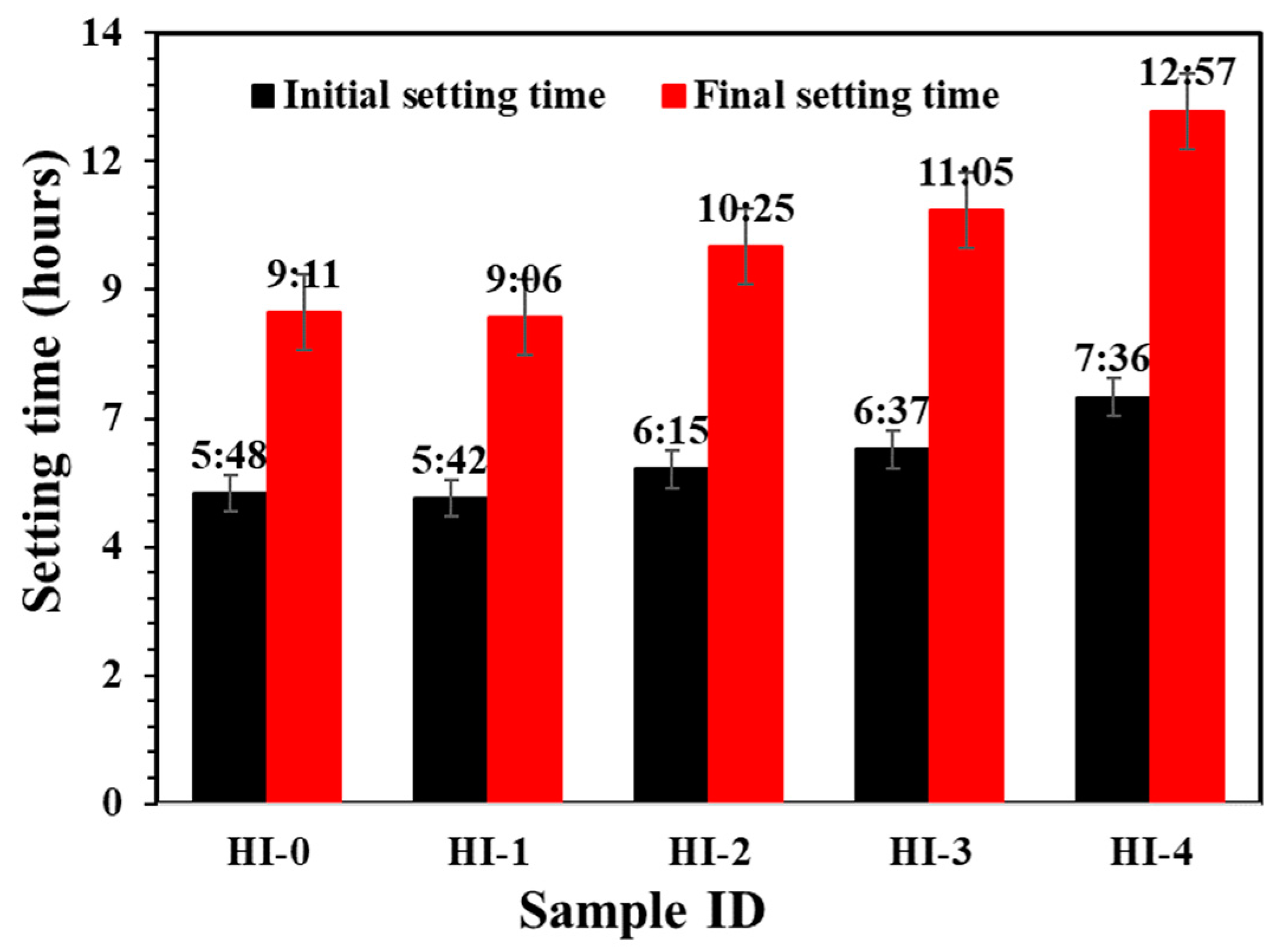
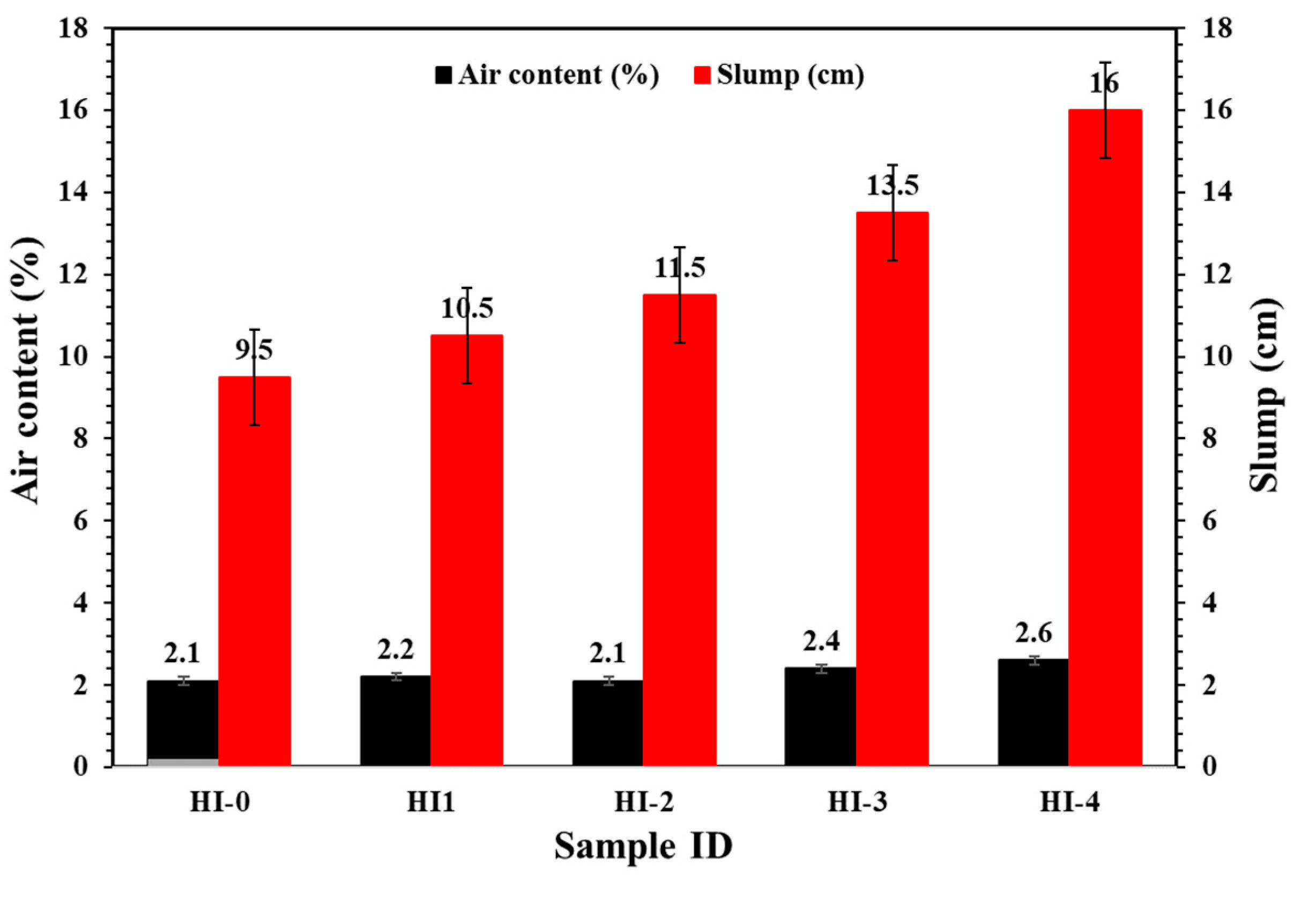
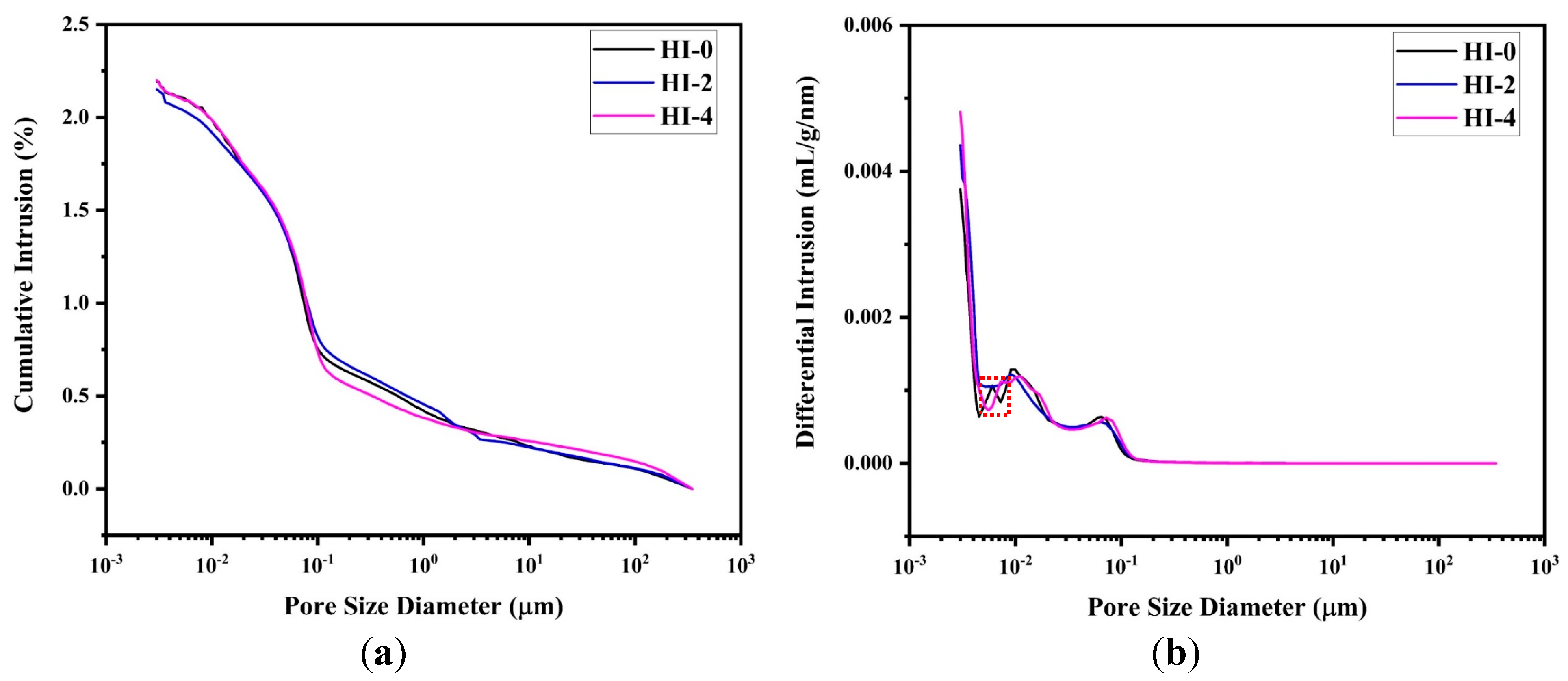
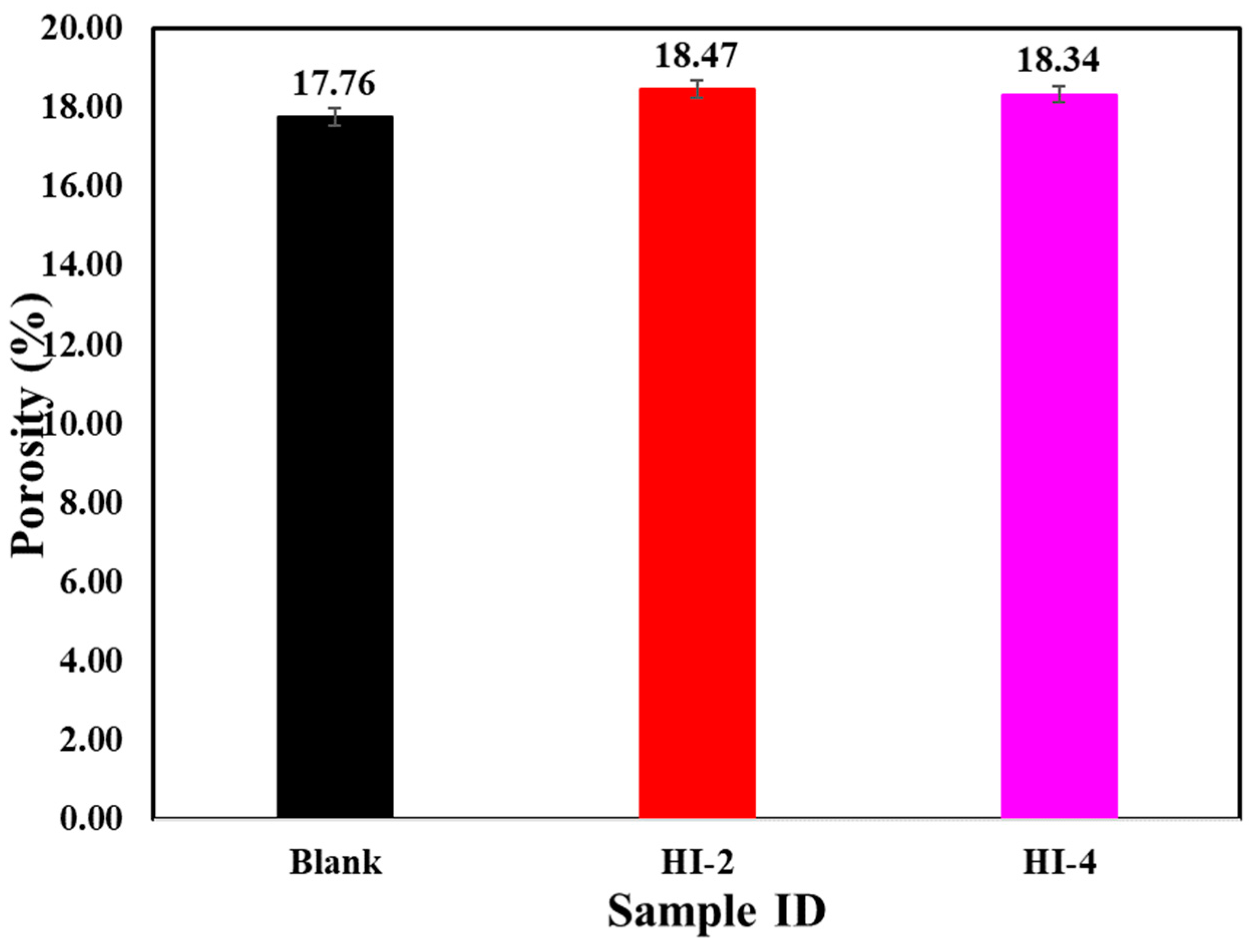
3.2.2. Electrochemical Impedance Spectroscopy (EIS)
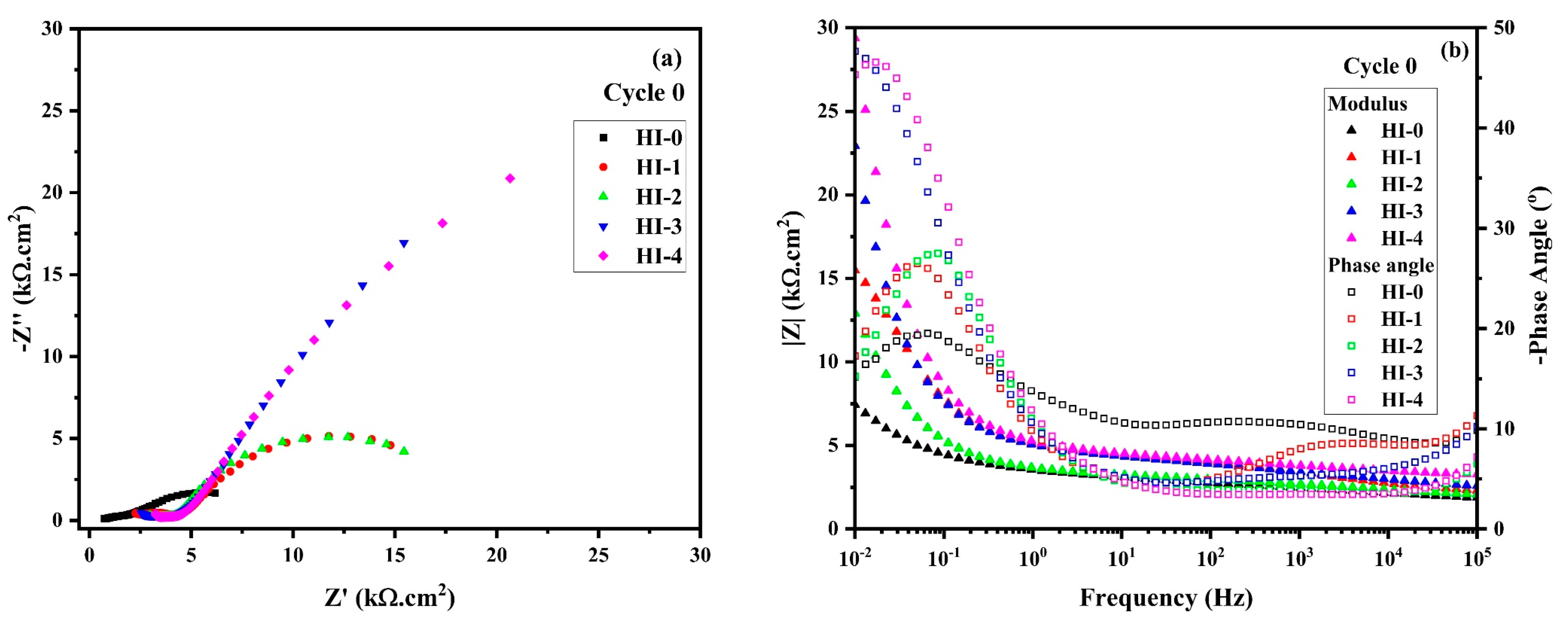
The Nyquist plot of RC specimens with different amounts of hybrid inhibitor after cycle 0 of the wet–dry condition is shown in
Figure 10a. It can be seen that HI-0 shows the smallest magnitude of complex-plane impedance due to the absence of an inhibitor. In this case, the oxide/hydroxide film can be formed onto the steel rebar in the alkaline condition of concrete. However, this film is unstable in wet-dry cycle once immersed in NaCl solution [
61]. On contrary, the RC specimens containing a hybrid inhibitor exhibit a larger magnitude of complex-plane impedance in comparison to HI-0, owing