Abstract
Marine ecosystems of temperate regions are highly modified by human activity and far from their original natural status. The North Sea, known as an intensively used area, has lost its offshore oyster grounds due to overexploitation in a relatively short time. Native oyster beds as a once abundant and ecologically highly important biogenic reef-type have vanished from the North Sea ecosystem in most areas of both their former distribution and magnitude. Worldwide, oyster stocks have been severely exploited over the past centuries. According to estimates, about 85% of the worldwide oyster reef habitats have been destroyed over the course of the last century. This loss of oyster populations has meant far more than just the loss of a valuable food resource. Oyster reefs represent a characteristic benthic community which offers a variety of valuable ecosystem services: better water quality, local decrease of toxic algal blooms, increase in nutrient uptake, increase of bentho-pelagic coupling, increase in species richness, increase of multidimensional biogenic structures which provide habitat, food, and protection for numerous invertebrate and fish species. The aim of oyster restoration is to promote redevelopment of this valuable missing habitat. The development of strategies, methods, and procedures for a sustainable restoration of the European oyster Ostrea edulis in the German North Sea is currently a focus of marine nature conservation. Main drivers for restoring this ecological key species are the enhancement of biodiversity and ecosystem services in the marine environment. Results of these investigations will support the future development and implementation of a large-scale and long-term German native oyster restoration programme to re-establish a healthy population of this once-abundant species now absent from the region.
1. Eradication from Coastal Ecosystems and Human Memory: Environmental Generational Amnesia as a Specific Challenge for Oyster Restoration
Oysters are important ecosystem engineers as they build up ecologically relevant hard structures by forming reef habitats. Worldwide oyster stocks have been exploited intensively over the past centuries. Ever more efficient harvesting techniques led to the destruction of oyster reefs and their associated species-rich fauna and flora. Biogenic temperate reefs are among the most threatened habitats globally and much of the continental shelf and some deeper ocean seafloors have been homogenized by bottom trawling and dredging (Airoldi et al. 2008).
There is clear evidence that the German North Sea not only encountered the loss of oyster reefs from its coastal ecosystems, but also the loss of an ecological baseline through the eradication of oyster reefs from coastal ecosystems and human memory (Alleway and Connell 2015). The tendency for younger generations to lose the past as a benchmark for interpreting the present is called “environmental generational amnesia” e.g., (Kahn and Friedman 1995; Kahn 2002; Papworth et al. 2009). It is based on the concept of shifting baselines (Pauly 1995) and, for the German situation, on the fact that oysters vanished around 70 years ago from their original habitat and commercial use of the species ended even longer ago, almost 100 years before today. As observers of these times left the system, the population’s perception of normality updated and past conditions were forgotten (Papworth et al. 2009), which is clearly the case for former oyster abundance in Germany. Even coastal communities in Germany no longer remember the former existence, vast distribution, and ecological relevance of the species. This contemporary state can be considered as an environmental generational amnesia and, in consequence, as the failure of adequate calibration of conservation and restoration goals. It accounts for altered expectations for food, economic value, and ecosystem services from coastal waters (Alleway and Connell 2015). The vast presence of oyster reefs in German waters as a key ecological system has been forgotten. This is something that eventually impairs the progress of restoration and recovery. It also reduces expectations and the potential value of these coastal ecosystems. In the marine environment, we lose or eventually lost species which we do not explicitly see. These may not be spectacular and might therefore not be on the agenda of the general public. Conservation and restoration efforts have to be linked closely to education programs, by respecting the historical and cultural relevance, and by explaining the ecological role and value of these species for our marine ecosystems.
2. Historical Distribution of Ostrea edulis in Europe and in the German North Sea: Reasons for its Drastic Decline
The European oyster Ostrea edulis (syn. European flat oyster, Flat oyster, Edible oyster, Belon oyster) is the native oyster species of European waters (Figure 1). Its natural distribution ranges from the Norwegian Sea in the North, around Ireland and Britain, the Iberian Peninsula to the Mediterranean Sea in the South. Historical records date back to the 13th century and state that natural beds of European oysters existed in subtidal coastal waters, but were also abundant in deeper waters and offshore, down to 50 m, e.g., in the North Sea and the eastern Channel (Figure 2) (Olsen 1883; Neild 1995; Lotze et al. 2005; Thurstan et al. 2013). Today, this species has disappeared along the German and Belgian coast and Ostrea edulis beds are under threat and/or decline in all the regions where they occur. The species was also believed to be extinct in the Dutch Wadden Sea since 1940, although small numbers have been discovered over the last two decades (de Bruyne et al. 2013). Ostrea edulis was nominated for inclusion on the OSPAR (Oslo–Paris-Commission) list with particular reference to global and regional importance, rarity, decline, role as a keystone species, sensitivity and threat, and as a priority for OSPAR Region II—Greater North Sea, Northeast Atlantic.

Figure 1.
The native European oyster Ostrea edulis, Genus Ostrea (flat oysters) (Picture taken by Pogoda).
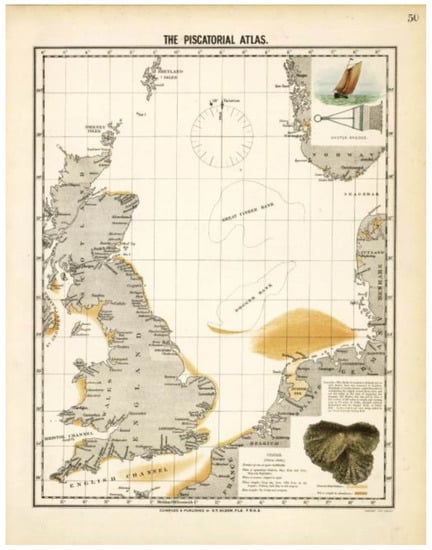
Figure 2.
