Review of the New Zealand Theileria orientalis Ikeda Type Epidemic and Epidemiological Research since 2012
Abstract
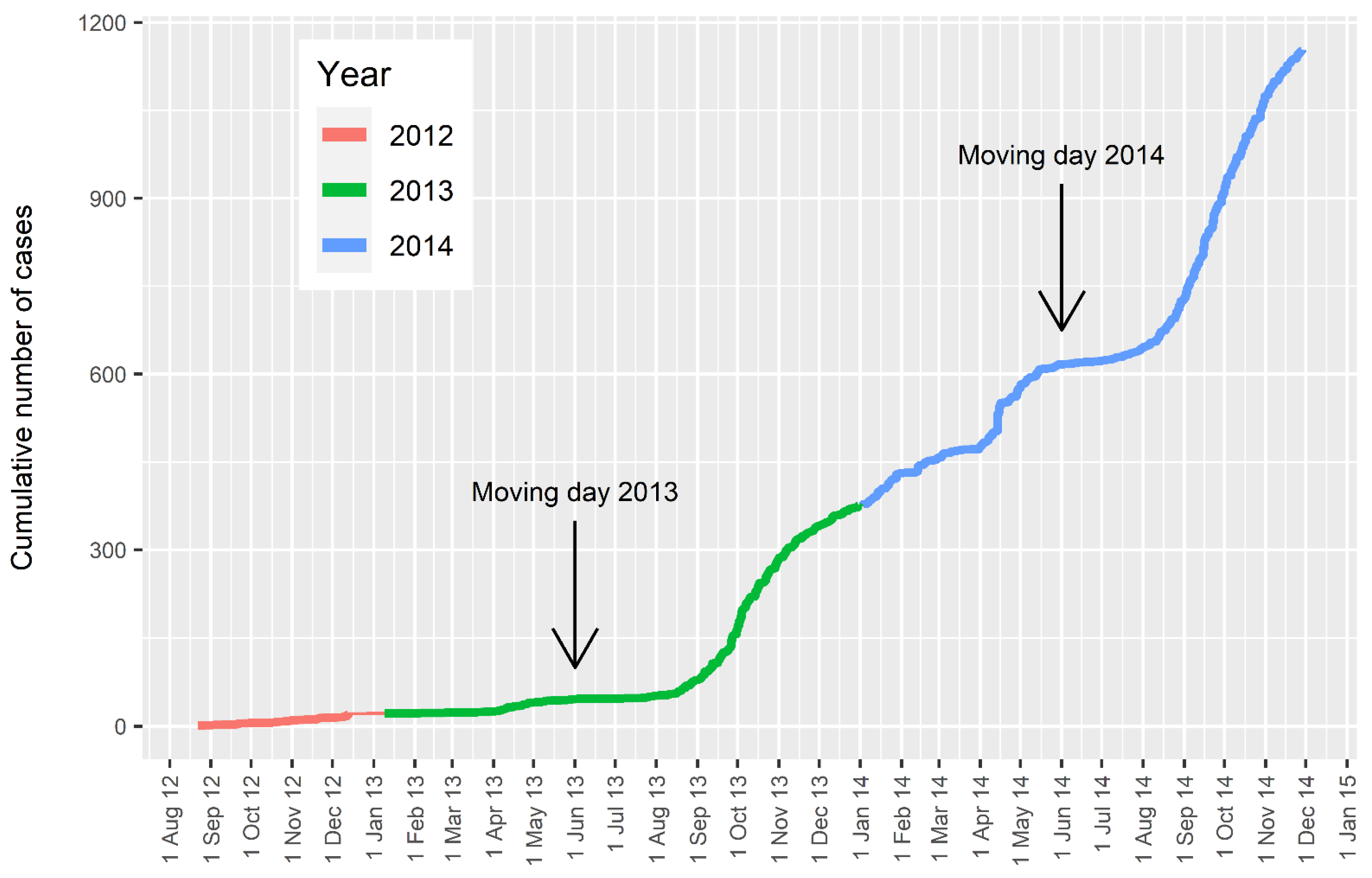
:1. The New Zealand Epidemic
2. Source of Infection
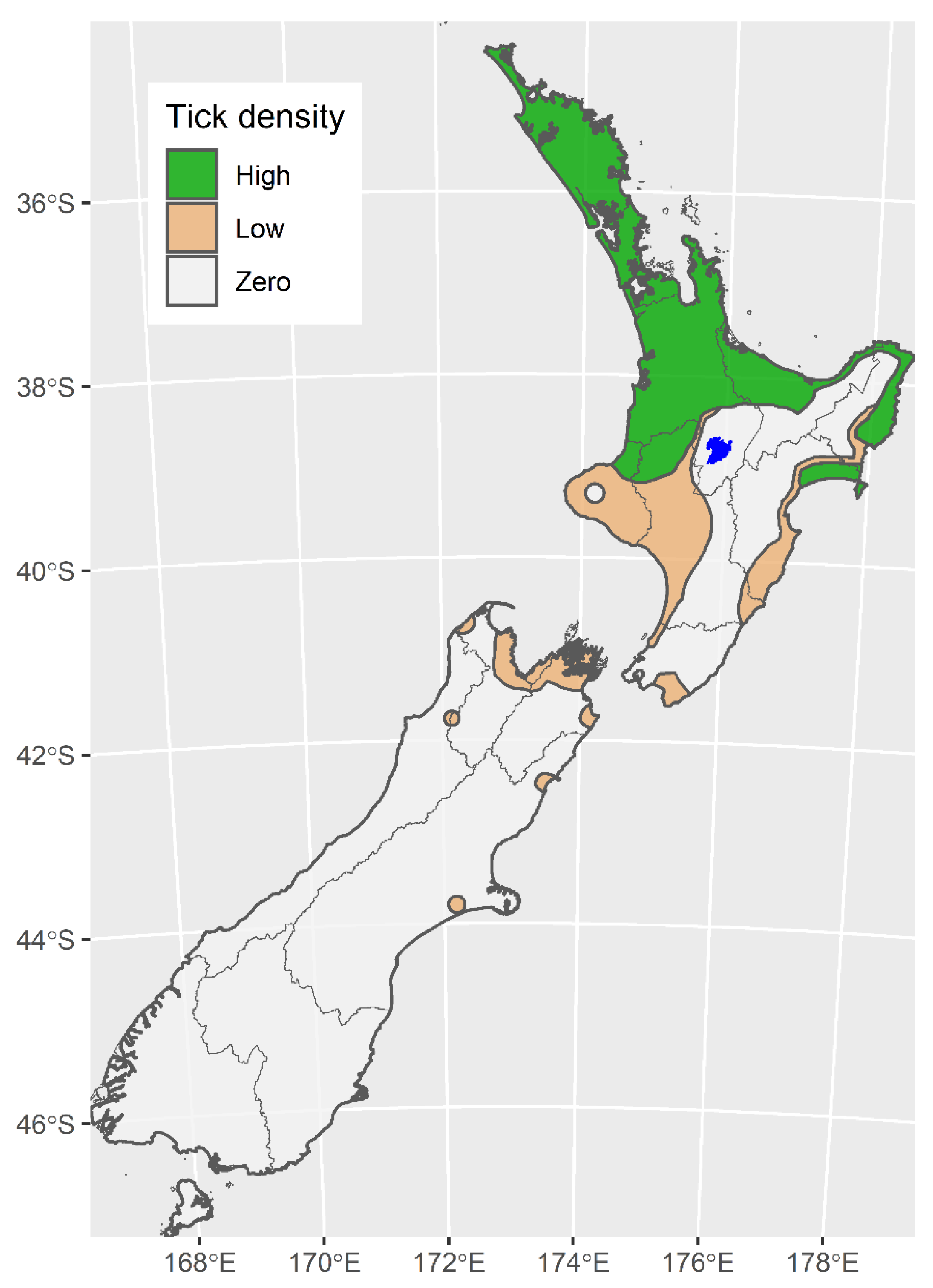
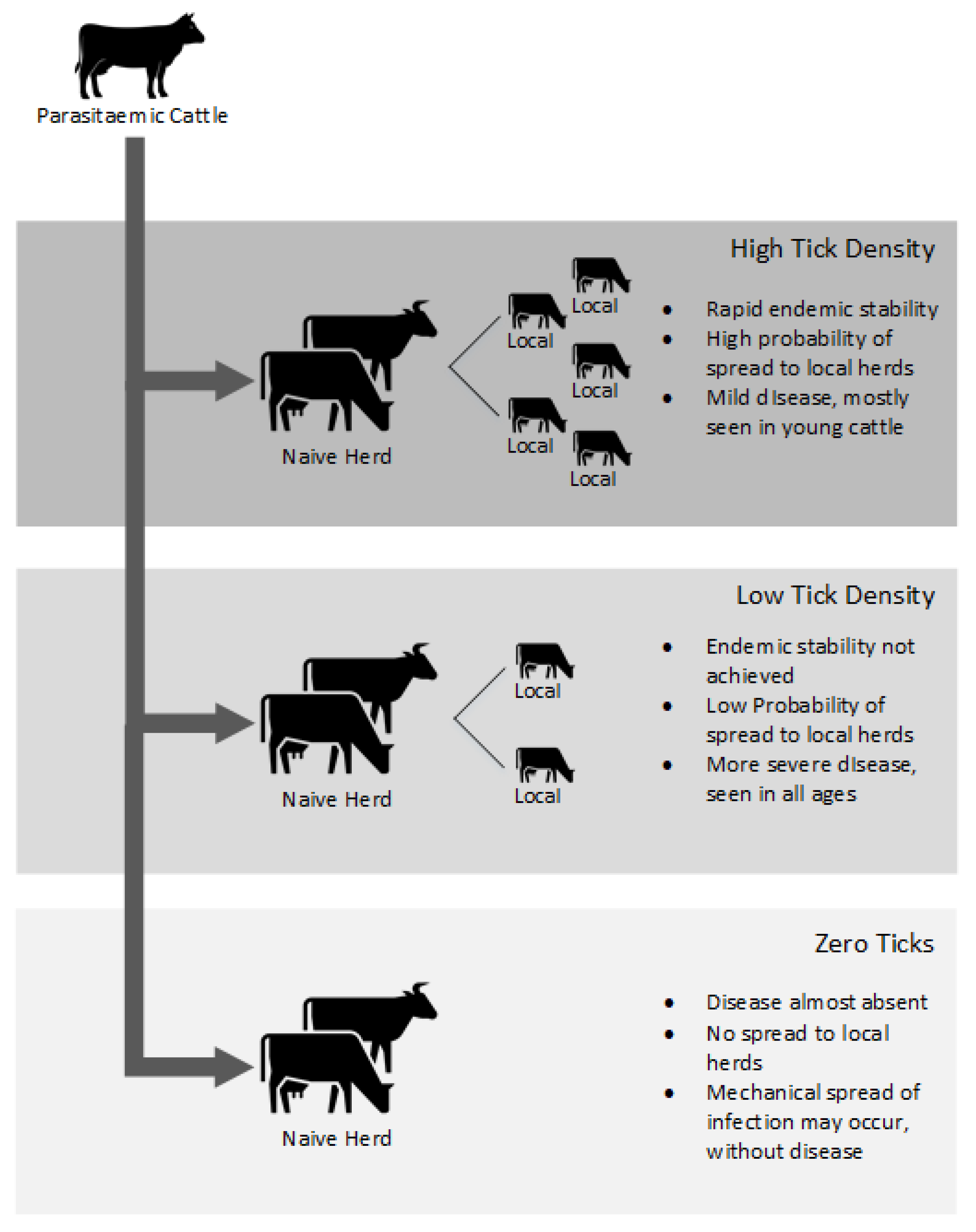
3. Tick Distribution
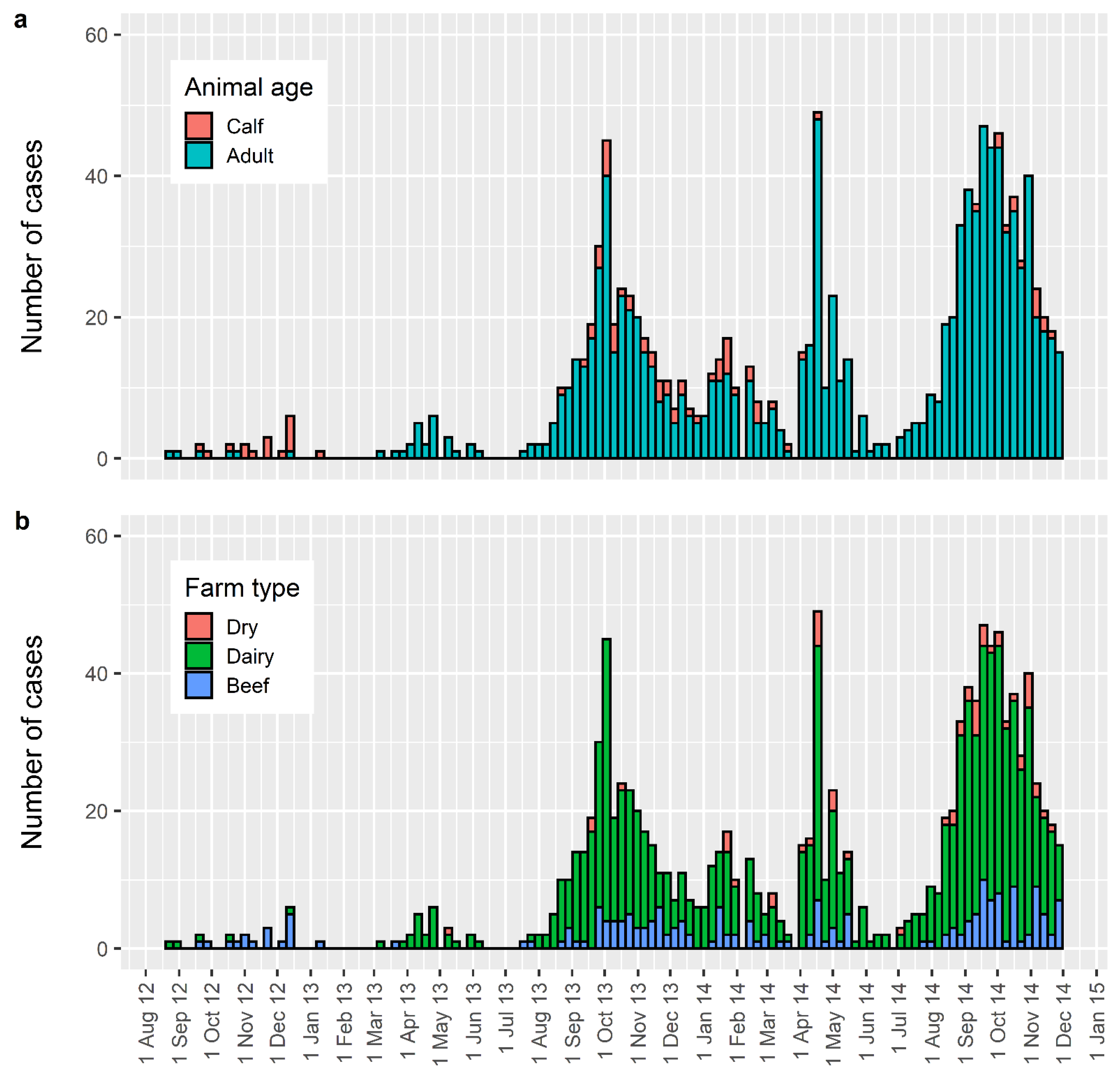
4. Spread of Disease
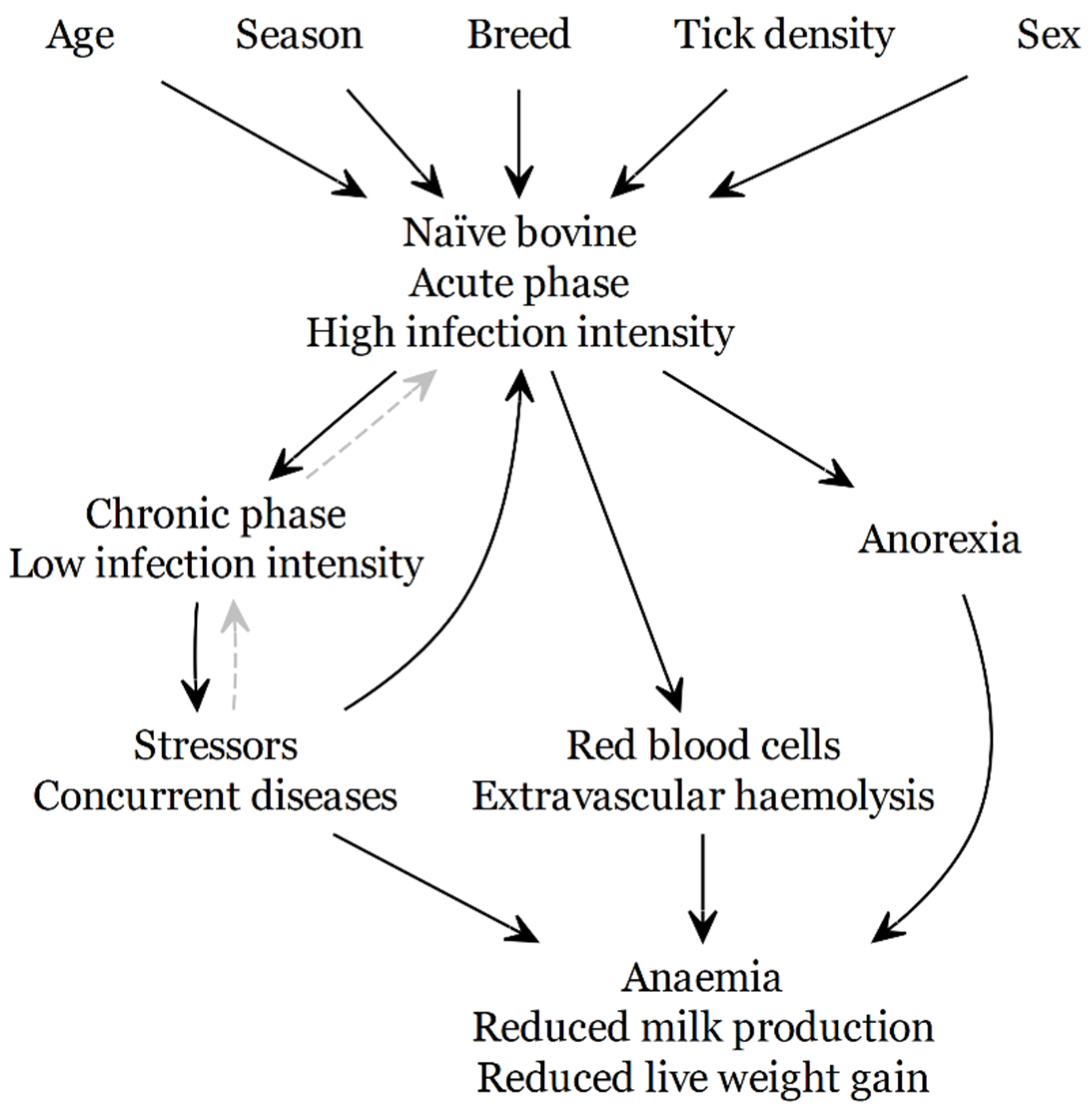
5. Impact of Disease
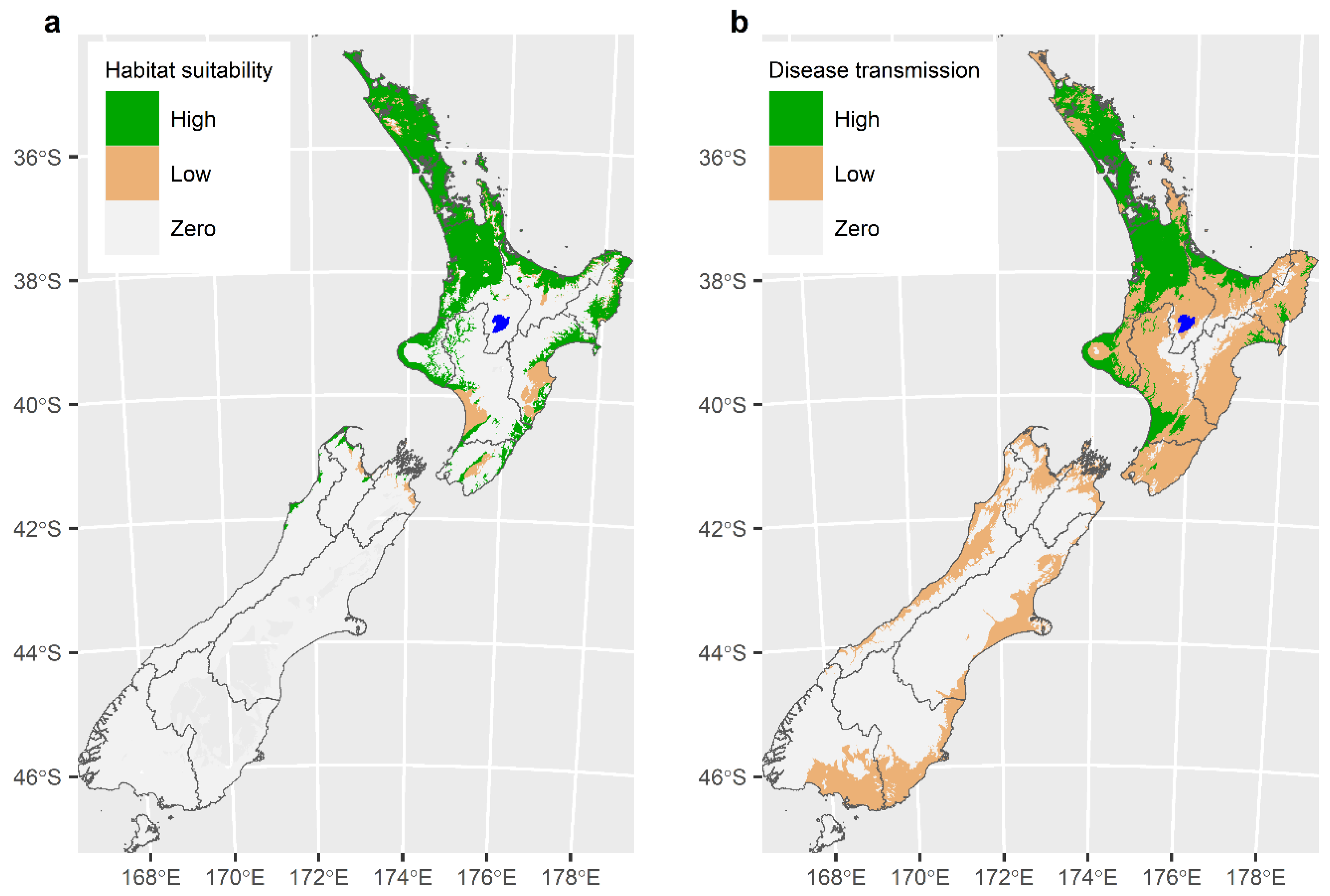
6. Modelling to Describe the Distribution of the Tick Haemaphysalis longicornis and the Likely Extent of the TABA Epidemic
7. Managing the New Zealand Epidemic
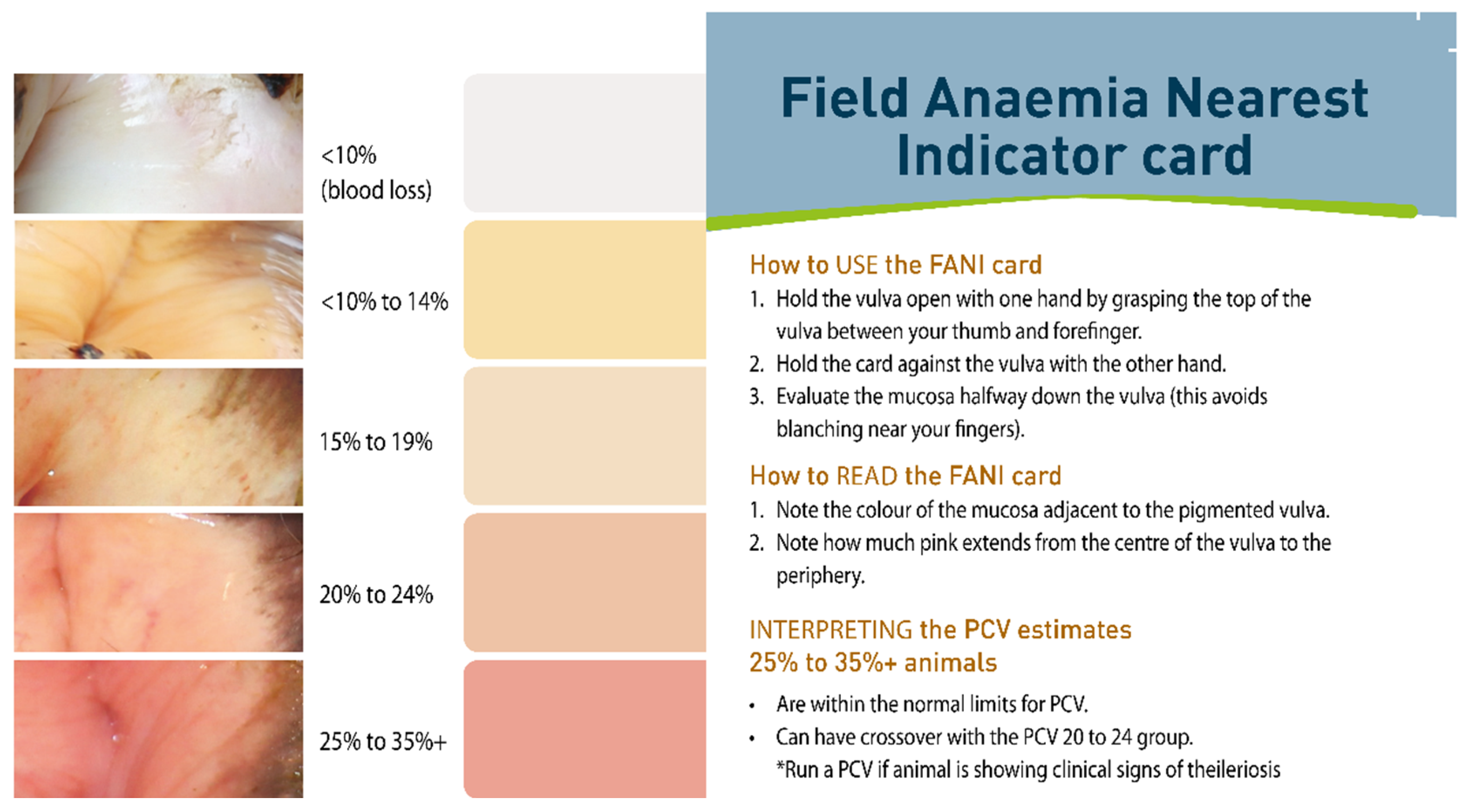
8. Control of Disease
9. Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulford, D.; McFadden, A.M.J.; Hamilton, J.; Donald, J. Investigation of the index case herd and identification of the genotypes of Theileria orientalis associated with outbreaks of bovine anaemia in New Zealand in 2012. N. Z. Vet. J. 2016, 64, 21–28. [Google Scholar] [CrossRef]