to the adsorption of inhibitor molecules and formation of the passive film. Alternatively, despite the same protection mechanism of the passive film, HI-1 and HI-2 specimens exhibit smaller magnitudes of complex-plane impedance compared to HI-3 and HI-4, attributed to the lower amount of hybrid inhibitor in the concrete mix. As the amount of inhibitor is increasing, the content of LA and TSP in concrete is increasing, which leads to the formation of a greater amount of P-zwitterion-(Cl)-Fe, Zwitterion-(Cl)-Fe, and FePO
4 onto the steel rebar. The chloride ions in the solution (10 wt. % NaCl to perform the corrosion test) reach the steel rebar surface and form aforementioned complexes, as described elsewhere [
16]. Alternatively, in the case of HI-0, there is no inhibitor; therefore, the chloride ions and wet–dry cycle accelerate the corrosion reaction. The magnitude of the low-frequency semi-arc is greater than the middle and high frequencies of the inhibitor-containing specimens, suggesting the charge transfer resistance (R
ct) is caused by film formation onto the steel rebar surface.
The Bode impedance modulus plot of RC specimens mixed with different amounts of hybrid inhibitor after 0 cycles of wet–dry test is shown in
Figure 10b. As it can be observed in
Figure 10b, the HI-0 specimen exhibits the lowest impedance modulus at the lowest frequency, whereas the hybrid inhibitor-containing specimens exhibit higher values compared to HI-0, owing to adsorption mechanism [
17,
62]. The high- and middle-frequency impedance values of all the specimens are found to be identical, attributed to the oxide film formation, whereas at the low frequency, the total impedance of inhibitor-containing