Piscatorial Atlas Map on the distribution of the European oyster in the North Sea (Olsen 1883).
In Europe, the native oyster, Ostrea edulis, was collected and consumed as an important food source since the Roman times. Shell middens of northern European coasts show an intensive use of oysters over many centuries (Gerlach 2001; Becker et al. 2012). The oyster fisheries’ continuous removal of adult oysters greatly weakened natural reproduction success of the species. In addition, ever more efficient harvesting techniques allowed the catches to increase and resulting shell removal in such high quantities led to the destruction of ecologically highly valuable biogenic structures (Möbius 1877; Yonge 1960). These biogenic oyster reefs had provided hard substrate for many associated life forms and had served as a fundamental settling substrate for oyster larvae itself. The loss of these structures induced alterations of the whole ecosystem and resulted in constant oyster recruitment failures due to the absence of natural settlement substrate (oyster shells). During industrialization, steam trawlers fished maximum yields as demand for oysters as a cheap food source was high. The development of railway infrastructure allowed the transportation of massive amounts of oysters to the big cities. During the 19th century, newly discovered reefs in the English Channel provided oysters that were a food of the poor (Neild 1995). In London alone, over 700 million oysters were consumed in 1864. One hundred years later, the national oyster production in Great Britain amounted to only 3 million (Gercken and Schmidt 2014). Overexploitation of the native oyster was an accepted practice all along the European coast and is well documented in fisheries reports, museums, and archives of Scotland, England, Wales, Ireland, France, Belgium, the Netherlands, and Germany. Consequently, this led to a Europe-wide decline of native oyster populations and its functional extinction in Belgium and Germany (Gercken and Schmidt 2014; OSPAR 2009).
In the German Bight, the historical distribution covered the North and East Frisian Wadden Sea, the Helgoland Oyster Bed and also the North Sea Oyster Ground, 100–150 nm offshore in 40 m deep water, covering an enormous area of approx. 20,000–25,000 km2 (Berghahn and Ruth 2005). Oyster harvesting had become an important economic factor and was traditionally performed with sailing cutters and iron dredges. Since the end of the 17th century, the destruction of oyster beds due to overfishing was a constant problem. (Gercken and Schmidt 2014). For the Helgoland oyster bed, yields decreased drastically within a short time. The highest yields (350,000 to 500,000 oysters harvested per annum) were achieved in the years 1875–1877. From then, a significant decline in yield began, resulting in the implementation of closed seasons from 1879 to 1882. According to an estimate from the Biological Institute of Helgoland (BAH), the Helgoland oyster bed comprised about 1.5 million individuals in 1900. The stock was aging, with the majority of shells already 20 to 30 years old, while juveniles were missing. This implies that only few live adult oysters were present, an insufficient spawning stock (Boos et al. 2004). The methods of overexploitation peaked with the use of paddle steamers which employed up to 6 dredges at the same time (Figure 3A,B). After its discovery in the mid-19th century, the oyster grounds in the open North Sea were also targeted by the oyster fishery, but no reliable harvest data exist for the area. (Möbius 1877) wrote: “Dutch and German fishermen operate here, especially in the oyster harvesting months of August, September and October, and pulling a trawl sometimes harvest around 1000 oysters. Sometimes large clumps of oysters are caught in the net.” These “Deep-sea oysters” were unloaded by Dutch, English, and German fishermen in their home ports. In addition to the loss of oyster beds in the North and East Frisian Wadden Sea, biogenic oyster reef habitats and their associated species-rich fauna and flora in these offshore areas of the German Bight also vanished. In Germany, the commercial fishery for the native oyster ended in the 1920s as it was no longer profitable. Since the 1950s, the oyster is believed to be extinct in German waters.
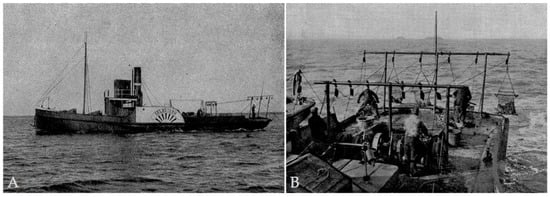
Figure 3.
Oyster trawler Gelbstern (A) and oyster dredges (B). Modified after Hagmeier and Kändler (1927). Neue Untersuchungen im nordfriesischen Wattenmeer und auf den fiskalischen Austernbänken. Aus der Biologischen Anstalt auf Helgoland und deren Zweiglaboratorium in List a. Sylt 16(6): 1–90.
