Surveillance of Fresh Artisanal Cheeses Revealed High Levels of Listeria monocytogenes Contamination in the Department of Quindío, Colombia
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sampling Site and Sampling
4.2. Isolation and Identification of Listeria monocytogenes
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Listeriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 2 August 2021).
- Centers for Disease Control and Prevention (CDC). Listeria (Listeriosis). Available online: https://www.cdc.gov/listeria/index.html (accessed on 2 August 2021).
- Arslan, F.; Meynet, E.; Sunbul, M.; Sipahi, O.R.; Kurtaran, B.; Kaya, S.; Inkaya, A.C.; Pagliano, P.; Sengoz, G.; Batirel, A.; et al. The clinical features, diagnosis, treatment, and prognosis of neuroinvasive listeriosis: A multinational study. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1213–1221. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive response of Listeria monocytogenes to the stress factors in the food processing environment. Front. Microbiol. 2021, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Paduro, C.; Montero, D.A.; Chamorro, N.; Carreño, L.J.; Vidal, M.; Vidal, R. Ten years of molecular epidemiology surveillance of Listeria monocytogenes in Chile 2008–2017. Food Microbiol. 2020, 85, 103280. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Outbreak Investigation of Listeria monocytogenes—Hispanic-Style Fresh and Soft Cheeses. February 2021. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-hispanic-style-fresh-and-soft-cheeses-february-2021 (accessed on 3 October 2021).
- Desai, A.N.; Anyoha, A.; Madoff, L.C.; Lassmann, B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019, 84, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón, A.; Mantilla, J.; Acosta, N.; Cortés, M.; Melo, N.; Correa, D.; Gamboa, Y.; Montoya, M.; Sarmiento, N.; Durango, A. Identificación de Riesgos Biológicos Asociados al Consumo de Leche Cruda Bovina en Colombia; Instituto Nacional de Salud (INS), Ministerio de Salud y Protección Social: Bogotá, Colombia, 2011; ISBN 978-958-13-0151-5.
- Instituto Nacional de Salud (INS). Boletín Epidemiológico Semanal: Reporte Técnico de Listeria monocytogenes Para Queso en Colombia; Ministerio de Salud y Protección Social: Bogotá, Colombia, 2019. [CrossRef]
- Crespo, M.D.P.; Vélez, J.D.; Castañeda, C.; Hoyos, F.; López, M.L.; Salazar, J.C. Aislamiento de Listeria monocytogenes en un hospital de tercer nivel. Colomb. Med. 1999, 30, 89–98. [Google Scholar]
- Cartwright, E.J.; Jackson, K.A.; Johnson, S.D.; Graves, L.M.; Silk, B.J.; Mahon, B.E. Listeriosis outbreaks and associated food vehicles, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Infectious Disease/CDC Update. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. Ann. Emerg. Med. 2013, 62, 536–537. [Google Scholar] [CrossRef]
- Koopmans, M.M.; Bijlsma, M.W.; Brouwer, M.C.; van de Beek, D.; van der Ende, A. Listeria monocytogenes meningitis in The Netherlands, 1985–2014: A nationwide surveillance study. J. Infect. 2017, 75, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Lamont, R.F.; Sobel, J.; Mazaki-Tovi, S.; Kusanovic, J.P.; Vaisbuch, E.; Kim, S.K.; Uldbjerg, N.; Romero, R. Listeriosis in human pregnancy: A systematic review. J. Périnat. Med. 2011, 39, 227–236. [Google Scholar] [CrossRef]
- Bille, J.; Blanc, D.; Schmid, H.; Boubaker, K.; Baumgartner, A.; Siegrist, H.H.; Tritten, M.L.; Lienhard, R.; Berner, D.; Anderau, R.; et al. Outbreak of human listeriosis associated with tomme cheese in northwest Switzerland, 2005. Eurosurveillance 2006, 11, 11–12. [Google Scholar] [CrossRef]
- Büla, C.; Bille, J.; Glauser, M.P. An epidemic of food-borne listeriosis in western Switzerland: Description of 57 cases involving adults. Clin. Infect. Dis. 1995, 20, 66–72. [Google Scholar] [CrossRef]
- Dalton, C.B.; Austin, C.C.; Sobel, J.; Hayes, P.S.; Bibb, W.F.; Graves, L.M.; Swaminathan, B.; Proctor, M.E.; Griffin, P.M. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 1997, 336, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M. Human listeriosis and animal models. Microbes Infect. 2007, 9, 1216–1225. [Google Scholar] [CrossRef]