- Lawrence, K.; McFadden, A.M.J.; Gias, E.; Pulford, D.; Pomroy, W. Epidemiology of the epidemic of bovine anaemia associated with Theileria orientalis (Ikeda) between August 2012 and March 2014. N. Z. Vet. J. 2016, 64, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Pulford, D.J.; Gias, E.; Bueno, I.M.; McFadden, A.M.J. Developing high throughput quantitative PCR assays for diagnosing Ikeda and other Theileria orientalis types common to New Zealand in bovine blood samples. N. Z. Vet. J. 2016, 64, 29–37. [Google Scholar] [CrossRef]
- McFadden, A.M.J.; Vink, D.; Pulford, D.; Lawrence, K.E.; Gias, E.; Heath, A.C.G.; McFadden, C.; Bingham, P. Monitoring an epidemic of Theileria-associated bovine anaemia (Ikeda) in cattle herds in New Zealand. Prev. Vet. Med. 2016, 125, 31–37. [Google Scholar] [CrossRef]
- McFadden, A.M.J.; Gias, E.; Heuer, C.; Stevens McFadden, F.J.; Pulford, D.J. Prevalence and spatial distribution of cattle herds infected with Theileria orientalis in New Zealand between 2012 and 2013. N. Z. Vet. J. 2016, 64, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Martin, B. Dairy Cattle Vets Newsletter; New Zealand Veterinary Association: Wellington, New Zealand, 2018; pp. 29–33. [Google Scholar]