Most of the examples of decline in the oyster fisheries relate to overfishing. Historic accounts place Ostrea edulis at the centre of important local fisheries, e.g., in Wales, the Solent, and The Firth of Forth in Scotland back to the Roman occupation of Britain. Fishing communities in South Wales describe a fishery supporting 200 vessels landing over 9 million oysters at its peak in the 19th century, many of which were transported to the London markets and beyond to the continent (Woolmer et al. 2011). The Solent was the largest remaining native oyster fishery in Europe. As recently as 1978, up to 450 boats made a living from catching oysters in the Solent, employing more than 700 men at sea. This period ended in 2013, when annual harvest had dropped from 200 tons to 20 tons over the course of five years, related to oysters evidently failing to reproduce. The fishery was closed in several areas of the Solent (Blue Marine Foundation 2016). The local oyster beds in the Firth of Forth on Scotland’s east coast once covered 166 km2 and were maintained into the 19th century as the most important oyster fishery of Scotland. Oysters were harvested for local markets, but also exported, particularly seed oysters, to other countries for supporting degraded stocks. The unrestrained exploitation led to a 99% decrease in the harvest over the course of 60 years, and by 1890, the fishery was no longer profitable (Thurstan et al. 2013). Today, no natural oyster beds are left in the Firth of Forth.
The main reason for the loss of formerly widespread oyster populations was overharvesting for centuries. Drastic declines can be connected to industrialisation: the consequent development of efficient harvesting techniques and the beginning of the railway transport era. In general, stocks have been exploited until a fishery was no longer economically viable. With the consequential loss of reef structures, intensified effects of other stressors, such as sedimentation, pollution (TBT, copper), diseases, and invasive species could be observed (Gercken and Schmidt 2014).
Smaller populations of Ostrea edulis still exist in Norway, Sweden, and in the Danish Limfjord. The Danish population increased over the last decades but has recently been affected by the Bonamia disease, an invasive protozoan parasite, which was formerly absent in Scandinavian populations. It infects the blood cells which results in elevated mortality rates, especially when water temperatures are high during summer, when food availability was low in the preceding winter, and/or when oyster densities are high (oyster cultivation, aquaculture). Both remaining Netherlands stocks and parts of the UK populations are also suffering from an infection with Bonamia ostreae, which arose for the first time in 1980 (Culloty and Mulcahy 2007; Laing et al. 2014). Further native oyster populations, all showing clear signs of decline or massive recruitment failures, exist in England along the coast of Cornwall, in estuaries of the counties of Sussex and Essex, the Thames, and in Wales and Scotland. In Ireland, Ostrea edulis still occurs along the West and North Coast and in fjord-like inlets, the so-called “sea loughs”, including Lough Foyle and Strangford Lough in Northern Ireland (Kennedy and Roberts 1999, 2006). In France, native European oysters occur mainly in Brittany along the Atlantic Coast, but mostly due to oyster farming activities. The only two remaining natural oyster banks are located in coastal waters off Brest and Quiberon. In Spain, Ostrea edulis mainly occurs in Galicia, but also mostly due to oyster farming (Bromley et al. 2016). Shellfish reefs, mainly flat oysters but also mussels, once occupied about 20% of the North Sea bottom (Olsen 1883). As in many other regions of the world, they have almost completely disappeared, due to overfishing, habitat destruction, and diseases (Beck et al. 2011).
3. Reasons for Restoration: Reef Habitat Protection and Biodiversity Enhancement
Native oyster species were once vital ecosystem engineers and relevant reef-builders. However, much of the continental shelf and some deeper ocean seafloors have been homogenized by bottom trawling and dredging (Airoldi et al. 2008). Today, these temperate biogenic reefs are among the most threatened habitats globally. According to the Habitats Directive for the protected habitat type “reef”, a favourable conservation status has to be preserved or restored. Reintroduction and restoration of Ostrea edulis in the German Bight contribute to the objectives of the OSPAR Convention on Protection of the Seas (OSPAR 2010), the EU Flora–Fauna–Habitat Directive (FFH Directive, 92/43/EWG) (Council of the European Union 1992) and the Marine Strategy Framework Directive (MSFD) (Council of the European Union 2008).
Oyster beds form specific reef-communities with associated algae, invertebrates, fish, and fish larvae as the three-dimensional structure provides habitat, food, settlement substrate, shelter, and spawning ground. (Möbius 1877) created the ecological term “biocoenosis” by describing the outstanding biodiversity and the ecological function of oyster beds in the German North Sea. Oyster beds and reefs are biodiversity hotspots, which provide a variety of ecosystems services and which must have played a key role in the North Sea ecosystem. As filter-feeding bivalves, they filter small particles of plankton and suspended organic particles with high efficiency. Selected particles are ingested. Surplus particles are ejected as pseudofaeces and are deposited on the seafloor. The capacity of water filtration enhances the water quality by removing particles from the water body and by reducing harmful algal blooms at local scales. Improved water clarity increases sunlight penetration, which results in higher growth rates of algae and an increase in oxygen availability, upgrading the complex system. Furthermore, the biodeposition of organic particles enriches surrounding sediments and leads to higher nitrogen fixation and removal rates (zu Ermgassen et al. 2016). The loss of oyster bed communities has contributed to associated declines in productivity of oysters, reef-associated invertebrates, and fish species, complex hard substrate and biological capacity for water filtration and shoreline protection.
An increase of biodiversity and of described ecosystem services is expected from the recovery of oyster beds. The biodiversity enhancement will be closely monitored and quantified. It represents the key ecosystem service and main driver for the restoration approach in the German North Sea.
4. Development of Restoration Guidelines
Notably, once-extensive oyster reefs are functionally extinct throughout the German Bight. Similar to other regions, where extensive oyster beds and reefs have vanished, humans and society are unaware that this loss has even occurred (Beck et al. 2011). These declines in Germany followed the global norm and are no surprise. Marine restoration initiatives were not on the agenda of natural resource management for a long time, but are gaining momentum in Europe and benefit from experiences in the US.
