Rickettsia lusitaniae in Ornithodoros Porcinus Ticks, Zambia
Abstract
:1. Introduction
2. Results
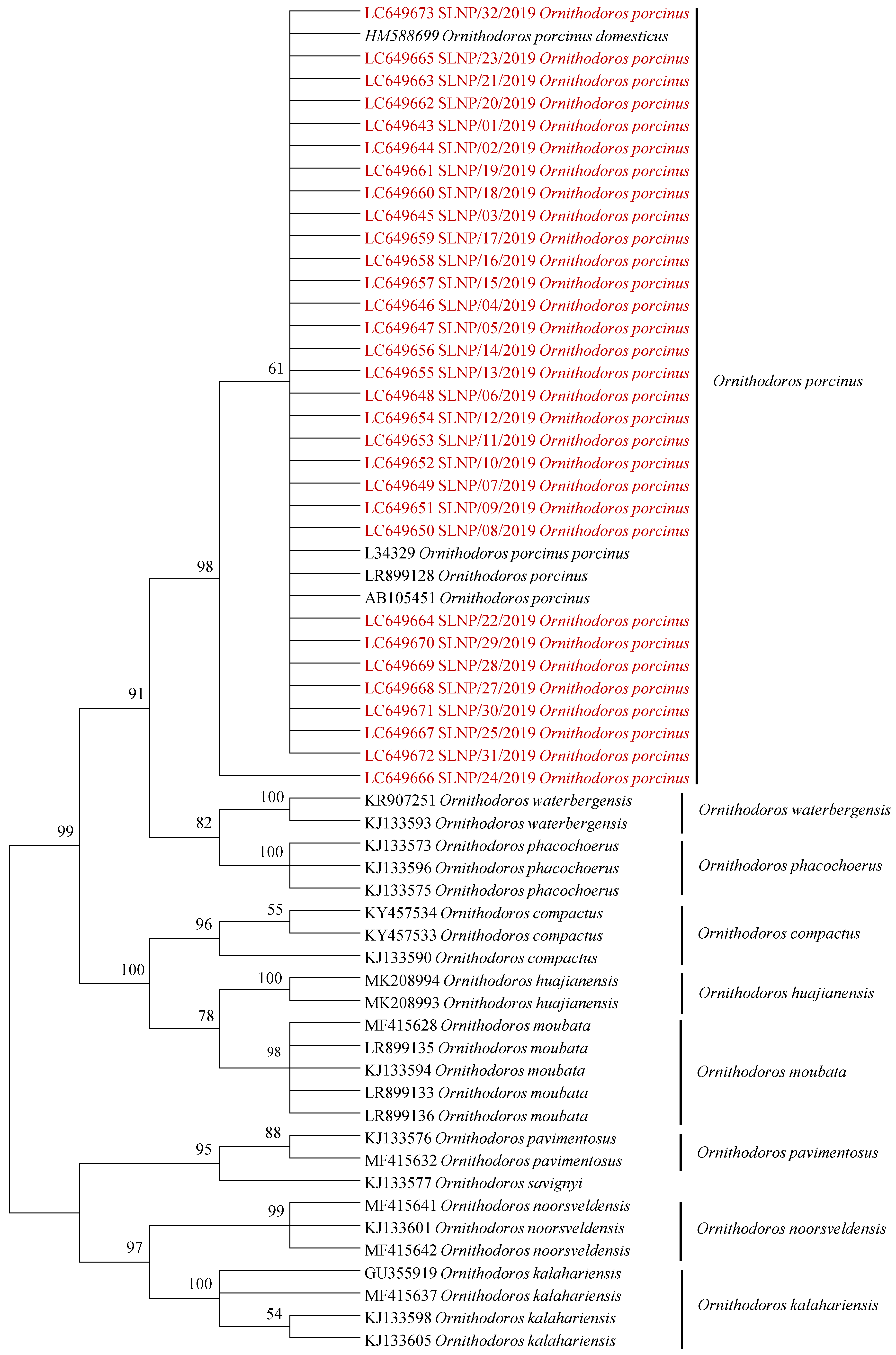
2.1. Tick Sampling and Identification
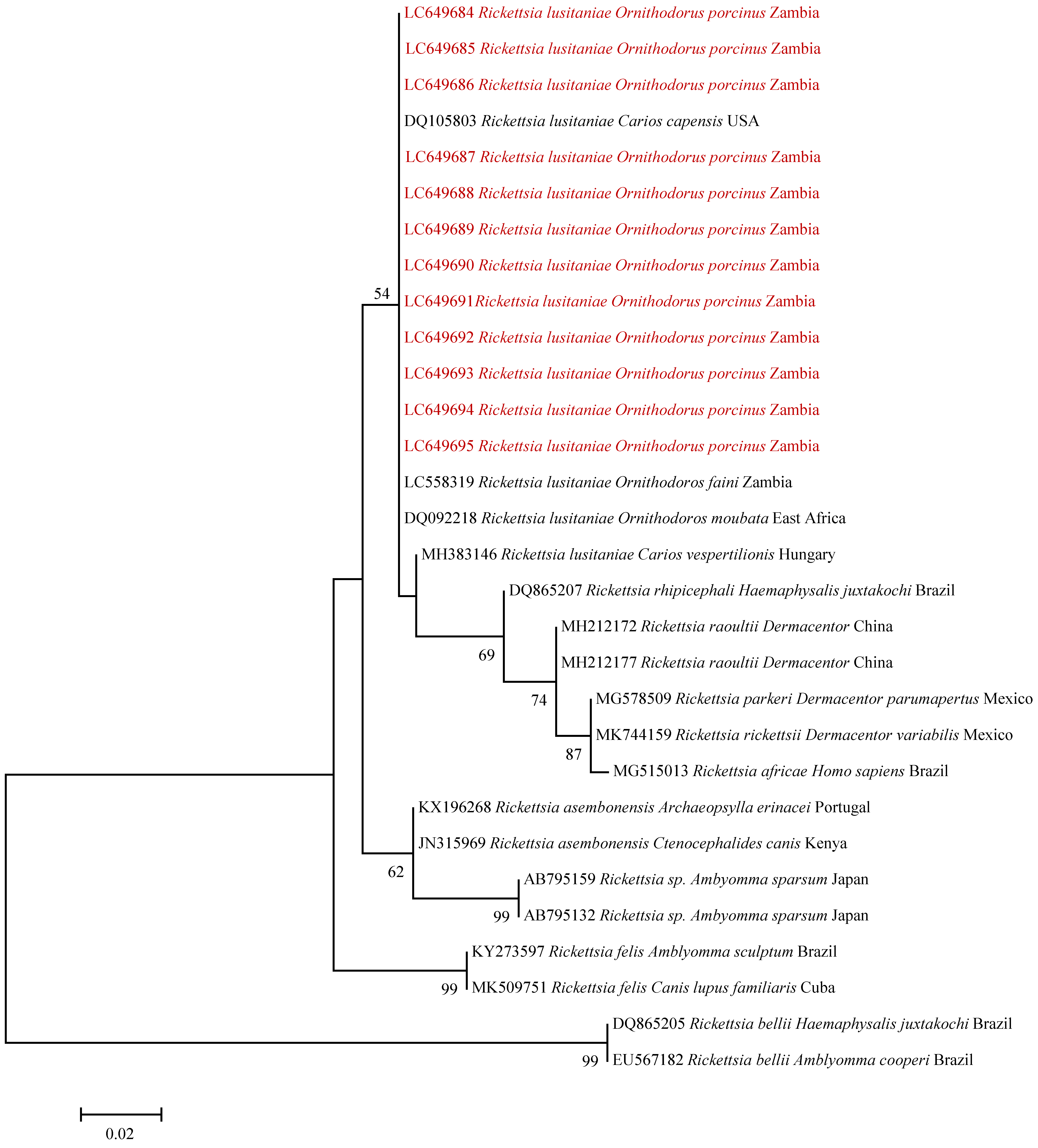
2.2. Rickettsia Screening and Identification
3. Discussion
4. Materials and Methods
4.1. Study Sites
4.2. Molecular Identification of Soft Ticks
4.3. Molecular Screening and Identification of Rickettsia Species
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelakis, E.; Richet, H.; Raoult, D. Rickettsia sibirica mongolitimonaeInfection, France, 2010–2014. Emerg. Infect. Dis. 2016, 22, 880–882. [Google Scholar] [CrossRef] [Green Version]
- Mediannikov, O.; Diatta, G.; Fenollar, F.; Sokhna, C.; Trape, J.-F.; Raoult, D. Tick-Borne Rickettsioses, Neglected Emerging Diseases in Rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, P.-E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene Sequence-Based Criteria for Identification of New Rickettsia Isolates and Description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef] [Green Version]
- Adem, P.V. Emerging and re-emerging rickettsial infections. Semin. Diagn. Pathol. 2019, 36, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Magnarelli, L.A. Biology of Ticks. Infect. Dis. Clin. N. Am. 2008, 22, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef] [PubMed]
- Buysse, M.; Duron, O. Two novel Rickettsia species of soft ticks in North Africa: ‘Candidatus Rickettsia africaseptentrionalis’ and ‘Candidatus Rickettsia mauretanica’. Ticks Tick-Borne Dis. 2020, 11, 101376. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Szőke, K.; Meli, M.L.; Sándor, A.D.; Görföl, T.; Estók, P.; Wang, Y.; Tu, V.T.; Kováts, D.; Boldogh, S.A.; et al. Molecular detection of vector-borne bacteria in bat ticks (Acari: Ixodidae, Argasidae) from eight countries of the Old and New Worlds. Parasites Vectors 2019, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Reeves, W.K.; Mans, B.J.; Durden, L.A.; Miller, M.M.; Gratton, E.M.; Laverty, T.M. Rickettsia hoogstraalii and a Rickettsiella from the Bat Tick Argas transgariepinus, in Namibia. J. Parasitol. 2020, 106, 663–669. [Google Scholar] [CrossRef]
