Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration
Abstract
:1. Introduction
2. Approaches of Plant Disease Control
3. Types and Mechanisms of Biological Control
3.1. Suppressing Pathogens
3.2. Compounds Priming, Inducing, or Strengthening Plant Defense Responses
3.3. Regulating the Ecosystem to Protect and Promote Natural Enemies or Competitors of Pathogens
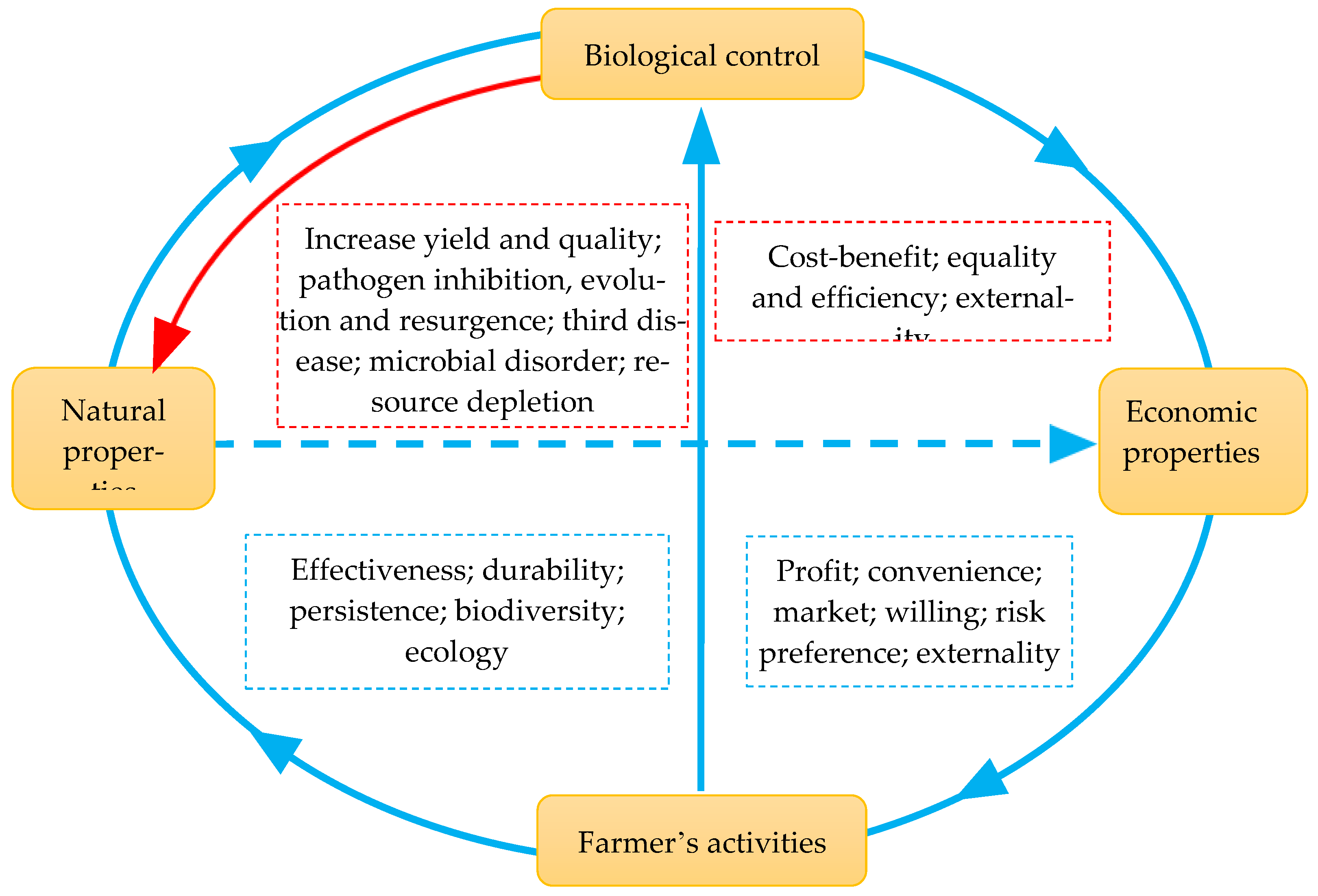
4. The Natural and Economic Considerations of Plant Disease Management with Biological Control Agents
4.1. Effectiveness
4.2. Durability
4.3. Ecological Sustainability
4.4. Economic and Practical Incentives
5. Enhance Efficiency of Biological Control through Eco-Evolutionary Principles
5.1. The Development of “Green” and Cost-Effective Biological Control Agents
5.2. Use Together with Other Disease-Control Approaches
5.3. Formulate a Dynamic Disease Management Program
6. Social Involvement
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Austin Bourke, P.M. Emergence of potato blight, 1843–1846. Nature 1964, 203, 805–808. [Google Scholar] [CrossRef]
- Padmanabhan, S.Y. The great Bengal famine. Annu. Rev. Phytopathol. 1973, 11, 11–24. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M. Ophiostoma novo-ulmi sp. nov., causative agent of current dutch elm disease pandemics. Mycopathologia 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies-A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- McGuire, S.; FAO, IFAD, and WFP. The state of food insecurity in the world 2015: Meeting the 2015 international hunger targets: Taking stock of uneven progress. Rome: FAO, 2015. Adv. Nutr. 2015, 6, 623–624. [Google Scholar] [CrossRef] [Green Version]
- Bisht, N.; Mishra, S.K.; Chauhan, P.S. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. J. Biol. Macromol. 2020, 143, 937–951. [Google Scholar] [CrossRef]
- Denes, T.E.; Molnar, I.; Rakosy-Tican, E. New insights into the interaction between cultivated potato and Phytophthora infestans. Studia Univ. Babes-Bolyai Biol. 2015, 60, 165–175. [Google Scholar]
- Zhang, N.; Yuan, S.; Zhao, C.; Park, R.F.; Wen, X.; Yang, W.; Liu, D. TaNAC35 acts as a negative regulator for leaf rust resistance in a compatible interaction between common wheat and Puccinia triticina. Mol. Genet. Genomics. 2021, 296, 279–287. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rafiqi, M.; Gan, P.H.P.; Hardham, A.R.; Jones, D.A.; Ellis, J.G. Effectors of biotrophic fungi and oomycetes: Pathogenicity factors and triggers of host resistance. New Phytol. 2009, 183, 993–1000. [Google Scholar] [CrossRef]
- Gassmann, W.; Bhattacharjee, S. Effector-triggered immunity signaling: From gene-for-gene pathways to protein-protein interaction networks. Mol. Plant Microbe Interact. 2012, 25, 862–868. [Google Scholar] [CrossRef] [Green Version]
- Stukenbrock, E.H.; McDonald, B.A. Population genetics of fungal and oomycete effectors involved in gene-for-gene interactions. Mol. Plant Microbe Interact. 2009, 22, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Peressotti, E.; Wiedemann-Merdinoglu, S.; Delmotte, F.; Bellin, D.; Di Gaspero, G.; Testolin, R.; Merdinoglu, D.; Mestre, P. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 2010, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Dodds, P.; Thrall, P. Recognition events and host-pathogen co-evolution in gene-for-gene resistance to flax rust. Funct. Plant Biol. 2009, 36, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Zhan, J.; Thrall, P.H.; Papaïx, J.; Xie, L.; Burdon, J.J. Playing on a pathogen’s weakness: Using evolution to guide sustainable plant disease control strategies. Annu. Rev. Phytopathol. 2015, 53, 19–43. [Google Scholar] [CrossRef]
