Morphological Characteristics of Alveolar and Cystic Echinococcosis Lesions in Human Liver and Bone
Abstract
:1. Introduction
2. Macroscopic Findings
- Biopsy or cytoaspirate of an unclear lesion, such as a liver lesion of unknown dignity and entity, by means of a cutting needle biopsy or a liquid aspirate for cytological evaluation. In this instance they must be beware of some characteristic histological features of the larval stage of the parasite that will allow them to differentiate echinococcal lesion from lesions due to infectious disease, e.g., tuberculosis, fungal infections or further forms of infectious abscesses, as well as neoplastic primary or secondary metastatic lesions.
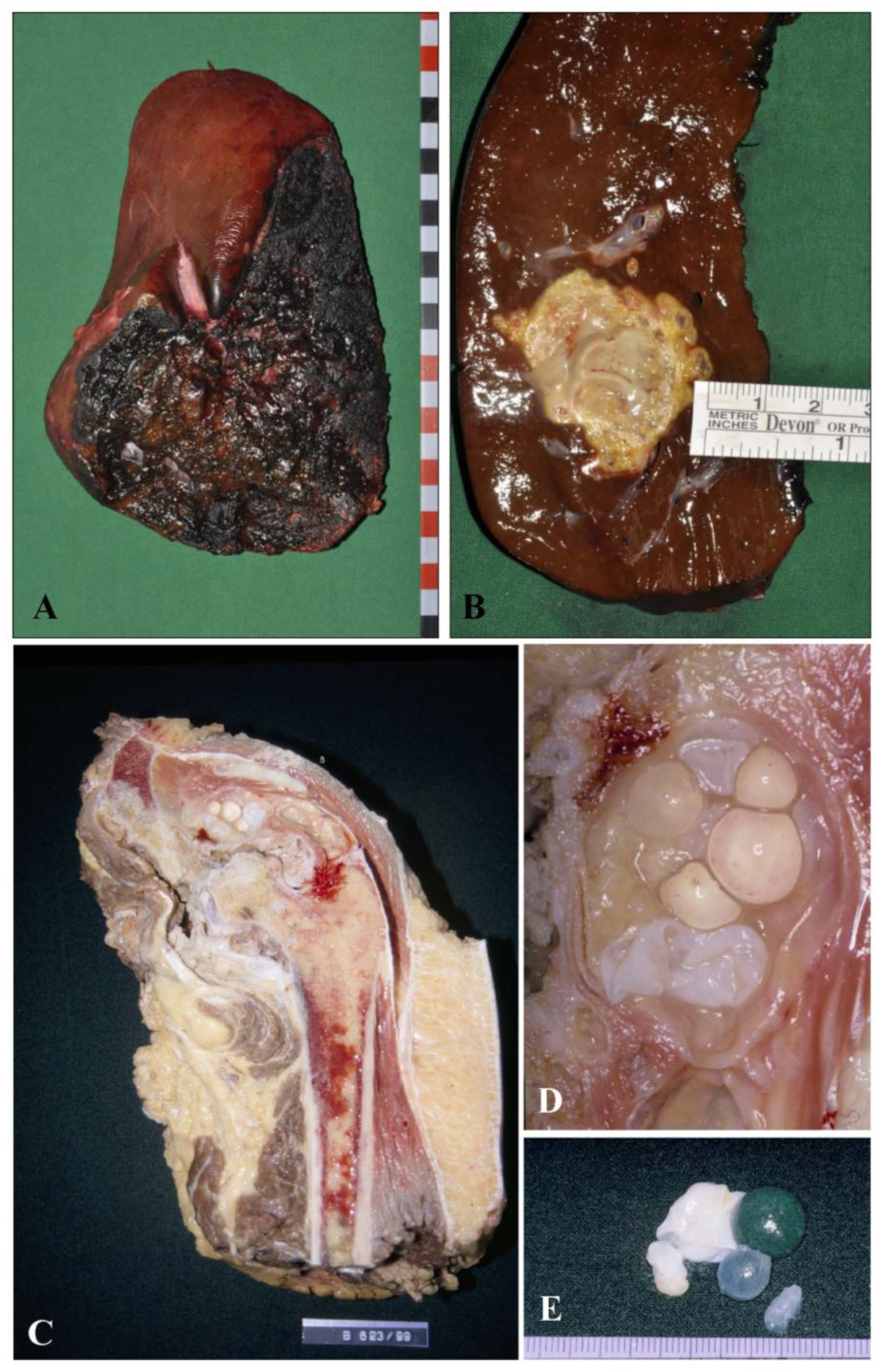
- Description of a resection specimen of e.g., a diagnostically defined liver manifestation of the larval stage of Echinococcus spp., already characterized during the diagnostic workup of the bioptic material prior to elective surgery. In this instance, the pathologist must define the specimen with the lesion by measuring the lesion in centimeters and weighing the specimen. After fixation in buffered formalin (4%) for at least 24 h, the pathologist has to prepare several tissue blocks of paraffin-embedded material from the sample for documenting and archiving the lesion and defining the resection borders; they should state the distance of the lesion from the resection line (Figure 1A,B). To document clearly the distance of the lesion from the resection, we suggest marking the resection line with ink during macroscopic description of the specimen. This will allow the pathologist to precisely measure the minimal distance of the lesion from the resection line. This distance should be given in mm in the final report.