- Qiu, Y.; Simuunza, M.; Kajihara, M.; Chambaro, H.; Harima, H.; Eto, Y.; Simulundu, E.; Squarre, D.; Torii, S.; Takada, A.; et al. Screening of tick-borne pathogens in argasid ticks in Zambia: Expansion of the geographic distribution of Rickettsia lusitaniae and Rickettsia hoogstraalii and detection of putative novel Anaplasma species. Ticks Tick Borne Dis. 2021, 12, 101720. [Google Scholar] [CrossRef]
- Lafri, I.; Leulmi, H.; Baziz-Neffah, F.; Lalout, R.; Mohamed, C.; Mohamed, K.; Parola, P.; Bitam, I. Detection of a novel Rickettsia sp. in soft ticks (Acari: Argasidae) in Algeria. Microbes Infect. 2015, 17, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Pader, V.; Nikitorowicz-Buniak, J.; Abdissa, A.; Adamu, H.; Tolosa, T.; Gashaw, A.; Cutler, R.R.; Cutler, S.J. Candidatus Rickettsia hoogstraalii in Ethiopian Argas persicus ticks. Ticks Tick-Borne Dis. 2012, 3, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Bakkes, D.K.; De Klerk, D.; Latif, A.A.; Mans, B.J. Integrative taxonomy of Afrotropical Ornithodoros (Ornithodoros) (Acari: Ixodida: Argasidae). Ticks Tick-Borne Dis. 2018, 9, 1006–1037. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Browning, P.; Scott, J.C. Ornithodoros moubata, a Soft Tick Vector for Rickettsia in East Africa? Ann. N. Y. Acad. Sci. 2006, 1078, 373–377. [Google Scholar] [CrossRef]
- Chitanga, S.; Chibesa, K.; Sichibalo, K.; Mubemba, B.; Nalubamba, K.S.; Muleya, W.; Changula, K.; Simulundu, E. Molecular Detection and Characterization of Rickettsia Species in Ixodid Ticks Collected From Cattle in Southern Zambia. Front. Vet. Sci. 2021, 8, 684487. [Google Scholar] [CrossRef]