Several constraints to restoration have to be considered, e.g., lack of broodstock and degraded habitats, which are probably less suitable for recruitment. Although, recent developments in water quality improvements as well as the existing knowledge on sustainable management and preservation practices increase the prospects of successful restoration. These aspects will be examined and discussed and aim at the development of strategies for the preservation of this endangered species in European waters.
Oysters have a limited dispersal range and need hard substrate to settle. The decrease of oyster stock resulted in very little natural hard substrate remaining on the North Sea bed. The absence of a critical mass for reproduction and recruitment prevents the natural return of native oysters, even if environmental conditions were favourable. Furthermore, bottom trawling for different target species (flat fish, shrimp) is still a common practice in the German Bight. Monitoring data show heavy trawling effort with severe impact on the seafloor (Cook et al. 2013; Eigaard et al. 2016). Except for the areas of the Wadden Sea National Parks, most of the seafloor can be considered as ‘extensively modified’ or ‘modified’. Due to that instability of the seafloor and sediments, no natural spatfall or successful recruitment would occur even if oyster larvae were abundant in German waters. Without active restoration procedures, the oyster reefs cannot return on a large scale.
Biological attributes of Ostrea edulis are important factors for successful restoration: the recruitment of European oyster stocks is irregular and sporadic, so that the number of individuals of a population naturally fluctuates. Successful recruitment is connected to mild water temperatures in summer. In the Danish Limfjord, oysters spawn in summer when water temperatures reach 15 °C. Due to the variability of recruitment success, stocks are very sensitive to overexploitation. They generally need a long time to recover from stock declines as they usually show moderate growth and reproduction rates compared to oysters of the genus Crassostrea (OSPAR 2009; Pogoda et al. 2011).
Certain densities of broodstock oysters and availability of shell substrate for settlement are important prerequisites for successful recruitment. Breeding will not be successful if the adults are too far apart and oyster larvae preferably settle on the shells of their own species or indigenous substrate (Kennedy and Roberts 1999, 2006). Flat oysters are found in between the stone on soft sediments (sand or silt), where the bottom is covered with empty mussel and oyster shells, and promote reef structure formation on soft bottoms (Kerckhof et al. 2018).
In many European regions, mass mortalities of Ostrea edulis occurred from the 1980s onwards, caused by Bonamia ostreae. It was initially spread by aquaculture-related movements of European oysters in the United States from California to Maine and Washington in the 1970s. Later, European oysters of these US populations were moved back to France and Spain to restock degraded oyster beds in Europe and increase commercial production (Bromley et al. 2016). Today Bonamia is present in many Ostrea edulis areas (Culloty and Mulcahy 2007; Haenen et al. 2011; Mortensen et al. 2016). The spread of this disease is an impressive example for the negative potential of animal or plant translocations. For centuries, many oyster species, e.g., Crassostrea gigas, C. angulata, and C. virginica have been traded for the recovery of depleted native oyster populations and of declining fishery. These oyster translocations for commercial purposes are, in sum, a major vector for disease and invasive species transfer around the globe (e.g., Bromley et al. 2016; Elton 1958; Ruesink et al. 2005; Wolff and Reise 2002). It is therefore crucial to prevent further transfer of pathogens and invasive species by following the International Union for Conservation of Nature (IUCN) guidelines and by developing and implementing recommendations for native oyster restoration in Europe (IUCN/SSC 2013; Pogoda et al. 2018). Accordingly, restoration attempts in Germany will be realized with Bonamia-free seed oysters, following strict biosecurity protocols. Although, there is the risk of an infection occurring in the course of the restoration process since Bonamia is present in all surrounding water bodies: in the Netherlands, in Denmark, and in parts of the UK. In consequence, oysters selected for restoration should exhibit a high genetic variability in order to possess the highest possible adaptability to stress factors and to ensure the preservation of the species-specific genetic diversity.
5. Current Efforts towards Regeneration of Oyster Stocks: The Special Situation in Germany—Restoring Deep Offshore Oyster Beds
In 2014, a feasibility study for the German North Sea (Gercken and Schmidt 2014) identified several open research questions, but basically came to the conclusion that the restoration of native European oyster habitats in the German North Sea is feasible. Based on this study and in view of ecosystem-related benefits of oyster reefs, the currently running testing and development project RESTORE addresses the regulatory framework for restoration, site selection, connectivity, and the biological monitoring of health and fitness of Ostrea edulis in the German North Sea. The project will identify the scientific and technological baselines for European oyster-stock recovery and is jointly conducted by the German Federal Agency for Nature Conservation (BfN), and the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI). Field studies at possible recovery sites provide the development of adequate technologies and allow studies on growth and health of the animals.
The biological and geographical magnitude of decline of oyster and oyster-created habitat means that restoration to historical abundance may not be possible (zu Ermgassen et al. 2016). In particular, the following tasks will be addressed, resembling several key elements that need increased attention in order to adjust the scale of marine restoration to levels that are ecologically, socially, and economically meaningful (Gillies et al. 2015):
- Development of a regulatory framework for species reintroduction
- Proof evidence on growth and fitness of oysters in the field (biological suitability)
- Identification of oyster seed suppliers for long-term restoration projects
- Hydrodynamic modelling of larval drift: Understanding of larval dispersal for future site connectivity
- Recommendations on adequate sites
- Recommendations on adequate technologies: substrate, reef design, and scale
- Build skills and experience in restoration methods
- Learn from terrestrial restoration initiatives
- Quantification and evaluation of ecosystem services from restored oyster habitats
- Build awareness, capacity, and confidence that key degraded coastal and marine habitats can be repaired
These fundamental aspects have to be addressed as preconditions for an appropriate large-scale and long-term restoration programme, reaching the size required to improve ecosystem services at the landscape scale.