- Pinner, R.W.; Schuchat, A.; Swaminathan, B.; Hayes, P.S.; Deaver, K.A.; Weaver, R.E.; Plikaytis, B.D.; Reeves, M.; Broome, C.V.; Wenger, J.D.; et al. Role of foods in sporadic listeriosis. JAMA 1992, 267, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Schuchat, A.; Deaver, K.A.; Wenger, J.D.; Plikaytis, B.D.; Mascola, L.; Pinner, R.W.; Reingold, A.L.; Broome, C.V. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. The Listeria study group. JAMA 1992, 267, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 19484–19491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, B.N.; Chakravarty, D.; Gardner, J.; Ricke, S.C.; Donaldson, J.R. Listeria monocytogenes response to anaerobic environments. Pathogens 2020, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Heisick, J.E.; Wagner, D.E.; Nierman, M.L.; Peeler, J.T. Listeria spp. found on fresh market produce. Appl. Environ. Microbiol. 1989, 55, 1925–1927. [Google Scholar] [CrossRef] [Green Version]
- Lorber, B. Listeriosis. Clin. Infect. Dis. 1997, 24, 1–9. [Google Scholar] [CrossRef]
- Vivant, A.-L.; Garmyn, D.; Piveteau, P. Listeria monocytogenes, a down-to-earth pathogen. Front. Cell. Infect. Microbiol. 2013, 3, 87. [Google Scholar] [CrossRef] [Green Version]
- Ricchi, M.; Scaltriti, E.; Cammi, G.; Garbarino, C.; Arrigoni, N.; Morganti, M.; Pongolini, S. Short communication: Persistent contamination by Listeria monocytogenes of bovine raw milk investigated by whole-genome sequencing. J. Dairy Sci. 2019, 102, 6032–6036. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.; Andrew, P.; Faleiro, M. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Rossi, M.L.; Paiva, A.; Tornese, M.; Chianelli, S.; Troncoso, A. Listeria monocytogenes outbreaks: A review of the routes that favor bacterial presence. Rev. Chil. Infectol. 2008, 25, 328–335. [Google Scholar]
- Uhlich, G.A.; Luchansky, J.B.; Tamplin, M.L.; Molina-Corral, F.J.; Anandan, S.; Porto-Fett, A.C. Effect of storage temperature on the growth of Listeria monocytogenes on Queso Blanco slices. J. Food Saf. 2006, 26, 202–214. [Google Scholar] [CrossRef]
- Lortal, S.; Licitra, G.; Valence, F. Wooden tools: Reservoirs of microbial biodiversity in traditional cheesemaking. Microbiol. Spectr. 2014, 2, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda, C.G.; Libre, U.; Peñaranda, R.Q.; Caicedo, I.B.; Trujillo, R.S. Modelo de quesería artesanal bajo un signo distintivo en el Caribe colombiano: Caso Atlántico. Rev. Lasallista Investig. 2017, 14, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Navas, J.S. Queso molido nariñense. Tecnol. Láctea Latinoam. 2010, 59, 56–59. [Google Scholar]
- Filiousis, G.; Johansson, A.; Frey, J.; Perreten, V. Prevalence, genetic diversity and antimicrobial susceptibility of Listeria monocytogenes isolated from open-air food markets in Greece. Food Control 2009, 20, 314–317. [Google Scholar] [CrossRef]
- Wu, S.; Wu, Q.; Zhang, J.; Chen, M.; Yan, Z.; Hu, H. Listeria monocytogenes prevalence and characteristics in retail raw foods in China. PLoS ONE 2015, 10, e0136682. [Google Scholar] [CrossRef]
- D’Amico, D.; Donnelly, C. Microbiological quality of raw milk used for small-scale artisan cheese production in Vermont: Effect of farm characteristics and practices. J. Dairy Sci. 2010, 93, 134–147. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, D.J.; Donnelly, C.W. Detection, isolation, and incidence of Listeria spp. in small-scale artisan cheese processing facilities: A methods comparison. J. Food Prot. 2009, 72, 2499–2507. [Google Scholar] [CrossRef]
- Carrascal-Camacho, A.; Hernandez, T.; Suárez, M.C.; Olivares Tenorio, M.; Sepulveda, M.; Muñoz, M.; Lizarazo, D. Evaluación de Riesgo de Listeria monocytogenes en Quseo Fresco en Colombia; Instituto Nacional de Salud (INS), Ministerio de Salud y Protección Social: Bogotá, Colombia, 2011; ISBN 978-958-13-0153-9.