- Lawrence, K.E.; McFadden, A.M.J.; Bingham, P.; Pulford, D.; Vink, W.; Pomroy, W. Prevalence studies for Theileria orientalis conducted during the early stages of the 2012 New Zealand epidemic of Theileria associated bovine anaemia. Vet. Parasitol. Reg. 2018, 13, 38–44. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Saunders, B.; Guy, L.A.; Brookbanks, E.; Charleston, W.; Uilenberg, G. Theileria orientalis, a blood parasite of cattle. First report in New Zealand. N. Z. Vet. J. 1984, 32, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.C.G. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J. 2016, 64, 10–20. [Google Scholar] [CrossRef]
- Myers, J.G. The Cattle-tick (Haemaphysalis bispinosa): Investigations during 1923–24. Bull. Dep. Agric. N. Z. 1924, 116, 1–105. [Google Scholar]
- Lawrence, K.E.; Summers, S.; Heath, A.C.G.; McFadden, A.M.J.; Pulford, D.; Tait, A.; Pomroy, W. Using a rule-based envelope model to predict the expansion of habitat suitability within New Zealand for the tick Haemaphysalis longicornis, with future projections based on two climate change scenarios. Vet. Parasitol. 2017, 243, 226–234. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Sanson, R.; McFadden, A.M.J.; Pulford, D.; Pomroy, W. The effect of month, farm type and latitude on the level of anaemia associated with Theileria orientalis Ikeda type infection in New Zealand cattle naturally infected at pasture. Res. Vet. Sci. 2018, 117, 233–238. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Summers, S.; Heath, A.C.G.; McFadden, A.M.J.; Pulford, D.; Pomroy, W. Predicting the potential environmental suitability for Theileria orientalis transmission in New Zealand cattle using maximum entropy niche modelling. Vet. Parasitol. 2016, 224, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Gedye, K.; McFadden, A.M.J.; Pulford, D.; Pomroy, W. An observational study of the vertical transmission of Theileria orientalis (Ikeda) in a New Zealand pastoral dairy herd. Vet. Parasitol. 