Historically, oyster restoration projects addressed the declines in fishery landings and were carried out in shallow coastal waters (0–10 m). Today this motivation shifted to a more nature conservation-orientated view (zu Ermgassen et al. 2016). Accordingly, the current activities in native oyster restoration in Germany clearly aim at biodiversity enhancement, species diversity (reintroduction and protection of highly endangered animals and plants), and habitat protection (conservation, restoration, and integration/cross-linking of valuable natural habitats). The first restoration trials in German waters will be carried out in deeper offshore areas of the North Sea (20–40 m), within the area of the historical North Sea Oyster Ground (Olsen 1883) in the Natura 2000 site Borkum Riffgrund. The restoration of biogenic reefs, namely native oyster reefs, is a designated nature conservation measure for this particular marine protected area.
As Ostrea edulis is considered extinct in the German North Sea, the restoration will follow the guidelines of the International Union for Conservation of Nature (IUCN) of a species reintroduction (IUCN/SSC 2013) and the Berlin Oyster Recommendation (Pogoda et al. 2018). Accordingly, a detailed regulatory framework for Ostrea edulis reintroduction is implemented by responsible German authorities to prevent disease and invasive species translocation (Pogoda and von Nordheim 2019; Pogoda et al. 2018). The German restoration initiative is connected to several other European oyster restoration attempts, such as the recently started native oyster restoration in the Netherlands, but also to ongoing research and restoration activities in the UK, Ireland, France, and Portugal. In 2017, ten European nations agreed to establish an active network for knowledge and technology transfer: the Native Oyster Restoration Alliance (NORA) will increase success of marine nature conservation by facilitating oyster reef restoration and protection. It addresses subjects like governance, site selection, reef design, seed oyster production, health status, monitoring, and management. In addition, the European projects benefit from the collaboration with US partners. The wide experiences of traditional restoration projects in the US, e.g., in the Chesapeake Bay area or along the Gulf and even the West Coast, provide an immensely valuable knowledge transfer in the fields of technology, socioecological perspectives, biology, hydrodynamics, and site selection (The Nature Conservancy (TNC) 2014; zu Ermgassen et al. 2016; Lipcius et al. 2015).
Appropriate site selection is an essential precondition for long-term restoration and includes critical success factors for developing and sustaining native European oyster reefs in the North Sea (Pogoda et al. 2011). Conditions in the former distribution areas of oysters in the North Sea may differ from the conditions in historical times of high oyster abundances. Changes were set in motion with the degradation of the oyster beds and habitat destruction by oyster fishery. Today, bottom trawling still prevents the return of the native European oyster. Mandatory site selection criteria are the absence of bottom trawling fishery, the presence of suitable substrate (coarse sand, oyster shells, stones, or artificial reef structures), and environmental conditions that allow oyster growth, survival, reproduction, and recruitment. The deeper offshore areas of the German Bight offer appropriate conditions for restoration attempts as important abiotic factors like temperature and salinity exhibit less variation than in coastal waters (Pogoda et al. 2011). Constantly high salinities in offshore areas also provide some protection against infection with the pathogen Marteilia refringens, which prefers lower salinities (Gercken and Schmidt 2014). Respecting these criteria secures robustness and suitability of restoration locations. As offshore areas of the North Sea are intensively used by a variety of different stakeholders (Jentoft and Knol 2014), site selection may also depend on marine spatial planning and potential multi-use sites, e.g., offshore wind farms, where fishery is completely excluded by German law (Kamermans et al. 2018). The marine protected area Borkum Riffgrund (Natura 2000 site) in the German exclusive economic zone is located in the historical Offshore Oyster Ground and therefore a target site for large-scale oyster restoration. Recovering broodstock concentrations in these deeper waters will be the basis for successful restoration in the future.
Sufficient seed oyster supply was identified as a key limiting factor for native oyster restoration projects in Europe within the project RESTORE. Translocation between sites of seed oysters or any other size classes from wild beds should be discouraged to avoid increasing the pressure on still-existing wild beds and reduce the risk of spreading invasive species and disease. Following the Berlin Oyster Recommendation (Pogoda et al. 2018), action should be undertaken to establish hatcheries and spatting ponds for the production of robust and genetically diverse Ostrea edulis seed. In Germany, the project PROCEED, funded by the German Federal Program for Biological Diversity, will now implement a hatchery for sustainable seed oyster production on the Island of Helgoland to support a long-term restoration program for Ostrea edulis and its diverse and highly valuable ecosystem services. In particular, the following tasks will be addressed:
- Establishment of a healthy broodstock and a sufficient seed oyster production for North Sea restoration and nature conservation measures
- Biological and technological research with direct application of results
- Facilitation of native oyster restoration and reinforcement projects in Europe by coordinating and supporting the NORA network
- Active knowledge transfer to raise and to improve general awareness for native species and the importance of restoration. The native oyster will be characterized as an ecological key player and oyster reefs will be used as an example to explain biodiversity and other ecosystem services as stabilizing factors for the wider ecosystem.