3. Histological Findings
- In AE the liver lesion is characterized by central necrosis of varying diameter; the necrosis may have similarities to that seen in tuberculosis; however, the area of the necrosis is generally larger in AE and the typical multinuclear giant of the tuberculosis infection occurs only rarely or not at all. Calcifications may be present within the necrosis; these calcifications are generally not solid but have the characteristics of an impregnation with a dot-like or granular pattern. Ossifications are not detected. Next follows an inner circle close to the necrotic zone characterized by epithelioid cells, macrophages, and granulocytes; the granulocytes are predominantly of the neutrophilic type; some eosinophilic granulocytes may be present as well as some giant cells. This is followed by an outer zone with numerous lymphocytes followed by hepatic tissue. Between the outer and inner a fibrotic layer of varying diameter is present.
- In CE there is no central necrosis; the lesion is characterized by a broad fibrotic capsule with a limited lymphocytic infiltrate around the fibrotic capsule.
4. Immunohistological Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckert, J.; Deplazes, P.; Kern, P. Alveolar echinococcosis (Echinococcus multilocularis) and other forms of echinococcosis (Echinococcus vogeli and Echinococcus oligarthrus). In Zoonoses; Brown, D., Palmer, S., Torgerson, P.R., Soulsby, E., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 671–701. [Google Scholar]
- Casulli, A. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob. Health 2020, 8, e470–e471. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, A.; Rausch, R.L. New aspects of neotropical polycystic (Echinococcus vogeli) and unicystic (Echinococcus oligarthrus) echinococcosis. Clin. Microbiol. Rev. 2008, 21, 380–401. [Google Scholar] [CrossRef] [Green Version]
- Vuitton, D.A.; McManus, D.P.; Rogan, M.T.; Romig, T.; Gottstein, B.; Naidich, A.; Tuxun, T.; Wen, H.; Menezes da Silva, A. World Association of Echinococcosis. International consensus on terminology to be used in the field of echinococcoses. Parasite 2020, 27, 41. [Google Scholar] [CrossRef] [PubMed]
- Barth, T.F.E.; Herrmann, T.S.; Tappe, D.; Stark, L.; Grüner, B.; Buttenschoen, K.; Hillenbrand, A.; Juchems, M.; Henne-Bruns, D.; Kern, P.; et al. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl. Trop. Dis. 2012, 6, e1877. [Google Scholar] [CrossRef] [PubMed]
- Buttenschoen, K.; Kern, P.; Reuter, S.; Barth, T.F. Hepatic infestation of Echinococcus multilocularis with extension to regional lymph nodes. Langenbeck’s Arch. Surg. 2009, 394, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Casulli, A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl. Trop. Dis. 2021, 15, e0009373. [Google Scholar] [CrossRef] [PubMed]
- Junghanss, T.; da Silva, A.M.; Horton, J.; Chiodini, P.L.; Brunetti, E. Clinical management of cystic echinococcosis: State of the art, problems, and perspectives. Am. J. Trop. Med. Hyg. 2008, 79, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Casaravilla, C.; Irigoín, F.; Lin, G.; Previato, J.O.; Ferreira, F. Understanding the laminated layer of larval Echinococcus I: Structure. Trends Parasitol. 2011, 27, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.A. Biology and systematics of Echinococcus. In Echinococcus and Hydatid Disease; Thompson, R.C.A., Ed.; CAB International: Wallingford, UK, 1995; pp. 1–50. [Google Scholar]