- Ocampo-Ibáñez, I.D.; González, C.; Moreno, S.L.; Calderón, C.; Flórez-Elvira, L.J.; Olaya, M.B.; Sánchez, S.P.R.; Lesmes, M.C. Presence of Listeria monocytogenes in fresh artisanal cheese marketed in Cali-Colombia. Acta Agron. 2019, 68, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Acuña, D.M.B.; González, A.M.B.; Msc, S.E.C. Determinación de Listeria monocytogenes en quesos blancos artesanales expendidos en la plaza de mercado de Cáqueza, Cundinamarca. Nova 2006, 4, 80. [Google Scholar] [CrossRef] [Green Version]
- Albarracin, F.Y.; Sarmiento, P.; Carrascal, A.; Mercado, M. Estimación de la proporcion de Listeria monocytogenes y Salmonella spp en quesos frescos (queso de hoja, cuajada) y queso Doble Crema producidos y comercializados en el Municipio de Pamplona, Norte de Santander. BISTUA—Rev. Fac. Cienc. Basicas 2006, 4, 30–41. [Google Scholar]
- Gallegos, J.G.; Arrieta, G.; Máttar, S.; Poutou, R.; Trespalacios, A.; Carrascal, A. Frecuencia de Listeria spp., en quesos colombianos costeños. Rev. MVZ Córdoba 2007, 12, 996–1012. [Google Scholar] [CrossRef]
- Martinez-Rios, V.; Dalgaard, P. Prevalence of Listeria monocytogenes in European cheeses: A systematic review and meta-analysis. Food Control 2018, 84, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Churchill, K.J.; Sargeant, J.M.; Farber, J.M.; O’Connor, A.M. Prevalence of Listeria monocytogenes in select ready-to-eat foods—Deli meat, soft cheese, and packaged salad: A systematic review and meta-analysis. J. Food Prot. 2019, 82, 344–357. [Google Scholar] [CrossRef]
- Gérard, A.; El-Hajjaji, S.; Niyonzima, E.; Daube, G.; Sindic, M. Prevalence and survival of Listeria monocytogenes in various types of cheese—A review. Int. J. Dairy Technol. 2018, 71, 825–843. [Google Scholar] [CrossRef]
- Pintado, C.; Oliveira, A.; Pampulha, M.; Ferreira, M. Prevalence and characterization of Listeria monocytogenes isolated from soft cheese. Food Microbiol. 2005, 22, 79–85. [Google Scholar] [CrossRef]
- Brito, J.R.F.; Santos, E.M.P.; Arcuri, E.F.; Lange, C.C.; Brito, M.A.V.P.; Souza, G.N.; Cerqueira, M.M.P.O.; Beltran, J.M.S.; Call, J.E.; Liu, Y.; et al. Retail survey of Brazilian milk and minas frescal cheese and a contaminated dairy plant to establish prevalence, relatedness, and sources of Listeria monocytogenes isolates. Appl. Environ. Microbiol. 2008, 74, 4954–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas-Barbosa, B.T.; Morales, A.L.-J.; Alaniz, R.; Ramírez-Álvarez, A.; Soltero-Ramos, J.P.; de la Mora-Quiroz, R.; Martin, P.; Jacquet, C. Presencia y persistencia de Listeria en cuatro queserias artesanales de Jalisco, México. e-CUCBA 2014, 2, 3–37. [Google Scholar]