6. Conclusions
Ecosystem services are defined as specific types: there are supporting, regulating, provisioning, and cultural services (Vilà et al. 2010). Today, we are aware that native oyster reefs provided multiple services out of all four types in the past and were therefore key elements of our marine coastal ecosystems (Coen et al. 2007). Human impact of extensive oyster fishery resulted in overexploitation and loss of oyster reefs. Human-induced large-scale transfers of oysters (e.g., within Europe, but also from and to the US, Africa, Australia, and Asia) to stabilize declining fisheries resulted in the spread of diseases and invasive species. In sum, this directly caused dramatic negative effects on the health and functionality of oyster reefs and the wider ecosystem. We actively shaped our coastal and shelf seas by driving native oyster beds to extinction and by destroying valuable biogenic hard substrate. The loss of these three-dimensional structures is critical and the ecological consequences of changes in biodiversity are complex (Tilman 1999). As humans show limited memories when it comes to environmental degradation, most cases of biodiversity decline are probably a lot worse than we assume or than existing data might suggest. This aspect must be respected when it comes to the management of marine protected areas and oyster reef restoration. By implementing successful restoration and mitigation measures, we will again actively shape our coastal and shelf seas in the future, certainly with corresponding positive effects for both humans and the North Sea ecosystem.
Future success will rely on a general upscale and connection of current restoration efforts as well as on a long-term sustainment of European oyster research and restoration. It is challenging and relevant to involve European-wide initiatives for the restoration of the native oyster and its habitat.
Funding
This research was funded by EU COST Action IS1403 Oceans Past Platform, grant number OPP COST Action IS1403-30556 and by the German Federal Agency for Nature Conservation (BfN), grant number FKZ 3516892001.
Acknowledgments
I thank the Marine Directorate of the German Federal Agency for Nature Conservation for the constant and substantial support of oyster restoration. Furthermore, I would like to thank the members of the Native Oyster Restoration Alliance (NORA) and the members of the Oceans Past Platform (OPP) for sharing their knowledge and thoughts. Janet H. Brown helped to improve the manuscript, thank you for your critical comments.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Airoldi, Laura, David Balata, and Michael W. Beck. 2008. The Gray Zone: Relationships between habitat loss and marine diversity and their applications in conservation. Journal of Experimental Marine Biology and Ecology 366: 8–15. [Google Scholar] [CrossRef]
- Alleway, Heidi K., and Sean D. Connell. 2015. Loss of an ecological baseline through the eradication of oyster reefs from coastal ecosystems and human memory. Conservation Biology 29: 795–804. [Google Scholar] [CrossRef] [PubMed]
- Beck, Michael W., Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew C. Kay, and et al. 2011. Oyster Reefs at Risk and Recommendations for Conservation, Restoration, and Management. BioScience 61: 107–16. [Google Scholar] [CrossRef]
- Becker, Cornelia, Angela von den Driesch, and Hans Christian Küchelmann. 2012. Mogador, eine Handelsstation am westlichen Rand der phönizischen und römischen Welt—Die Tierreste. In Current Discoveries from Outside and within: Field Explorations and Critical Comments from the Lab. Edited by Documenta Archaeobiologiae. Rahden: VML Verlag Marie Leidorf, pp. 11–159. [Google Scholar]
- Berghahn, Rüdiger, and Maarten Ruth. 2005. The disappearance of oysters from the Wadden Sea: A cautionary tale for no-take zones. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 91–104. [Google Scholar] [CrossRef]
- Blue Marine Foundation. 2016. A Blue Vision for the Solent and the Native Oyster. Available online: https://www.bluemarinefoundation.com/2016/06/07/9204/ (accessed on 1 November 2018).
- Boos, Karin, Cornelia Buchholz, Friedrich Buchholz, and Lars Gutow. 2004. Bericht über die Zusammensetzung des Helgoländer Makrozoobenthos im Vergleich historischer und aktueller Quellen—Klassifizierungsvorschlag nach der WRRL und Empfehlungen zum Monitoring. Edited by Landesamt für Natur und Umwelt des Landes Schleswig-Holstein. Flintbek: LANU, 75p. [Google Scholar]
- Bromley, Cass, Ciarán McGonigle, Elizabeth C. Ashton, and David Roberts. 2016. Bad moves: Pros and cons of moving oysters—A case study of global translocations of Ostrea edulis Linnaeus, 1758 (Mollusca: Bivalvia). Ocean & Coastal Management 122: 103–15. [Google Scholar]
- Coen, Loren D., Robert D. Brumbaugh, David Bushek, Ray Grizzle, Mark W. Luckenbach, Martin H. Posey, Sean P. Powers, and S. Gregory Tolley. 2007. Ecosystem services related to oyster restoration. Marine Ecology Progress Series 341: 303–7. [Google Scholar] [CrossRef]