- Kaur, B.; Bhatia, D.; Mavi, G.S. Eighty years of gene-for-gene relationship and its applications in identification and utilization of R genes. J. Genet. 2021, 100, 50. [Google Scholar] [CrossRef]
- Keane, P.J. Horizontal or generalized resistance to pathogens in plants. Plant Pathol. 2012, 327–362. [Google Scholar] [CrossRef] [Green Version]
- Kapsa, J.S. Important threats in potato production and integrated pathogen/pest management. Potato Res. 2008, 51, 385–401. [Google Scholar] [CrossRef]
- Burdon, J.; Barrett, L.G.; Yang, L.N.; He, D.C.; Zhan, J. Maximizing world food production through disease control. BioScience 2020, 70, 126–128. [Google Scholar] [CrossRef]
- Cai, J.Y.; Xiong, J.J.; Yu, H.; Ruifa, H. Pesticide overuse in apple production and its socioeconomic determinants: Evidence from Shaanxi and Shandong provinces, China. J. Clean. Prod. 2021, 315, 128–179. [Google Scholar] [CrossRef]
- Bragard, C.; Caciagli, P.; Lemaire, O.; Lopez-Moya, J.J.; MacFarlane, S.; Peters, D.; Susi, P.; Torrance, L. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 2013, 51, 177–201. [Google Scholar] [CrossRef]
- de Souza Vandenberghe, L.P.; Garcia, L.M.B.; Rodrigues, C.; Camara, M.C.; de Melo Pereira, G.V.; de Oliveira, J.; Soccol, C.R. Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, W. Recent Advances in synthetic chemical inducers of plant immunity. Front. Plant Sci. 2018, 9, 1613. [Google Scholar] [CrossRef] [Green Version]
- Zhan, J.; McDonald, B.A. Thermal adaptation in the fungal pathogen Mycosphaerella graminicola. Mol. Ecol. 2011, 20, 1689–1701. [Google Scholar] [CrossRef]
- Sommerhalder, R.J.; McDonald, B.A.; Mascher, F.; Zhan, J. Sexual recombinants make a significant contribution to epidemics caused by the wheat pathogen Phaeosphaeria nodorum. Phytopathology 2010, 100, 855–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.N.; Pan, Z.C.; Zhu, W.; Wu, E.J.; He, D.C.; Yuan, X.; Qin, Y.Y.; Wang, Y.; Chen, R.S.; Thrall, P.H. Enhanced agricultural sustainability through within-species diversification. Nat. Sustain. 2019, 2, 46–52. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Alvindia, D.G. Revisiting hot water treatments in controlling crown rot of banana cv. Bungulan. Crop Prot. 2012, 33, 59–64. [Google Scholar] [CrossRef]
- Alvindia, D.G. An integrated approach with hot water treatment and salt in the control of crown rot disease and preservation of quality in banana. Int. J. Pest Manag. 2013, 59, 271–278. [Google Scholar] [CrossRef]
- Miguel, A.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Xu, Y.L.; Li, S.X.; Zhu, L.; Song, J. Developing suppressive soil for root diseases of soybean with continuous long-term cropping of soybean in black soil of Northeast China. Acta. Agric. Scand. B Soil Plant Sci. 2015, 63, 279–285. [Google Scholar] [CrossRef]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Shi, H.; Chern, M.; Yu, H.; Yi, H.; He, M.; Yin, J.; Zhu, X.; Li, Y.; et al. A single transcription factor promotes both yield and immunity in rice. Science 2018, 361, 1026–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, S. An analysis of the biopesticide market now and where it is going. Outlooks Pest Manag. 2015, 26, 203–206. [Google Scholar] [CrossRef]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invertebr. Pathol. 2019, 165, 74–81. [Google Scholar] [CrossRef]
