut Microbiota and Improves Metabolic Syndrome in High-Carbohydrate, High-Fat Diet-Fed Rats. Pathogens 2021, 10, 1369. https://doi.org/10.3390/pathogens10111369
Bhandarkar NS, Mouatt P, Majzoub ME, Thomas T, Brown L, Panchal SK. Coffee Pulp, a By-Product of Coffee Production, Modulates Gut Microbiota and Improves Metabolic Syndrome in High-Carbohydrate, High-Fat Diet-Fed Rats. Pathogens. 2021; 10(11):1369. https://doi.org/10.3390/pathogens10111369
Chicago/Turabian StyleBhandarkar, Nikhil S., Peter Mouatt, Marwan E. Majzoub, Torsten Thomas, Lindsay Brown, and Sunil K. Panchal. 2021. "Coffee Pulp, a By-Product of Coffee Production, Modulates Gut Microbiota and Improves Metabolic Syndrome in High-Carbohydrate, High-Fat Diet-Fed Rats" Pathogens 10, no. 11: 1369. https://doi.org/10.3390/pathogens10111369
APA StyleBhandarkar, N. S., Mouatt, P., Majzoub, M. E., Thomas, T., Brown, L., & Panchal, S. K. (2021). Coffee Pulp, a By-Product of Coffee Production, Modulates Gut Microbiota and Improves Metabolic Syndrome in High-Carbohydrate, High-Fat Diet-Fed Rats. Pathogens, 10(11), 1369. https://doi.org/10.3390/pathogens10111369