- Cook, Robert, Jose M. Fariñas-Franco, Fiona R. Gell, Rohan H. F. Holt, Terry Holt, Charles Lindenbaum, Joanne S. Porter, Ray Seed, Lucie R. Skates, Thomas B. Stringell, and et al. 2013. The Substantial First Impact of Bottom Fishing on Rare Biodiversity Hotspots: A Dilemma for Evidence-Based Conservation. PLoS ONE 8: e69904. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union. 1992. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of wild Fauna and Flora. Brussels: European Parliament, pp. 7–50. [Google Scholar]
- Council of the European Union. 2008. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Brussels: European Parliament, pp. 19–40. [Google Scholar]
- Culloty, Sarah C., and Maire F. Mulcahy. 2007. Bonamia ostreae in the native oyster Ostrea edulis—A review. Marine Environment and Health Series 29: 1–36. [Google Scholar]
- de Bruyne, Rykel H., Sylvia J. van Leeuwen, Adriaan W. Gmelig Meyling, and Rogier Daan. 2013. Schelpdieren van het Noordzeegebied. Ecologische atlas van de mariene weekdieren (Mollusca). Lisse: Utrecht & Stichting Anemoon Uitgeverij Tirion, 414p. [Google Scholar]
- Eigaard, Ole R., Francois Bastardie, Mike Breen, Grete E. Dinesen, Niels T. Hintzen, Pascal Laffargue, Lars O. Mortensen, J. Rasmus Nielsen, Hans C. Nilsson, Finbarr G. O’Neill, and et al. 2016. Estimating seabed pressure from demersal trawls, seines, and dredges based on gear design and dimensions. ICES Journal of Marine Science 73: i27–43. [Google Scholar] [CrossRef]
- Elton, Charles S. 1958. The Ecology of Invasions by Animals and Plants. Chicago: The University of Chicago Press. [Google Scholar]
- Gercken, Jens, and Andreas Schmidt. 2014. Current Status of the European Oyster (Ostrea edulis) and Possibilities for Restoration in the German North Sea. Bfn-Skripten 379. Bonn: Bundesamt für Naturschutz BfN, 107p. [Google Scholar]
- Gerlach, Gudrun. 2001. Zu Tisch bei den alten Römern. In Sonderheft Archäologie in Deutschland. Stuttgart: Theiss, 112p. [Google Scholar]
- Gillies, Chris L., James A. Fitzsimons, Simon Branigan, Lynne Hale, Boze Hancock, Colin Creighton, Heidi Alleway, Melanie J. Bishop, Simon Brown, Dean Chamberlain, and et al. 2015. Scaling-up marine restoration efforts in Australia. Ecological Management & Restoration 16: 84–85. [Google Scholar]
- Hagmeier, Arthur, and Rudolf Kändler. 1927. Neue Untersuchungen im nordfriesischen Wattenmeer und auf den fiskalischen Austernbänken. Aus der biologischen Anstalt auf Helgoland und deren Zweiglaboratorium in List a. Sylt. Helgoland: Littmann, vol. 16, pp. 1–90. [Google Scholar]
- Haenen, Olga, Marc Engelsma, and Steven van Beurden. 2011. Ziekten van vissen, schaal- en schelpdieren, van belang voor de Nederlandse aquacultuur. Wageningen: Central Veterinary Institute. [Google Scholar]
- IUCN/SSC. 2013. Guidelines for Reintroductions and Other Conservation Translocations. Gland: IUCN Species Survival Commission, 57p. [Google Scholar]
- Jentoft, Svein, and Maaike Knol. 2014. Marine spatial planning: Risk or opportunity for fisheries in the North Sea? Maritime Studies 13: 1. [Google Scholar] [CrossRef]
- Kahn, Peter H., Jr. 2002. Children’s affiliations with nature: Structure, development, and the problem of environmental generational amnesia. In Children and Nature: Psychological, Sociocultural, and Evolutionary Investigations. Cambridge: MIT Press, pp. 93–116. [Google Scholar]
- Kahn, Peter H., and Batya Friedman. 1995. Environmental views and values of children in an inner city Black community. Child Development 66: 1403–17. [Google Scholar] [CrossRef]
- Kamermans, Pauline, Brenda Walles, Marloes Kraan, Luca van Duren, Frank Kleissen, Tom van der Have, Aad Smaal, and Marnix Poelman. 2018. Offshore Wind Farms as Potential Locations for Flat Oyster (Ostrea edulis) Restoration in the Dutch North Sea. Sustainability 10: 3942. [Google Scholar] [CrossRef]
- Kennedy, Richard J., and David Roberts. 1999. A Survey of the Current Status of the Flat Oyster Ostrea edulis in Strangford Lough, Northern Ireland, with a View to the Restoration of Its Oyster Beds. Biology and Environment: Proceedings of the Royal Irish Academy 99B: 79–88. [Google Scholar]
- Kennedy, Richard J., and David Roberts. 2006. Commercial oyster stocks as a potential source of larvae in the regeneration of Ostrea edulis in Strangford Lough, Northern Ireland. Journal of the Marine Biological Association of the United Kingdom 86: 153–59. [Google Scholar] [CrossRef]
- Kerckhof, Francis, Joop W. P. Coolen, Bob Rumes, and Stephen Degraer. 2018. Recent findings of wild European flat oysters Ostrea edulis (Linnaeus, 1758) in Belgian and Dutch offshore waters: New perspectives for offshore oyster reef restoration in the southern North Sea. Belgian Journal of Zoology 148: 13–24. [Google Scholar] [CrossRef]
- Laing, Ian, Peter L. Dunn, Edmund J. Peeler, Stephen W. Feist, and Matt Longshaw. 2014. Epidemiology of Bonamia in the UK, 1982 to 2012. Diseases of Aquatic Organisms 110: 101–11. [Google Scholar] [CrossRef]