- Jones, A.W. Ancient egyptian model for the biological control of Schistosomiasis. Proc. Okla. Acad. Sci. 1975, 55, 136–142. [Google Scholar]
- Waage, J.K.; Greathead, D.J. Biological control: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B 1988, 318, 111–128. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Holtappels, D.; Lavigne, R.; Huys, I.; Wagemans, J. Protection of Phage Applications in Crop Production: A Patent Landscape. Viruses 2019, 11, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, Y.; Gisi, U.; Mosinger, E. Systemic resistance of potato plants against Phytophthora infestans induced by unsaturated fatty acids. Physiol. Mol. Plant. Pathol. 1991, 38, 255–263. [Google Scholar] [CrossRef]
- El-mohamedy, R.; Shafeek, M.; El-Samad, E.; Salama, D.; Rizk, F. Field application of plant resistance inducers (PRIs) to control important root rot diseases and improvement growth and yield of green bean (Phaseolus vulgaris L.). Aust. J. Crop. Sci. 2017, 11, 496–505. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Tan, X.L.; Zhang, Z.F.; Zhu, J.Y.; Tian, H.G.; Liu, T.X. Infection of powdery mildew reduces the fitness of grain aphids (Sitobion avenae) through restricted nutrition and induced defense response in wheat. Front. Plant. Sci. 2018, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Wees, S.V.; Bakker, P. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef]
- Alvindia, D.G. The antagonistic action of Trichoderma harzianum strain DGA01 against anthracnose-causing pathogen in mango cv. ‘Carabao’. Biocontrol Sci. Technol. 2018, 28, 591–602. [Google Scholar] [CrossRef]
- Hou, Q.; Kolodkin-Gal, I. Harvesting the complex pathways of antibiotic production and resistance of soil bacilli for optimizing plant microbiome. FEMS Microbiol. Ecol. 2020, 96, 142. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Brescia, F.; Vlassi, A.; Bejarano, A.; Seidl, B.; Marchetti-Deschmann, M.; Schuhmacher, R.; Puopolo, G. Characterisation of the antibiotic profile of Lysobacter capsici AZ78, an effective biological control agent of plant pathogenic microorganisms. Microorganisms 2021, 9, 1320. [Google Scholar] [CrossRef]
- Kim, H.; Rim, S.O.; Bae, H. Antimicrobial potential of metabolites extracted from ginseng bacterial endophyte Burkholderia stabilis against ginseng pathogens. Biol. Control 2019, 128, 24–30. [Google Scholar] [CrossRef]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016, 92, fiw114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant. Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr.Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bélanger, R.R.; Labbé, C.; Lefebvre, F.; Teichmann, B. Mode of action of biocontrol agents: All that glitters is not gold. Can. J. Plant. Pathol. 2012, 34, 469–479. [Google Scholar] [CrossRef]
- Raio, A.; Puopolo, G. Pseudomonas chlororaphis metabolites as biocontrol promoters of plant health and improved crop yield. World J. Microbiol. Biotechnol. 2021, 37, 99. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Shoda, M. Bacterial control of plant diseases. J. Biosci. Bioeng. 2000, 89, 515–521. [Google Scholar] [CrossRef]