specimens is gradually increased, while HI-3 and HI-4 exhibit a significant increment in the value. It is attributed to the R
ct caused at low frequency. In the case of a higher amount of inhibitor, the LA and TSP both are in high amounts, which leads to the formation of the P-zwitterion-(Cl)-Fe and Zwitterion-(Cl)-Fe complexes onto the steel rebar in the presence of chloride ions. The HI-4 specimen exhibits the highest impedance modulus, suggesting its corrosion resistance. In this case, the excessive amount of LA repels the water molecules [
63] from the steel rebar surface, reduces the cathodic reaction [
64], and the chloride ions in the studied solution reach the steel rebar. The chloride ions first interact with Fe due to being smaller in size compared to zwitterions, i.e., LA, which restrict the direct adsorption of zwitterion [
65], followed by the interaction of zwitterion with chloride to form a Zwitterion-(Cl)-Fe complex [
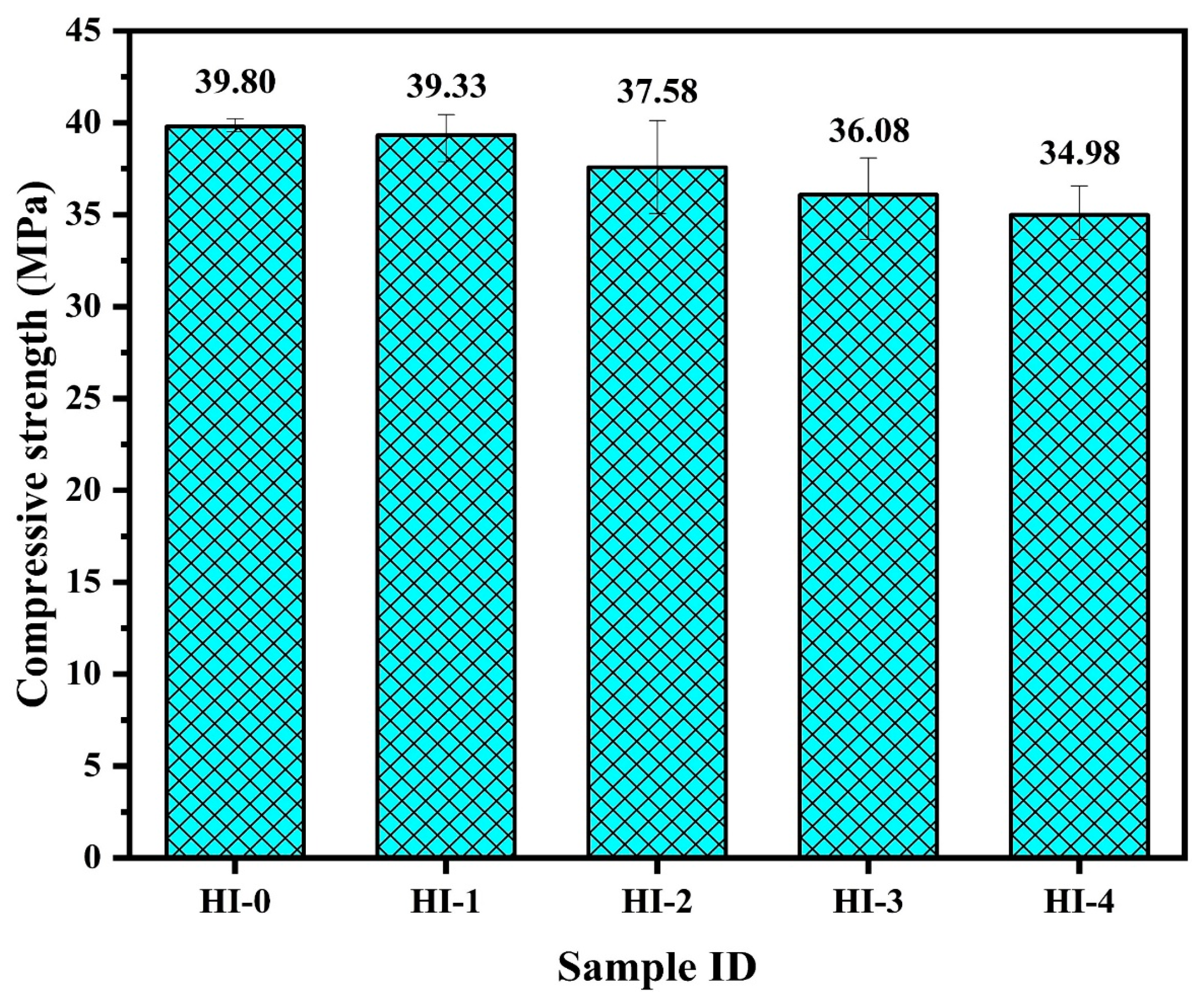
62]. The phosphate ions in the inhibitor react with zwitterion because the size of phosphate is greater than chloride, thus, it forms a P-Zwitterion-(Cl)-Fe complex. This complex has a greater stability than the former one. However, the compressive strength result of the HI-4 specimen does not meet the demands of mechanical property in accordance with KS F 2561 [
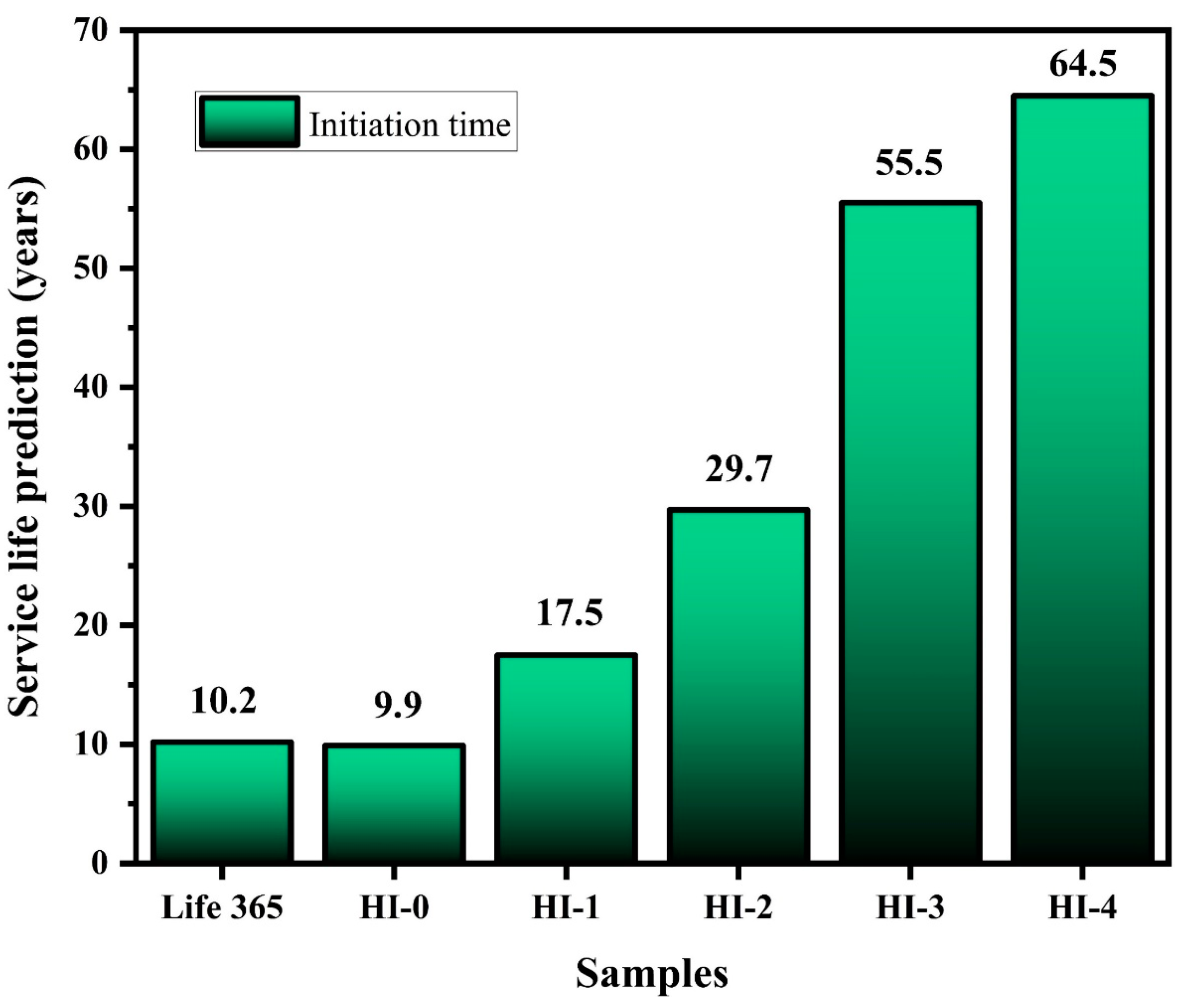
57]. Thus, if considering the mechanical properties, it can be said that the HI-3 specimen could be used in the RC structure, otherwise, if we control the mechanical properties by altering the W/C ratio, then HI-4 is the best concrete mix design for the higher corrosion protection in the aggressive condition.