- Kinde, H.; Mikolon, A.; Rodriguez-Lainz, A.; Adams, C.; Walker, R.L.; Cernek-Hoskins, S.; Treviso, S.; Ginsberg, M.; Rast, R.; Harris, B.; et al. Recovery of Salmonella, Listeria monocytogenes, and Mycobacterium bovis from cheese entering the United States through a noncommercial land port of entry. J. Food Prot. 2007, 70, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Tirloni, E.; Bernardi, C.; Pomilio, F.; Torresi, M.; de Santis, E.P.L.; Scarano, C.; Stella, S. Occurrence of Listeria spp. and Listeria monocytogenes isolated from PDO taleggio production plants. Foods 2020, 9, 1636. [Google Scholar] [CrossRef]
- Montero, D.; Bodero, M.; Riveros, G.; Lapierre, L.; Gaggero, A.; Vidal, R.M.; Vidal, M. Molecular epidemiology and genetic diversity of Listeria monocytogenes isolates from a wide variety of ready-to-eat foods and their relationship to clinical strains from listeriosis outbreaks in Chile. Front. Microbiol. 2015, 6, 384. [Google Scholar] [CrossRef] [Green Version]
- Bernini, V.; Bottari, B.; Dalzini, E.; Sgarbi, E.; Lazzi, C.; Neviani, E.; Gatti, M. The presence, genetic diversity and behaviour of Listeria monocytogenes in blue-veined cheese rinds during the shelf life. Food Control 2013, 34, 323–330. [Google Scholar] [CrossRef]
- Arslan, S.; Özdemir, F. Prevalence and antimicrobial resistance of Listeria spp. in homemade white cheese. Food Control 2008, 19, 360–363. [Google Scholar] [CrossRef]
- Di Pinto, A.; Novello, L.; Montemurro, F.; Bonerba, E.; Tantillo, G.M. Occurrence of Listeria monocytogenes in ready-to-eat foods from supermarkets in Southern Italy. New Microbiol. 2010, 33, 249–252. [Google Scholar]
- Pesavento, G.; Ducci, B.; Nieri, D.; Comodo, N.; Nostro, A.L. Prevalence and antibiotic susceptibility of Listeria spp. isolated from raw meat and retail foods. Food Control 2010, 21, 708–713. [Google Scholar] [CrossRef]
- Parisi, A.; Latorre, L.; Fraccalvieri, R.; Miccolupo, A.; Normanno, G.; Caruso, M.; Santagada, G. Occurrence of Listeria spp. in dairy plants in Southern Italy and molecular subtyping of isolates using AFLP. Food Control 2013, 29, 91–97. [Google Scholar] [CrossRef]