- English-Loeb, G.; Norton, A.P.; Gadoury, D.; Seem, R.; Wilcox, W. Biological Control of grape powdery mildew using mycophagous mites. Plant. Dis. 2007, 91, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Gafni, A.; Calderon, C.E.; Harris, R.; Buxdorf, K.; Dafa-Berger, A.; Zeilinger-Reichert, E.; Levy, M. Biological control of the cucurbit powdery mildew pathogen Podosphaera xanthii by means of the epiphytic fungus Pseudozyma aphidis and parasitism as a mode of action. Front. Plant. Sci. 2015, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Héloir, M.C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant. Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherm, H.; Ngugi, H.K.; Savelle, A.T.; Edwards, J.R. Biological control of infection of blueberry flowers caused by Monilinia vaccinii-corymbosi. Biol. Control. 2004, 29, 199–206. [Google Scholar] [CrossRef]
- Aysan, Y.; Karatas, A.; Cinar, O. Biological control of bacterial stem rot caused by Erwinia chrysanthemi on tomato. Crop. Prot. 2003, 22, 807–811. [Google Scholar] [CrossRef]
- Cotty, P.J.; Bhatnagar, D. Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl. Environ. Microbiol. 1994, 60, 2248–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, A. Foliar application of effective microorganisms on pea as an alternative fertilizer. Agron. Sustain. Dev. 2006, 26, 257–262. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; Gardener, B.M. Biological Control of Plant Pathogens. Plant Health Instr. 2006, 2, 1117–1142. [Google Scholar] [CrossRef] [Green Version]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant. Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi. J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant. Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, P.; Unestam, T. Differential influence of ectomycorrhizae on plant growth and disease resistance in Pinus sylvestris seedlings. J. Phytopathol. 2008, 120, 104–120. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [Green Version]
- He, D.C.; Zhan, J.S.; Xie, L.H. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 15, 705–715. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Liechty, Z.; Santos-Medellin, C.; Edwards, J.; Nguyen, B.; Mikhail, D.; Eason, S.; Phillips, G.; Sundaresan, V. Comparative analysis of root microbiomes of rice cultivars with high and low methane emissions reveals differences in abundance of methanogenic archaea and putative upstream fermenters. mSystems 2020, 5, e00897-19. [Google Scholar] [CrossRef] [Green Version]
- He, D.C.; Burdon, J.J.; Xie, L.H.; Zhan, J. Triple bottom-line consideration of sustainable plant disease management: From economic, sociological and ecological perspectives. J. Integr. Agric. 2021, 20, 2581–2591. [Google Scholar] [CrossRef]
- Zheng, R.; Zhan, J.; Liu, L.; Ma, Y.; Wang, Z.; Xie, L.; He, D.C. Factors and minimal subsidy associated with tea farmers’ willingness to adopt ecological pest management. Sustainability 2019, 11, 6190. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H.; et al. Genetic diversity and disease control in rice. Nature 2000, 406, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.M.; Johnson, S.A.; Mooers, A.O.; M’Gonigle, L.K. Using historical data to estimate bumble bee occurrence: Variable trends across species provide little support for community-level declines. Biol. Conserv. 2021, 257, 109141. [Google Scholar] [CrossRef]