The Bode phase angle plot of RC specimens with different amounts of hybrid inhibitor at cycle 0 is depicted in
Figure 10b. The HI-0 specimens exhibit two times, constant at high-to-middle and low frequency. The high- and middle-frequency capacitive loop suggests the formation of oxide film onto the steel rebar, owing to the hydration of the cement, where some hydration product deposits and causes the barrier. However, at the low frequency, the phase angle maxima are found to be lowest and the maximum can be reached at around −20°. This result suggests that the corrosion protection is mostly exerted by the steel rebar. Alternatively, in the case of inhibitor specimens, the phase angle maxima mostly shifted towards higher angle at the lowest frequency, attributed to the adsorption of the inhibitor molecules on the steel rebar. Therefore, resistance is caused at the steel rebar/concrete interface. As the inhibitor amount is increased, the phase angle maxima shifted towards a higher angle and are found around −50° at the lowest frequency for HI-3 and HI-4 specimens.
The Nyquist plot of RC specimens with different amounts of hybrid inhibitor after 7 cycles of wet–dry test is depicted in
Figure 11a. All the RC specimens were immersed in 10 wt. % NaCl, where a significant amount of aggressive chloride ions exists, which perturb the film; therefore, a reduction in the magnitude of complex-plane impedance is observed. The HI-0 specimen exhibits a large reduction in the magnitude of complex-plane impedance compared to cycle 0, owing to the absence of the inhibitor. Alternatively, it also exhibits a smaller radius in complex-plane impedance than those of hybrid inhibitor-containing specimens. The film formed on the HI-0 specimen is unstable, and heterogeneous in the presence of aggressive chloride ions, which influence the hydration reaction of the oxide film and causes the cracking [
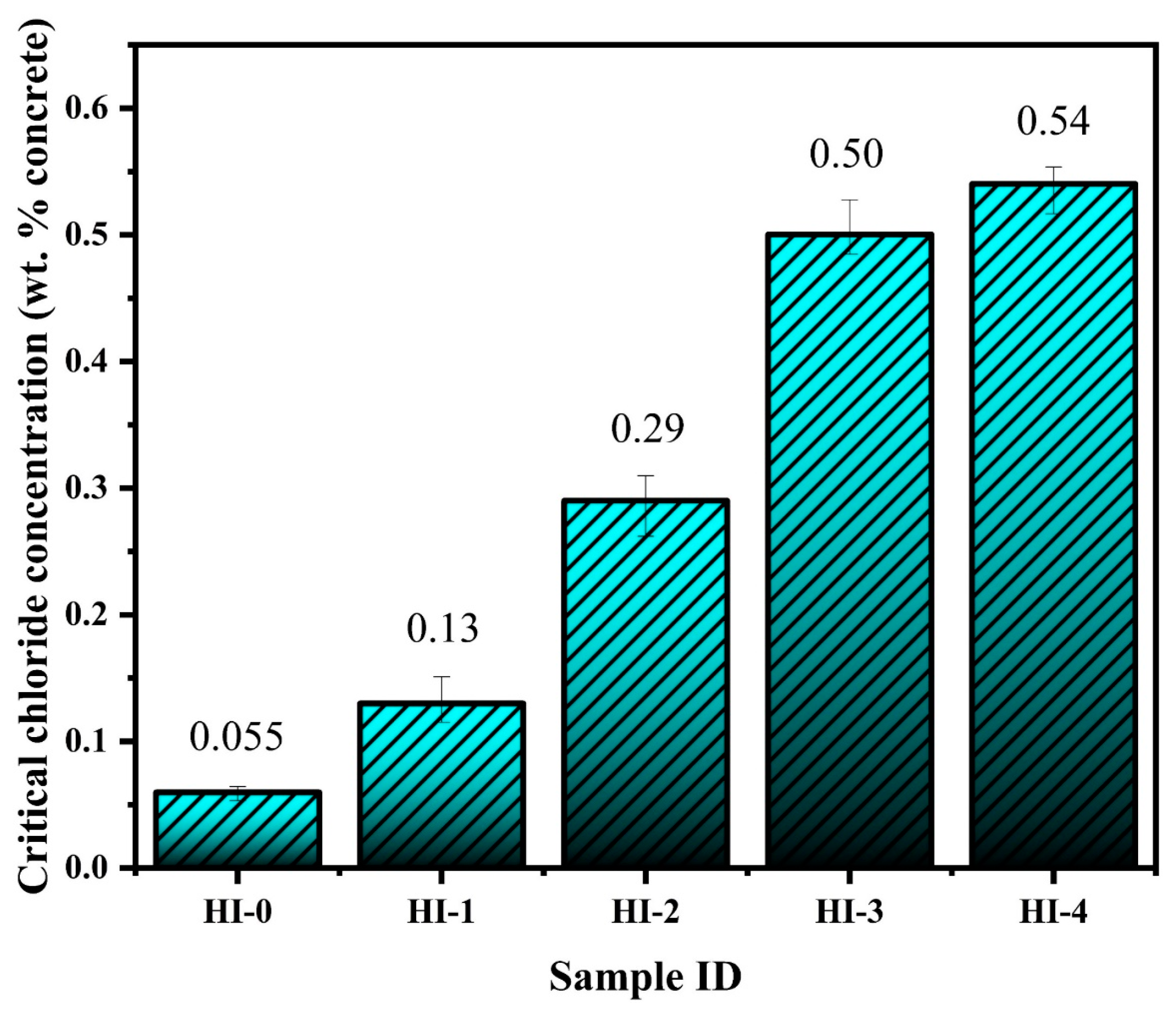
66]. The low-frequency semi-circle magnitude of the HI-0 specimen is significantly reduced, suggesting the deterioration of the steel rebar in an accelerated condition. There is a certain chloride threshold of the inhibitor, and earlier we have found that this hybrid inhibitor exhibits 6 wt. % NaCl in the concrete pore solution [
24], but, in the present study, we have considered 10 wt. % NaCl as well as wet–dry conditions to accelerate the reaction. Therefore, the inhibitor-containing specimens also exhibit a reduction in the magnitude of complex-plane impedance plots, as shown in
Figure 11a. Moreover, the complex-plane semi-arc of the inhibitor-containing specimens mostly exhibited Z’
real, suggesting the formation of uniform film, but it is defective owing to the Cl
− ions, which attenuate the properties of the adsorbed layer onto the steel rebar surface. As it can be seen, the HI-3 and HI-4 specimens exhibit the same magnitude of complex-plane impedance, suggesting that they are exhibiting identical corrosion resistance properties. The low-frequency complex-plane magnitude is greater than the high-frequency, suggesting that the inhibitor molecules are providing corrosion protection by forming P-Zwitterions-(Cl)-Fe and Zwitterion-(Cl)-Fe complexes film onto the steel rebar. Alternatively, in the case of HI-1 and HI-2 specimens, the inhibitor amount is not significant enough to form greater P-Zwitterions-(Cl)-Fe and Zwitterions-(Cl)-Fe complexes to resist the attack of chloride ions in accelerated conditions for corrosion protection.