2016, 218, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Rawdon, T.; Tham, K.; Johnstone, A.; Seddon, D.; Ellmers, F. The investigation of an unusual presentation of Theileria orientalis in a mature steer. In Proceedings of the 14th FAVA Congress and the Food Safety & Biosecurity, and Epidemiology & Animal Health Management Branches of the New Zealand Veterinary Association, Napier, New Zealand, 26–28 June 2006; pp. 107–115. [Google Scholar]
- McFadden, A.M.J.; Rawdon, T.; Meyer, J.; Makin, J.; Morley, C.; Clough, R.; Tham, K.; Müllner, P.; Geysen, D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N. Z. Vet. J. 2011, 59, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gias, E.; Pulford, D.J.; Lawrence, K.E.; McFadden, A.M.J. Application of quantitative PCR assays for diagnosing Ikeda and other Theileria orientalis types to examine associations between severity of anaemia and parasitaemia in bovine anaemia outbreaks. N. Z. Vet. J. 2016, 64, 60–64. [Google Scholar] [CrossRef]
- Watts, J.; Playford, M.; Hickey, K. Theileria orientalis: A review. N. Z. Vet. J. 2016, 64, 3–9. [Google Scholar] [CrossRef]
- Vink, W.D.; Lawrence, K.E.; McFadden, A.M.J.; Bingham, P. An assessment of the herd-level impact of the Theileria orientalis (Ikeda) epidemic of cattle in New Zealand, 2012–2013: A mixed methods approach. N. Z. Vet. J. 2016, 64, 48–54. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Lawrence, B.; Hickson, R.E.; Hewitt, C.; Gedye, K.; Fermin, L.; Pomroy, W. Associations between Theileria orientalis Ikeda type infection and the growth rates and haematocrit of suckled beef calves in the North Island of New Zealand. N. Z. Vet. J. 2019, 67, 66–73. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Forsyth, S.F.; Vaatstra, B.L.; McFadden, A.M.J.; Pulford, D.J.; Govindaraju, K.; Pomroy, W.E. Cluster analysis of the clinical histories of cattle affected with bovine anaemia associated with Theileria orientalis Ikeda type infection. N. Z. Vet. J. 2017, 6, 305–312. [Google Scholar] [CrossRef]
- Thompson, J. Theileriasis in New Zealand. Surveillance 1991, 18, 21–23. [Google Scholar]
- Lawrence, K.; Forsyth, S.; Vaatstra, B.; McFadden, A.; Pulford, D.; Govindaraju, K.; Pomroy, W. Clinical haematology and biochemistry profiles of cattle naturally infected with Theileria orientalis Ikeda type in New Zealand. N. Z. Vet. J. 2018, 66, 21–29. [Google Scholar] [CrossRef]
- McFadden, A.M.J.; Hart, M.; Bueno, I.; Ha, H.; Heath, A.C.G.; Pulford, D. Monitoring Theileria orientalis (Ikeda)-associated bovine anaemia in affected cattle over time. Vet. Parasitol. 2017, 245, 29–33. [Google Scholar] [CrossRef]
- McDougall, S.; Pearson, I.; Perry, M.; Compton, C. Effect of Theileria orientalis ikeda on reproductive performance of a dairy herd. In Proceedings of the Society of Dairy Cattle Veterinarians of the NZVA Annual Conference, Hamilton, New Zealand, 16–20 June 2014; pp. 103–118. [Google Scholar]
- Lawrence, K.E.; Gedye, K.; Pomroy, W. A longitudinal study of the effect of Theileria orientalis Ikeda type infection on three New Zealand dairy farms naturally infected at pasture. Vet. Parasitol. 2019, 276, 108977. [Google Scholar] [CrossRef]
- Stewart, P. Knowledge on Theileria epidemic shared. VetScript 2014, 27, 24–34. [Google Scholar]
- Theileria Working Group. Theileria Veterinary Handbook 2; Ministry for Primary Industries: Wellington, New Zealand, 2015; pp. 1–32.