- Pellegrino, E.; Gamper, H.A.; Ciccolini, V.; Ercoli, L. Forage rotations conserve diversity of arbuscular mycorrhizal fungi and soil fertility. Front. Microbiol. 2019, 10, 2969. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, J.; Li, D.; Wu, F.; Zhou, X. Rotations with Indian mustard and wild rocket suppressed cucumber fusarium wilt disease and changed rhizosphere bacterial communities. Microorganisms 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebreton, L.; Lucas, P.; Dugas, F.; Guillerm, A.Y.; Schoeny, A.; Sarniguet, A. Changes in population structure of the soilborne fungus Gaeumannomyces graminis var. tritici during continuous wheat cropping. Environ. Microbiol. 2004, 6, 1174–1185. [Google Scholar] [CrossRef]
- Trivedi, P.; He, Z.; Van Nostrand, J.D.; Albrigo, G.; Zhou, J.; Wang, N. Huanglongbing alters the structure and functional diversity of microbial communities associated with citrus rhizosphere. ISME J. 2012, 6, 363–383. [Google Scholar] [CrossRef] [Green Version]
- Jeon, C.W.; Kim, D.R.; Bae, E.J.; Kwak, Y.S. Changes in bacterial community structure and enriched functional bacteria associated with turfgrass monoculture. Front. Bioeng. Biotechnol. 2021, 8, 530067. [Google Scholar] [CrossRef]
- Campanella, V.; Miceli, C. Biological control of fusarium wilt of ustica landrace lentil. Crop. Prot. 2021, 145, 105635. [Google Scholar] [CrossRef]
- Haddoudi, I.; Cabrefiga, J.; Mora, I.; Mhadhbi, H.; Montesinos, E.; Mrabet, M. Biological control of fusarium wilt caused by Fusarium equiseti in Vicia faba with broad spectrum antifungal plant-associated Bacillus spp. Biol. Control 2021, 160, 1049–9644. [Google Scholar] [CrossRef]
- Hassan, M.N.; Afghan, S.; Hafeez, F.Y. Biological control of red rot in sugarcane by native pyoluteorin-producing Pseudomonas putida strain NH-50 under field conditions and its potential modes of action. Pest. Manag. Sci. 2011, 67, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Del Bianco, M.; Kepinski, S. Context, specificity, and self-organization in auxin response. Cold Spring Harb. Perspect Biol. 2011, 3, a001578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Garrido, C.; Roudet, J.; Aveline, N.; Davidou, L.; Dupin, S.; Fermaud, M. Microbial antagonism toward Botrytis bunch rot of grapes in multiple field tests using one Bacillus ginsengihumi Strain and formulated biological control products. Front. Plant. Sci. 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Holah, J.; Alexander, H. Soil pathogenic fungi have the potential to affect the co-existence of two tallgrass prairie species. J. Ecol. 2001, 87, 598–608. [Google Scholar] [CrossRef]
- Mark, G.; Morrissey, J.P.; Higgins, P.; O’gara, F. Molecular-based strategies to exploit Pseudomonas biocontrol strains for environmental biotechnology applications. FEMS Microbiol. Ecol. 2006, 56, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, V.; Guigon, C.; Berlanga, D.; Ojeda, D. Complete control of Penicillium expansum on apple fruit using a combination of antagonistic yeast Candida oleophila. Chil. J. Agric. Res. 2014, 74, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant. Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Brown, M.W.; Mathews, C.R. Conservation biological control of rosy apple aphid, Dysaphis plantaginea (Passerini), in eastern North America. Environ. Entomol. 2007, 36, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Roossinck, M.J.; García-Arenal, F. Ecosystem simplification, biodiversity loss and plant virus emergence. Curr. Opin. Virol. 2015, 10, 56–62. [Google Scholar] [CrossRef]
- Li, H.; Leifert, C. Development of resistance in Botryotinia fuckeliana (de Barry) whetzel against the biological control agent Bacillus subtilis CL27. J. Plant. Dis. Prot. 1994, 101, 414–418. [Google Scholar]