- Reinehr, M.; Micheloud, C.; Grimm, F.; Kronenberg, P.A.; Grimm, J.; Beck, A.; Nell, J.; Meyer Zu Schwabedissen, C.; Furrer, E.; Müllhaupt, B.; et al. Pathology of Echinococcosis: A Morphologic and Immunohistochemical Study on 138 Specimens with Focus on the Differential Diagnosis Between Cystic and Alveolar Echinococcosis. Am. J. Surg. Pathol. 2020, 44, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Gottstein, B. A monoclonal antibody against Echinococcus multilocularis Em2 antigen. Parasitology 1991, 103 Pt 1, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hülsmeier, A.J.; Gehrig, P.M.; Geyer, R.; Sack, R.; Gottstein, B.; Deplazes, P.; Köhler, P. A major Echinococcus multilocularis antigen is a mucin-type glycoprotein. J. Biol. Chem. 2002, 277, 5742–5748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D.A. Immunology of Alveolar and Cystic Echinococcosis (AE and CE). Adv. Parasitol. 2017, 96, 1–54. [Google Scholar] [PubMed]
- Dezsényi, B.; Strausz, T.; Makrai, Z.; Csomor, J.; Danka, J.; Kern, P.; Rezza, G.; Barth, T.F.; Casulli, A. Autochthonous human alveolar echinococcosis in a Hungarian patient. Infection 2017, 45, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Ricken, F.J.; Nell, J.; Grüner, B.; Schmidberger, J.; Kaltenbach, T.; Kratzer, W.; Hillenbrand, A.; Henne-Bruns, D.; Deplazes, P.; Moller, P.; et al. Albendazole increases the inflammatory response and the amount of Em2-positive small particles of Echinococcus multilocularis (spems) in human hepatic alveolar echinococcosis lesions. PLoS Negl. Trop. Dis. 2017, 11, e0005636. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.; Nell, J.; Hillenbrand, A.; Henne-Bruns, D.; Schmidberger, J.; Kratzer, W.; Gruener, B.; Graeter, T.; Reinehr, M.; Weber, A.; et al. Immunohistological detection of small particles of Echinococcus multilocularis and Echinococcus granulosus in lymph nodes is associated with enlarged lymph nodes in alveolar and cystic echinococcosis. PLoS Negl. Trop. Dis. 2020, 14, e0008921. [Google Scholar] [CrossRef] [PubMed]
- Hillenbrand, A.; Beck, A.; Kratzer, W.; Graeter, T.; Barth, T.F.E.; Schmidberger, J.; Möller, P.; Henne-Bruns, D.; Gruener, B. Impact of affected lymph nodes on long-term outcome after surgical therapy of alveolar echinococcosis. Langenbeck’s Arch. Surg. 2018, 403, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Stijnis, K.; Dijkmans, A.C.; Bart, A.; Brosens, L.A.; Muntau, B.; Schoen, C.; Barth, T.F.; van Gulik, T.; van Gool, T.; Grobusch, M.P.; et al. Echinococcus vogeli in immigrant from Suriname to the Netherlands. Emerg. Infect. Dis. 2015, 21, 528–530. [Google Scholar] [CrossRef] [PubMed]


| Criteria | AE | CE |
|---|---|---|
| Macroscopy | Multiple Small Vesicles | Generally Single but Larger Cyst (or Multiple Cysts) |
| Microscopic features | ||
| Laminated layer (PAS staining) | Slender (below 1 mm) with weak striation | Thick (up to 3 mm) with strong to moderate striation |
| Central necrosis | Abundant | Little or none |
| Growth pattern | Tubular | Pseudocystic |
| Delineation from adjacent tissue | Poorly delineated | Pseudocystic lesion with thick fibrous capsule |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barth, T.F.E.; Casulli, A. Morphological Characteristics of Alveolar and Cystic Echinococcosis Lesions in Human Liver and Bone. Pathogens 2021, 10, 1326. https://doi.org/10.3390/pathogens10101326
Barth TFE, Casulli A. Morphological Characteristics of Alveolar and Cystic Echinococcosis Lesions in Human Liver and Bone. Pathogens. 2021; 10(10):1326. https://doi.org/10.3390/pathogens10101326
Chicago/Turabian StyleBarth, Thomas F. E., and Adriano Casulli. 2021. "Morphological Characteristics of Alveolar and Cystic Echinococcosis Lesions in Human Liver and Bone" Pathogens 10, no. 10: 1326. https://doi.org/10.3390/pathogens10101326
APA StyleBarth, T. F. E., & Casulli, A. (2021). Morphological Characteristics of Alveolar and Cystic Echinococcosis Lesions in Human Liver and Bone. Pathogens, 10(10), 1326. https://doi.org/10.3390/pathogens10101326