- Moonga, L.C.; Hayashida, K.; Nakao, R.; Lisulo, M.; Kaneko, C.; Nakamura, I.; Eshita, Y.; Mweene, A.S.; Namangala, B.; Sugimoto, C.; et al. Molecular detection of Rickettsia felis in dogs, rodents and cat fleas in Zambia. Parasites Vectors 2019, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chitimia-Dobler, L.; Dobler, G.; Schaper, S.; Kattner, S.; Wölfel, S.; Küpper, T. First detection of Rickettsia conorii ssp. caspia in Rhipicephalus sanguineus in Zambia. Parasitol. Res. 2017, 116, 3249–3251. [Google Scholar] [CrossRef]
- Andoh, M.; Sakata, A.; Takano, A.; Kawabata, H.; Fujita, H.; Une, Y.; Goka, K.; Kishimoto, T.; Ando, S. Detection of Rickettsia and Ehrlichia spp. in Ticks Associated with Exotic Reptiles and Amphibians Imported into Japan. PLoS ONE 2015, 10, e0133700. [Google Scholar] [CrossRef]
- Brinkerhoff, R.J.; Clark, C.; Ocasio, K.; Gauthier, D.T.; Hynes, W.L. Factors affecting the microbiome of Ixodes scapularis and Amblyomma americanum. PLoS ONE 2020, 15, e0232398. [Google Scholar] [CrossRef]
- Swei, A.; Kwan, J.Y. Tick microbiome and pathogen acquisition altered by host blood meal. ISME J. 2016, 11, 813–816. [Google Scholar] [CrossRef]
- Fryxell, R.T.T.; DeBruyn, J. The Microbiome of Ehrlichia-Infected and Uninfected Lone Star Ticks (Amblyomma americanum). PLoS ONE 2016, 11, e0146651. [Google Scholar] [CrossRef] [Green Version]
- Van Treuren, W.; Ponnusamy, L.; Brinkerhoff, R.J.; Gonzalez, A.; Parobek, C.M.; Juliano, J.J.; Andreadis, T.G.; Falco, R.C.; Ziegler, L.B.; Hathaway, N.; et al. Variation in the Microbiota of Ixodes Ticks with Regard to Geography, Species, and Sex. Appl. Environ. Microbiol. 2015, 81, 6200–6209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawlena, H.; Rynkiewicz, E.C.; Toh, E.; Alfred, A.; Durden, L.A.; Hastriter, M.W.; Nelson, D.E.; Rong, R.; Munro, D.; Dong, Q.; et al. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J. 2013, 7, 221–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, T.C.; Pulscher, L.A.; Caddell, L.; Von Fricken, M.E.; Anderson, B.D.; Gonchigoo, B.; Gray, G.C. Evidence for transovarial transmission of tick-borne rickettsiae circulating in Northern Mongolia. PLoS Negl. Trop. Dis. 2018, 12, e0006696. [Google Scholar] [CrossRef] [Green Version]