The Bode impedance modulus plot of RC specimens with different amounts of hybrid inhibitor immersed in 10 wt. % NaCl solution after 7 cycles of wet–dry test is depicted in
Figure 11b. There is a reduction in the total impedance of all the specimens after 7 cycles of wet–dry, attributed to the accelerated test where the specimens were immersed in 10 wt. % NaCl solution for the corrosion studies. The excessive amount of Cl
− ions ingress through the pore matrix of the concrete and reach the steel rebar surface and attenuate the passive film. However, the inhibitor-containing specimens exhibit a higher total impedance. As the inhibitor amount is increased, the total impedance is also increased, but, while comparing with 1 cycle of wet–dry test, it is lower. The protection by the hybrid inhibitor might be provided by the formation of complexes onto the steel rebar, where phosphate ions and LA (zwitterion) interact with chloride ions and Fe. If the amount of inhibitor is higher, then the possibility for the formation of complexes is greater. Moreover, these concentrations of inhibitor can sustain a certain amount of chloride ions in the concrete. The wet–dry condition accelerates the corrosion reaction; therefore, this is another reason for the reduction in the total impedance.
The Bode phase angle plots of RC specimens immersed in 10 wt. % NaCl after 7 cycles of wet–dry test are demonstrated in
Figure 11b. The phase angle maxima of all the specimens are gradually decreased at low frequency, suggesting the breakdown of passive film, owing to the significant amount of chloride ions as well as wet–dry cycles. However, the HI-0 specimen exhibits the lowest phase angle maxima and the maximum is found at −7° on 0.01 Hz. This result suggests that neither the steel rebar nor passive film provide corrosion protection attributed to the accelerated condition, where it breaks down and initiates the corrosion reaction at the steel rebar/concrete interface. On the other hand, the inhibitor-containing specimen phase angle maxima are found at a higher angle compared to HI-0, suggesting the charge transfer resistance (
Rct). It means corrosion protection is provided by R
ct where film is being formed.
The Nyquist plot of RC specimens after 15 cycles of wet–dry test is shown in
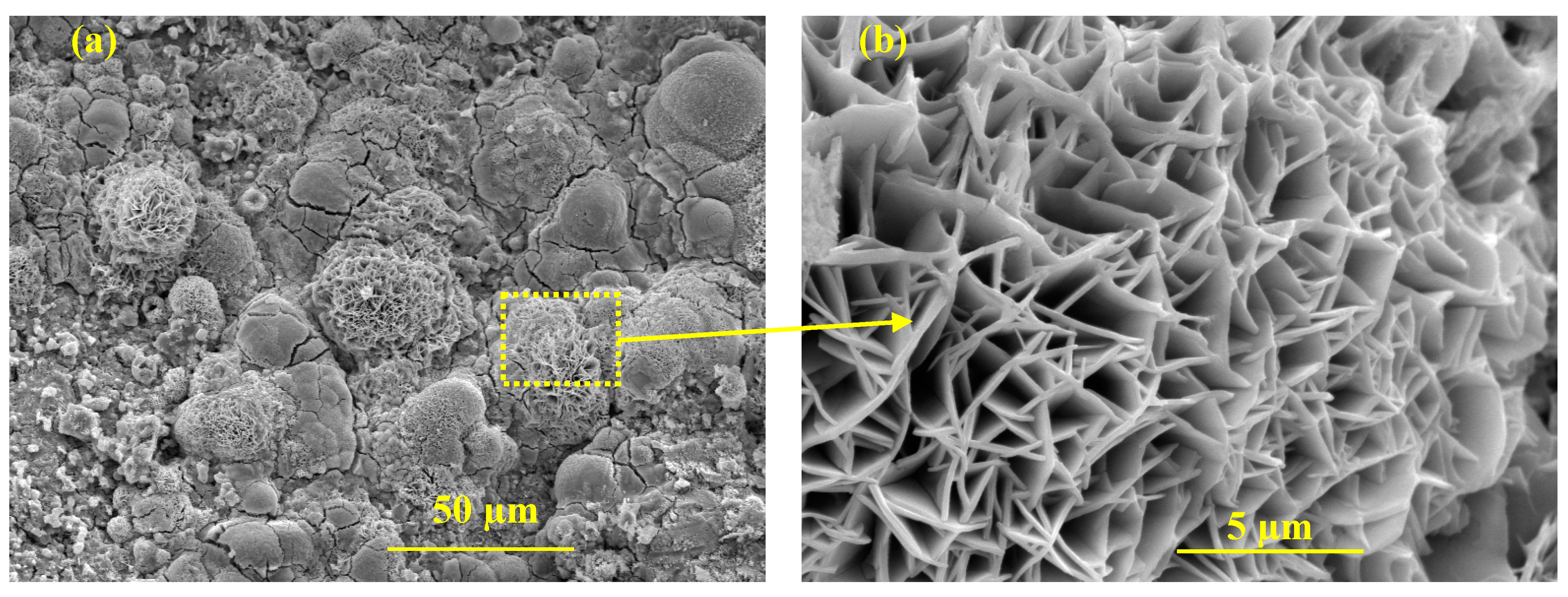
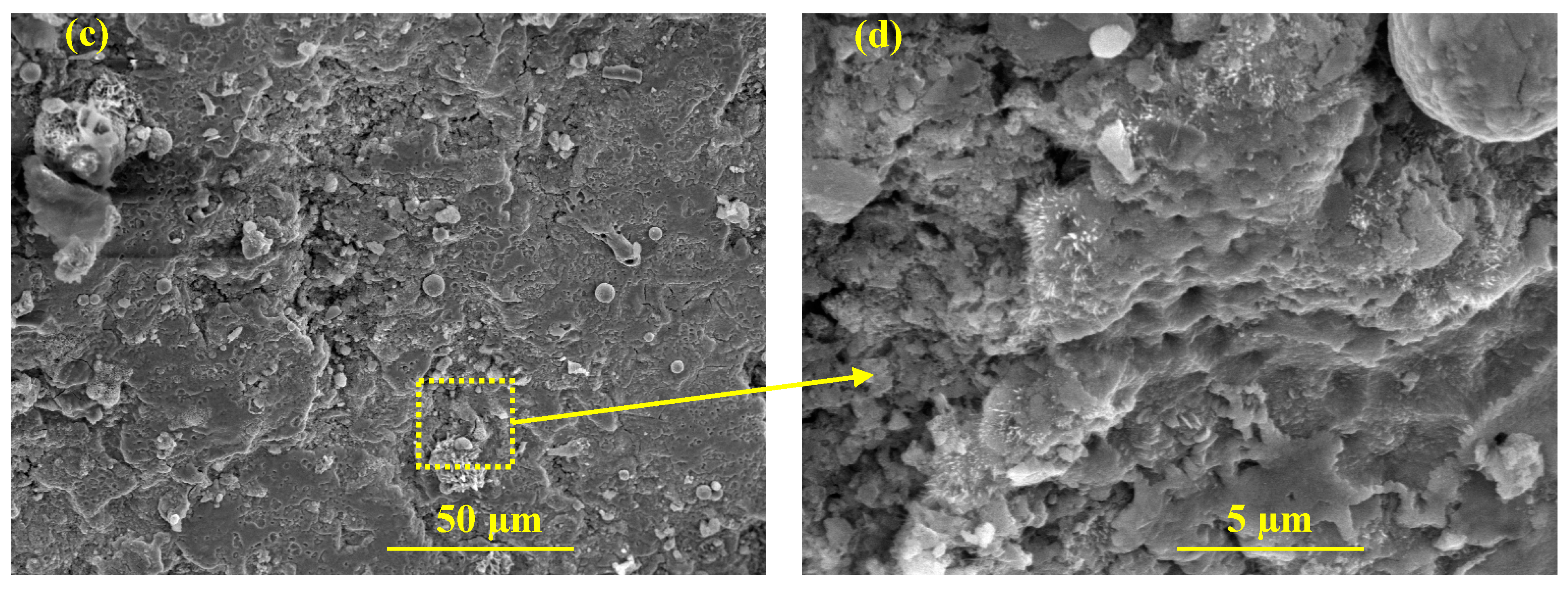