- Ajouz, S.; Nicot, P.; Bardin, M. Adaptation to pyrrolnitrin in Botrytis cinerea and cost of resistance. Plant. Pathol. 2010, 59, 556–566. [Google Scholar] [CrossRef]
- Berling, M.; Blachere-Lopez, C.; Soubabere, O.; Lery, X.; Bonhomme, A.; Sauphanor, B.; Lopez-Ferber, M. Cydia pomonella granulovirus genotypes overcome virus resistance in the codling moth and improve virus efficiency by selection against resistant hosts. Appl. Environ. Microbiol. 2009, 75, 925–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, J.; McDonald, B. Field-based experimental evolution of three cereal pathogens using a mark-release-recapture strategy. Plant. Pathol. 2013, 62, 106–114. [Google Scholar] [CrossRef]
- Kering, K.; Kibii, B.; Wei, H. Mini review: Biocontrol of phytobacteria with bacteriophage cocktails. Pest. Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [Green Version]
- Zhan, J.; Thrall, P.H.; Burdon, J.J. Achieving sustainable plant disease management through evolutionary principles. Trends Plant. Sci. 2014, 19, 570–575. [Google Scholar] [CrossRef]
- Settele, J.; Settle, W.H. Conservation biological control: Improving the science base. Proc. Natl. Acad. Sci. USA 2018, 115, 8241–8243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, D.; Kehoe, R.; van Veen, F.J.F. Experimental evidence for the population-dynamic mechanisms underlying extinction cascades of carnivores. Curr. Biol. 2015, 25, 3106–3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, M.; Fernandes, G.W.; Lewis, O.T.; Morris, R.J. Experimentally reducing species abundance indirectly affects food web structure and robustness. J. Anim. Ecol. 2017, 86, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, R.; Warner, K.D.; Jonsson, M.; Wratten, S.D. Economics and adoption of conservation biological control. Biol. Control 2008, 45, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Gaba, S.; Gabriel, E.; Chadœuf, J.; Bonneu, F.; Bretagnolle, V. Herbicides do not ensure for higher wheat yield, but eliminate rare plant species. Sci. Rep. 2016, 6, 30112. [Google Scholar] [CrossRef]
- Kraiss, H.; Cullen, E.M. Insect growth regulator effects of azadirachtin and neem oil on survivorship, development and fecundity of Aphis glycines (Homoptera: Aphididae) and its predator, Harmonia axyridis (Coleoptera: Coccinellidae). Pest. Manag. Sci. 2008, 64, 660–668. [Google Scholar] [CrossRef]
- Mills, N.J.; Heimpel, G.E. Could increased understanding of foraging behavior help to predict the success of biological control? Curr. Opin. Insect Sci. 2018, 27, 26–31. [Google Scholar] [CrossRef]
- He, D.C.; Ma, Y.L.; Li, Z.Z.; Zhong, C.S.; Cheng, Z.B.; Zhan, J. Crop rotation enhances agricultural sustainability: From an empirical evaluation of eco-economic benefits in rice production. Agriculture 2021, 11, 91. [Google Scholar] [CrossRef]
- Breakfield, N.; Collett, D.P.; Frodyma, M. Plant growth-promoting microbes—An industry view. Emerg. Top. Life Sci. 2021, 5, 317–324. [Google Scholar] [CrossRef]
- Syed, A.B.; Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant. Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Vanhove, M.; Sicard, A.; Ezennia, J.; Leviten, N.; Almeida, R.P.P. Population structure and adaptation of a bacterial pathogen in California grapevines. Environ. Microbiol. 2020, 22, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.H.; Reshi, Z.A. Vesicular arbuscular mycorrhizal (VAM) fungi- as a major biocontrol agent in modern sustainable agriculture system. Russ. Agric. Sci. 2017, 43, 138–143. [Google Scholar] [CrossRef]