- Lawrence, K.E.; Gibson, M.; Hickson, R.E.; Gedye, K.; Hoogenboom, A.; Fermin, L.; Draganova, I.; Pomroy, W. Experimental infection of Friesian bulls with Theileria orientalis (Ikeda) and effects on the haematocrit, live weight, rectal temperature and activity. Vet. Parasitol. Reg. 2018, 14, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Lawrence, K.E.; Hickson, R.E.; How, R.; Gedye, K.; Jones, G.; Hoogenboom, A.; Draganova, I.; Smith, S.; Pomroy, W. Effects of Theileria orientalis Ikeda type infection on libido and semen quality of bulls. Anim. Reprod. Sci. 2020, 214, 106312. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.C.G. An Investigation into the Temperature and Humidity Preferenda of Ixodid Ticks: And Their Distribution in Relation to Bioclimatic Zones in Australia. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 1974. [Google Scholar]
- Neilson, F.J.A. An Investigation into the Ecology, Biology, Distribution and Control of Haemaphysalis longicornis Neumann, 1901. Master’s Thesis, Massey University, Palmerston North, New Zealand, 1980. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Vink, D.; O’Driscoll, A.; Briggs, S.; Moors, D.; Lawrence, K.E. Farm-level management of Theileria orientalis Ikeda outbreaks in dairy cattle. VetScript 2013, 26, 34–38. [Google Scholar]
- Theileria Working Group. Theileria Veterinary Handbook; Ministry for Primary Industries: Wellington, New Zealand, 2014.
- Briggs, S. Blood transfusion in cattle. In Proceedings of the Society of Dairy Cattle Veterinarians of the NZVA Annual Conference, Hamilton, New Zealand, 16–20 June 2014. [Google Scholar]
- Heath, A.C.G. The role of ticks, biting flies and lice in the transmission of theilerosis. VetScript 2013, 26, 13–14. [Google Scholar]
- Lawrence, K.E.; McFadden, A.M.J.; Pulford, D. The epidemic to date. VetScript 2013, 26, 12–13. [Google Scholar]
- McFadden, A.M.J.; Pomroy, W.; Marchant, R.; Heath, A.C.G.; King, C.; Lawrence, K.E.; MacPherson, N. Farm management strategies to mitigate effects of Theileria-associated bovine anaemia. VetScript 2014, 27, 20–23. [Google Scholar]
- Watts, J.; Pulford, D.; Heath, A.C.G. Theileria: The risks and the ticks. VetScript 2013, 26, 9–11. [Google Scholar]
- Riddle, G. Theileriosis on beef farms in the Bay of Islands area. In Proceedings of the Society of Sheep and Beef Cattle Veterinarians of the NZVA, Hamilton, New Zealand, 21–24 June 2016. [Google Scholar]
- Lawrence, K.E.; Hickson, R.E.; Wang, B.; Gedye, K.; Fraser, K.; Pomroy, W.E. The efficacy of toltrazuril treatment for reducing the infection intensity of Theileria orientalis Ikeda type in dairy calves. Vet. Parasitol. 2020, 282, 109124. [Google Scholar] [CrossRef] [PubMed]
- Rankin, D. Theileria from the trenches. VetScript 2015, 28, 20–21. [Google Scholar]
- McDougall, S.; Hillerton, J.; Pegram, D. Concentrations of buparvaquone in milk and tissue of dairy cows. N. Z. Vet. J. 2016, 64, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Gedye, K.; Hickson, R.E.; Wang, B.; Carvalho, L.; Zhao, Y.; Pomroy, W.E. The role of sheep (Ovis aries) in maintaining Theileria orientalis Ikeda type infection. Vet. Parasitol. 2021, 291, 109391. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.; Burgh, S.; Dinh, T.H.H.H.; Rolls, P.; Carter, P. Merozoites of Theileria orientalis buffeli reduce the parasitaemia of T. orientalis ikeda following tick challenge. Vet. Parasitol. 2021, 298, 109532. [Google Scholar] [CrossRef]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 2018, 55, 757–759. [Google Scholar] [CrossRef]
- Oakes, V.J.; Yabsley, M.J.; Schwartz, D.; LeRoith, T.; Bissett, C.; Broaddus, C.; Schlater, J.L.; Todd, S.M.; Boes, K.M.; Brookhart, M.; et al. Theileria orientalis Ikeda Genotype in Cattle, Virginia, USA. Emerg. Infect. Dis. 2019, 25, 1653–1659. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, K.; Gedye, K.; McFadden, A.; Pulford, D.; Heath, A.; Pomroy, W. Review of the New Zealand Theileria orientalis Ikeda Type Epidemic and Epidemiological Research since 2012. Pathogens 2021, 10, 1346. https://doi.org/10.3390/pathogens10101346
Lawrence K, Gedye K, McFadden A, Pulford D, Heath A, Pomroy W. Review of the New Zealand Theileria orientalis Ikeda Type Epidemic and Epidemiological Research since 2012. Pathogens. 2021; 10(10):1346. https://doi.org/10.3390/pathogens10101346
Chicago/Turabian StyleLawrence, Kevin, Kristene Gedye, Andrew McFadden, David Pulford, Allen Heath, and William Pomroy. 2021. "Review of the New Zealand Theileria orientalis Ikeda Type Epidemic and Epidemiological Research since 2012" Pathogens 10, no. 10: 1346. https://doi.org/10.3390/pathogens10101346