- Rantsiou, K.; Alessandria, V.; Urso, R.; Dolci, P.; Cocolin, L. Detection, quantification and vitality of Listeria monocytogenes in food as determined by quantitative PCR. Int. J. Food Microbiol. 2008, 121, 99–105. [Google Scholar] [CrossRef]
- Cabedo, L.; Picart i Barrot, L.; Teixido i Canelles, A. Prevalence of Listeria monocytogenes and Salmonella in ready-to-eat food in Catalonia, Spain. J. Food Prot. 2008, 71, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Auer, B.; Trittremmel, C.; Hein, I.; Schoder, D. Survey on the Listeria contamination of ready-to-eat food products and household environments in Vienna, Austria. Zoonoses Public Health 2007, 54, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Dambrosio, A.; Quaglia, N.C.; Saracino, M.; Malcangi, M.; Montagna, C.; Quinto, M.; Lorusso, V.; Normanno, G. Microbiological quality of burrata cheese produced in Puglia region: Southern Italy. J. Food Prot. 2013, 76, 1981–1984. [Google Scholar] [CrossRef]
- Rosengren, A.; Fabricius, A.; Guss, B.; Sylvén, S.; Lindqvist, R. Occurrence of foodborne pathogens and characterization of Staphylococcus aureus in cheese produced on farm-dairies. Int. J. Food Microbiol. 2010, 144, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vitela, M.R.; Mendoza-Bernardo, M.; Castro-Rosas, J.; Gomez-Aldapa, C.A.; Garay-Martinez, L.E.; Navarro-Hidalgo, V.; Villarruel-López, A. Incidence of Salmonella, Listeria monocytogenes, Escherichia coli O157:H7, and staphylococcal enterotoxin in two types of mexican fresh cheeses. J. Food Prot. 2012, 75, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Rosshaug, P.S.; Detmer, A.; Ingmer, H.; Larsen, M.H. Modeling the growth of Listeria monocytogenes in soft blue-white cheese. Appl. Environ. Microbiol. 2012, 78, 8508–8514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabil, R.M.S.; Prahesti, K.I.; Yuliati, F.N. Behaviour of Listeria monocytogenes in pasteurization milk during refrigerator storage. Eur. J. Sustain. Dev. 2019, 8, 264. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- Lyytikäinen, O.; Autio, T.; Maijala, R.; Ruutu, P.; Honkanen-Buzalski, T.; Miettinen, M.; Hatakka, M.; Mikkola, J.; Anttila, V.; Johansson, T.; et al. An outbreak of Listeria monocytogenes serotype 3a infections from butter in Finland. J. Infect. Dis. 2000, 181, 1838–1841. [Google Scholar] [CrossRef] [Green Version]