- Fedele, G.; Brischetto, C.; Rossi, V. Biocontrol of Botrytis cinerea on grape berries as influenced by temperature and humidity. Front. Plant. Sci. 2020, 11, 1232. [Google Scholar] [CrossRef]
- Robin, D.C.; Marchand, P.A. Evolution of the biocontrol active substances in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest. Manag. Sci. 2019, 75, 950–958. [Google Scholar] [CrossRef]
- Law, J.W.; Ser, H.L.; Khan, T.M.; Chuah, L.H.; Pusparajah, P.; Chan, K.G.; Goh, B.H.; Lee, L.H. The potential of Streptomyces as biocontrol agents against the rice blast fungus, Mmagnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotolo, C.; De Miccolis Angelini, R.M.; Dongiovanni, C.; Pollastro, S.; Fumarola, G.; Di Carolo, M.; Perrelli, D.; Natale, P.; Faretra, F. Use of biocontrol agents and botanicals in integrated management of Botrytis cinerea in table grape vineyards. Pest. Manag. Sci. 2018, 74, 715–725. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef]
- Shtienberg, D.; Elad, Y. Incorporation of weather forecasting in integrated, biological-chemical management of Botrytis cinerea. Phytopathology 1997, 87, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Hu, X.; Xu, X. Dispersal of Bacillus subtilis and its effect on strawberry phyllosphere microbiota under open field and protection conditions. Sci. Rep. 2016, 6, 22611. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Claflin, S.B.; Jones, L.E.; Thaler, J.S.; Power, A.G. Crop-dominated landscapes have higher vector-borne plant virus prevalence. J. Appl. Ecol. 2017, 54, 1190–1198. [Google Scholar] [CrossRef] [Green Version]
- Wu, E.J.; Wang, Y.P.; Yahuza, L.; He, M.H.; Sun, D.L.; Huang, Y.M.; Liu, Y.C.; Yang, L.N.; Zhu, W.; Zhan, J. Rapid adaptation of the Irish potato famine pathogen Phytophthora infestans to changing temperature. Evol. Appl. 2020, 13, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.N.; Nkurikiyimfura, O.; Pan, Z.C.; Wang, Y.P.; Waheed, A.; Chen, R.S.; Burdon, J.J.; Sui, Q.J.; Zhan, J. Plant diversity ameliorates the evolutionary development of fungicide resistance in an agricultural ecosystem. J. Appl. Ecol. 2021. [Google Scholar] [CrossRef]
- Zhan, J.; Mundt, C.C.; Hoffer, M.E.; McDonald, B.A. Local adaptation and effect of host genotype on the rate of pathogen evolution: An experimental test in a plant pathosystem. J. Evol. Biol. 2002, 15, 634–647. [Google Scholar] [CrossRef]
- Burdon, J.J.; Zhan, J. Climate change and disease in plant communities. PLoS Biol. 2020, 18, e3000949. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elgawad, M.M.M.; Askary, T.H. Factors affecting success of biological agents used in controlling the plant-parasitic nematodes. Egypt J. Biol. Pest. Control 2020, 30, 17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. https://doi.org/10.3390/pathogens10101311
He D-C, He M-H, Amalin DM, Liu W, Alvindia DG, Zhan J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens. 2021; 10(10):1311. https://doi.org/10.3390/pathogens10101311
Chicago/Turabian StyleHe, Dun-Chun, Meng-Han He, Divina M. Amalin, Wei Liu, Dionisio G. Alvindia, and Jiasui Zhan. 2021. "Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration" Pathogens 10, no. 10: 1311. https://doi.org/10.3390/pathogens10101311
APA StyleHe, D.-C., He, M.-H., Amalin, D. M., Liu, W., Alvindia, D. G., & Zhan, J. (2021). Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens, 10(10), 1311. https://doi.org/10.3390/pathogens10101311