- Chambaro, H.M.; Sasaki, M.; Sinkala, Y.; Gonzalez, G.; Squarre, D.; Fandamu, P.; Lubaba, C.; Mataa, L.; Shawa, M.; Mwape, K.E.; et al. Evidence for exposure of asymptomatic domestic pigs to African swine fever virus during an inter-epidemic period in Zambia. Transbound. Emerg. Dis. 2020, 67, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Vial, L.; Penrith, M.; Sánchez, R.P.; Etter, E.; Albina, E.; Michaud, V.; Roger, F. Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res. 2013, 173, 212–227. [Google Scholar] [CrossRef]
- Black, W.C.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [Green Version]
- Walton, G.A. A taxonomic review of the Ornithodoros moubata (murray) 1877 (sensu walton, 1962) species group in africa. Recent Adv. Acarol. 1979, 2, 491–500. [Google Scholar] [CrossRef]
- Walton, G.A. The Ornithodorus moubata super species problem in relation to human relapsing fever epidemiology. Symp. Zool. Soc. Lond. 1962, 6, 83–156. [Google Scholar]
- Moonga, L.; Hayashida, K.; Kawai, N.; Nakao, R.; Sugimoto, C.; Namangala, B.; Yamagishi, J. Development of a Multiplex Loop-Mediated Isothermal Amplification (LAMP) Method for Simultaneous Detection of Spotted Fever Group Rickettsiae and Malaria Parasites by Dipstick DNA Chromatography. Diagnostics 2020, 10, 897. [Google Scholar] [CrossRef]
- Gaowa; Ohashi, N.; Aochi, M.; Wuritu, D.; Wu, D.; Yoshikawa, F.; Kawamori, F.; Honda, T.; Fujita, H.; Takada, N.; et al. Rickettsiae in Ticks, Japan, 2007–2011. Emerg. Infect. Dis. 2013, 19, 338–340. [Google Scholar] [CrossRef]
- Labruna, M.B.; Whitworth, T.; Bouyer, D.H.; McBride, J.; Camargo, L.M.A.; Camargo, E.P.; Popov, V.; Walker, D.H. Rickettsia bellii and Rickettsia amblyommii in Amblyomma Ticks from the State of Rondônia, Western Amazon, Brazil. J. Med. Èntomol. 2004, 41, 1073–1081. [Google Scholar] [CrossRef]