- Delgado, A.B.M.; Chaves, J.A.; Rodríguez, E.C.; Realpe, M.E.; Muñoz, Á.B. Listeria monocytogenes en manipuladores de alimentos: Un nuevo enfoque para tener en cuenta en los peligros de la industria alimentaria. Biomédica 2012, 33, 283–291. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization (ISO). ISO 11290-1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method; International Organization for Standardization (ISO): Geneva, Switzerland, 2017. [Google Scholar]
- Food and Drug Administration (FDA). Control of Listeria monocytogenes in Ready-To-Eat Foods: Guidance for Industry; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2017. [Google Scholar]
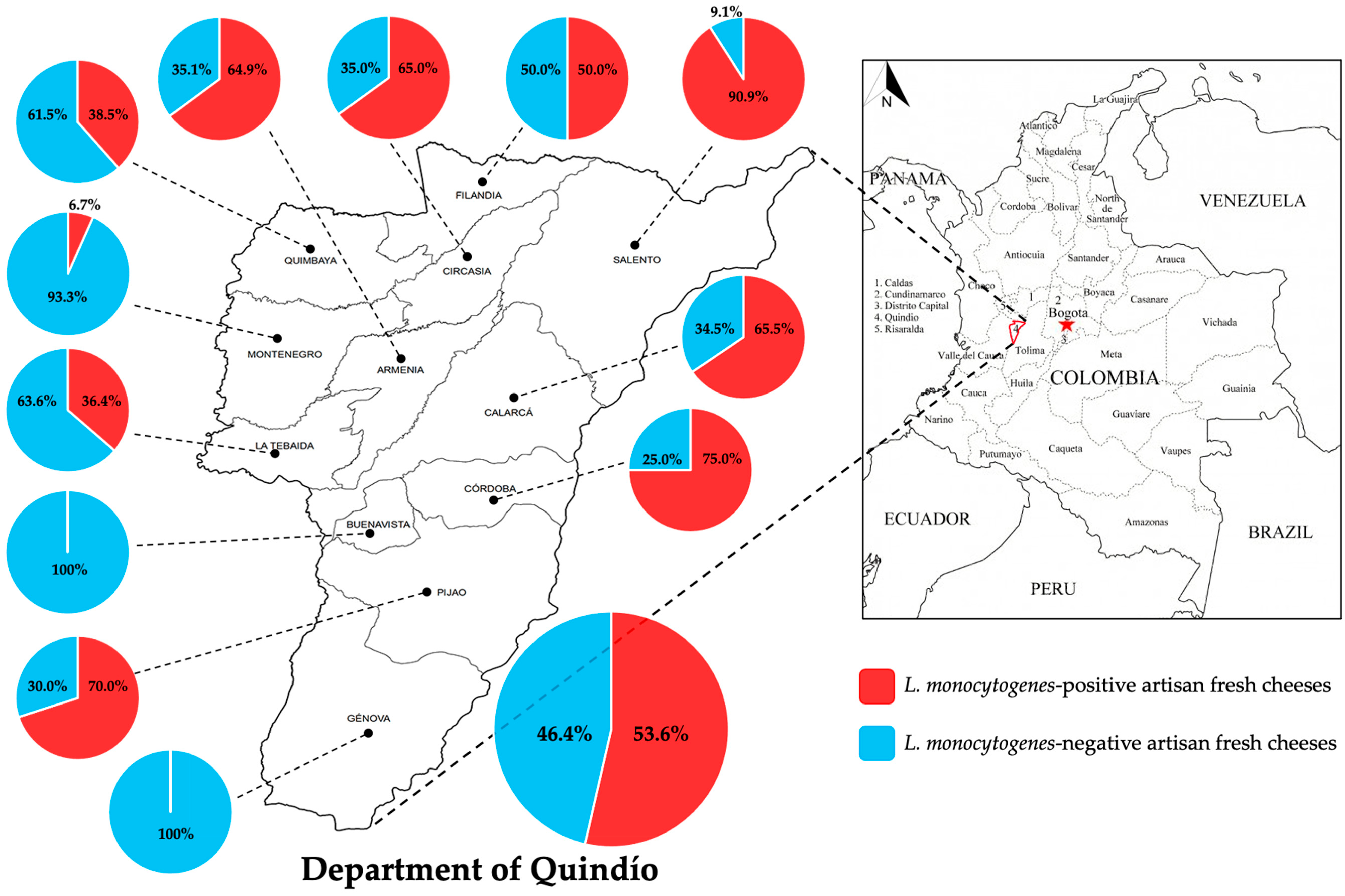
| Municipality 1 | Positive Samples | Negative Samples | Total Samples | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Armenia | 24 | 23.1 | 13 | 14.4 | 37 |
| Buenavista | 0 | 0.0 | 2 | 2.2 | 2 |
| Calarcá | 19 | 18.3 | 10 | 11.1 | 29 |
| Circasia | 13 | 12.5 | 7 | 7.8 | 20 |
| Córdoba | 6 | 5.8 | 2 | 2.2 | 8 |
| Filandia | 15 | 14.4 | 15 | 16.7 | 30 |
| Génova | 0 | 0.0 | 8 | 8.9 | 8 |
| La Tebaida | 4 | 3.8 | 7 | 7.8 | 11 |
| Montenegro | 1 | 1.0 | 14 | 15.6 | 15 |
| Pijao | 7 | 6.7 | 3 | 3.3 | 10 |
| Quimbaya | 5 | 4.8 | 8 | 8.9 | 13 |
| Salento | 10 | 9.6 | 1 | 1.1 | 11 |
| Total | 104 | 100 | 90 | 100 | 194 |
| Type of Cheese 1 | Positive Samples | Negative Samples | Total Samples | Prevalence | ||
|---|---|---|---|---|---|---|
| N | % | N | % | (%) | ||
| Queso Campesino | 41 | 39.4 | 36 | 40.0 | 77 | 53.2 |
| Queso Costeño | 8 | 7.7 | 6 | 6.7 | 14 | 57.1 |
| Queso Cuajada | 55 | 52.9 | 48 | 53.3 | 103 | 53.4 |
| Total | 104 | 100 | 90 | 100 | 194 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Bedoya, E.; Trujillo-Alzate, Y.A.; Ocampo-Ibáñez, I.D. Surveillance of Fresh Artisanal Cheeses Revealed High Levels of Listeria monocytogenes Contamination in the Department of Quindío, Colombia. Pathogens 2021, 10, 1341. https://doi.org/10.3390/pathogens10101341
Jaramillo-Bedoya E, Trujillo-Alzate YA, Ocampo-Ibáñez ID. Surveillance of Fresh Artisanal Cheeses Revealed High Levels of Listeria monocytogenes Contamination in the Department of Quindío, Colombia. Pathogens. 2021; 10(10):1341. https://doi.org/10.3390/pathogens10101341
Chicago/Turabian StyleJaramillo-Bedoya, Elizabeth, Yenny Alexandra Trujillo-Alzate, and Iván Darío Ocampo-Ibáñez. 2021. "Surveillance of Fresh Artisanal Cheeses Revealed High Levels of Listeria monocytogenes Contamination in the Department of Quindío, Colombia" Pathogens 10, no. 10: 1341. https://doi.org/10.3390/pathogens10101341