- Lipcius, Romuald, Russell Burke, Danielle McCulloch, Sebastian Schreiber, David Schulte, Rochelle Seitz, and Jian Shen. 2015. Overcoming restoration paradigms: Value of the historical record and metapopulation dynamics in native oyster restoration. Frontiers in Marine Science 2. [Google Scholar] [CrossRef]
- Lotze, Heike K., Karsten Reise, Boris Worm, Justus van Beusekom, Mette Busch, Anneli Ehlers, Dirk Heinrich, Richard C. Hoffmann, Poul Holm, Charlotte Jensen, and et al. 2005. Human transformations of the Wadden Sea ecosystem through time: A synthesis. Helgoland Marine Research 59: 84–95. [Google Scholar] [CrossRef]
- Möbius, Karl A. 1877. Die Austern und die Austernwirtschaft. Berlin: Hepel und Parey Verlag von Wiegandt, 126p. [Google Scholar]
- Mortensen, Stein, Åsa Strand, Torjan Bodvin, Anders Alfjorden, Cecilie K. Skår, Anders Jelmert, Anna Aspán, Lisbeth Sælemyr, Lars J. Naustvoll, and Jon Albretsen. 2016. Summer mortalities and detection of ostreid herpesvirus microvariant in Pacific oyster Crassostrea gigas in Sweden and Norway. Diseases of Aquatic Organisms 117: 171–76. [Google Scholar] [CrossRef]
- Neild, Robert Ralph. 1995. The English, the French, and the Oyster. London: London Quiller Press, p. 212. [Google Scholar]
- Olsen, Ole Theodor. 1883. The Piscatorial Atlas of the North Sea, English and St. George’s Channels. London: Taylor and Francis. [Google Scholar]
- OSPAR. 2009. Background document for Ostrea edulis and Ostrea edulis beds. In Biodiversity Series 428. Edited by Jan Haelters and Francis Kerckhof. Paris: OSPAR Commission, 22p. [Google Scholar]
- OSPAR. 2010. The North-East Atlantic Environment Strategy-Strategy of the OSPAR Commission for the Protection of the Marine Environment of the North-East Atlantic 2010–2020. Paris: OSPAR Commission, pp. 1–27. [Google Scholar]
- Papworth, Sarah K., Janna Rist, Lauren Coad, and Eleanor J. Milner-Gulland. 2009. Evidence for shifting baseline syndrome in conservation. Conservation Letters 2: 93–100. [Google Scholar] [CrossRef]
- Pauly, Daniel. 1995. Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology & Evolution 10: 430. [Google Scholar]
- Pogoda, Bernadette, Janet H. Brown, Boze Hancock, and Henning von Nordheim, eds. 2018. Berlin Oyster Recommendation on the Future of Native Oyster Restoration in Europe. Paper presented at Kick-Off Workshop “Native Oyster Restoration in Europe—Current Activities and future Perspectives”, Berlin, Germany, 1–3 November 2017. [Google Scholar]
- Pogoda, Bernadette, Bela H. Buck, and Wilhelm Hagen. 2011. Growth performance and condition of oysters (Crassostrea gigas and Ostrea edulis) farmed in an offshore environment (North Sea, Germany). Aquaculture 319: 484–92. [Google Scholar] [CrossRef]
- Pogoda, Bernadette, and Henning von Nordheim. 2019. Native oyster and habitat restoration in the German North Sea: Defining a conservation framework. Unpublished paper. [Google Scholar]
- Ruesink, Jennifer L., Hunter S. Lenihan, Alan C. Trimble, Kimberly W. Heiman, Fiorenza Micheli, James E. Byers, and Matthew C. Kay. 2005. Introduction of non-native oysters: Ecosystem effects and restoration implications. Annual Review of Ecology and Systematics 36: 643–89. [Google Scholar] [CrossRef]
- The Nature Conservancy (TNC). 2014. Inventory of Shellfish Restoration Permitting & Programs in the Coastal States. Edited by Mississippi-Alabama Sea Grant Legal Program. Troy: Troy University, 189p. [Google Scholar]
- Thurstan, Ruth H., Julie P. Hawkins, Lee Raby, and Callum M. Roberts. 2013. Oyster (Ostrea edulis) extirpation and ecosystem transformation in the Firth of Forth, Scotland. Journal for Nature Conservation 21: 253–61. [Google Scholar] [CrossRef]
- Tilman, David. 1999. The Ecological Consequences of Changes in Biodiversity: A Search for General Principles. Ecology 80: 1455–74. [Google Scholar] [CrossRef]
- Vilà, Montserrat, Corina Basnou, Petr Pyšek, Melanie Josefsson, Piero Genovesi, Stephan Gollasch, Wolfgang Nentwig, Sergej Olenin, Alain Roques, David Roy, and et al. 2010. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment 8: 135–44. [Google Scholar] [CrossRef]
- Wolff, Wim J., and Karsten Reise. 2002. Oyster Imports as a Vector for the Introduction of Alien Species into Northern and Western European Coastal Waters. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Edited by Erkki Leppäkoski, Stephan Gollasch and Sergej Olenin. Dordrecht: Springer. [Google Scholar]
- Woolmer, Andrew P., Martin Syvret, and Andrew FitzGerald. 2011. Restoration of Native Oyster, Ostrea edulis, in South Wales: Options and Approaches. CCW Contract Science Report No. 960. Newtown: Countryside Council for Wales, 93p. [Google Scholar]
- Yonge, Charlotte M. 1960. Oysters. London: Collins, 209p. [Google Scholar]
- zu Ermgassen, Philine S. E., Boze Hancock, Bryan DeAngelis, Jennifer Greene, Elisabeth Schuster, Mark Spalding, and Robert Brumbaugh. 2016. Setting Objectives for Oyster Habitat Restoration Using Ecosystem Services: A Manager’s Guide. Arlington: TNC, 76p. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).