- Burket, C.T.; Vann, C.N.; Pinger, R.R.; Chatot, C.L.; Steiner, F.E. Minimum Infection Rate of Ambylomma americanum (Acari: Ixodidae) by Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae in Southern Indiana. J. Med. Èntomol. 1998, 35, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, E.S.G. EpiTools Epidemiological Calculators. Ausvet. 2018. Available online: http://epitools.ausvet.com.au (accessed on 4 June 2021).
- Cowling, D.W.; Gardner, I.A.; Johnson, W.O. Comparison of methods for estimation of individual-level prevalence based on pooled samples. Prev. Vet. Med. 1999, 39, 211–225. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitanga, S.; Chambaro, H.M.; Moonga, L.C.; Hayashida, K.; Yamagishi, J.; Muleya, W.; Changula, K.; Mubemba, B.; Simbotwe, M.; Squarre, D.; et al. Rickettsia lusitaniae in Ornithodoros Porcinus Ticks, Zambia. Pathogens 2021, 10, 1306. https://doi.org/10.3390/pathogens10101306
Chitanga S, Chambaro HM, Moonga LC, Hayashida K, Yamagishi J, Muleya W, Changula K, Mubemba B, Simbotwe M, Squarre D, et al. Rickettsia lusitaniae in Ornithodoros Porcinus Ticks, Zambia. Pathogens. 2021; 10(10):1306. https://doi.org/10.3390/pathogens10101306
Chicago/Turabian StyleChitanga, Simbarashe, Herman M. Chambaro, Lavel C. Moonga, Kyoko Hayashida, Junya Yamagishi, Walter Muleya, Katendi Changula, Benjamin Mubemba, Manyando Simbotwe, David Squarre, and et al. 2021. "Rickettsia lusitaniae in Ornithodoros Porcinus Ticks, Zambia" Pathogens 10, no. 10: 1306. https://doi.org/10.3390/pathogens10101306
APA StyleChitanga, S., Chambaro, H. M., Moonga, L. C., Hayashida, K., Yamagishi, J., Muleya, W., Changula, K., Mubemba, B., Simbotwe, M., Squarre, D., Fandamu, P., Nalubamba, K. S., Qiu, Y., Hirofumi, S., & Simulundu, E. (2021). Rickettsia lusitaniae in Ornithodoros Porcinus Ticks, Zambia. Pathogens, 10(10), 1306. https://doi.org/10.3390/pathogens10101306