Figure 12a. Cycle 15 is selected as the last cycle for the electrochemical dynamic and kinetics observation of the passive film onto the steel rebar in RC structures due to the active corrosion of HI-0. The HI-0 specimen was broken down to investigate the morphology of the passive film by SEM and determined the chloride threshold. After 15 cycles of wet–dry test, the Nyquist plot magnitude of HI-0 specimen is slightly increased compared to 7 cycles attributed to the formation of oxide/corrosion products onto the steel rebar/concrete interface as confirmed by LPR. This oxide film caused resistance to corrosion. The identical inference can be drawn for HI-1 where the inhibitor amount is limited, and in the accelerated condition, it could not provide longer protection. However, the complex-plane magnitude of the HI-1 specimen is greater than HI-0, suggesting that even with a limited amount of inhibitor, it is better than HI-0 after 15 cycles of wet–dry as well as immersing them in 10 wt. % NaCl solution for the electrochemical studies. Alternatively, from HI-2 to HI-4, the magnitude of the complex impedance plot is slightly decreased, attributed to the effect of chloride ions, which attenuate the passive film and cause the defects. However, the low-frequency capacitive loop of inhibitor-containing specimens is higher compared to middle- and high-, suggesting that passive film at steel rebar/concrete interface provides protection owing to the adsorption of the inhibitor molecules. In this case, there is a possibility for the formation of Zwitterions-(Cl)-Fe and P-zwitterions-(Cl)-Fe complexes onto the steel rebar. However, it is very difficult to determine the formation of these complexes onto the ribbed steel bar by analytical techniques rather than assumption. Moreover, our earlier publications confirm the formation of these complexes in a simulated concrete pore solution [
16,
24].
The Bode impedance modulus plot of RC specimens with different amounts of hybrid inhibitor after 15 cycles of wet–dry is depicted in
Figure 12b. The HI-0 specimen continuously shows the lowest total impedance value due to the destruction of the oxide/hydroxide film, induced by chloride ions. It can be seen that there is a large difference in total impedance between HI-0 and hybrid inhibitor-containing specimens, attributed to the formation of P-zwitterions-(Cl)-Fe and Zwitterions-(Cl)-Fe complexes, which are more durable and protective than iron oxide/hydroxide film. However, the amount of hybrid inhibitor mixed in the concrete matrix also influences the corrosion resistance property against chloride ions; evidentially, the total impedance values are considerably higher than without inhibitor, i.e., HI-0. In addition, it is also evident that the high amount of inhibitor leads to the formation of greater P-zwitterions-(Cl)-Fe and Zwitterion-(Cl)-Fe complexes as the adsorbed film of HI-3 and HI-4 than that of HI-1 and HI-2. Paradoxically, the HI-4 specimen contains a two-times higher inhibitor amount compared to HI-3, where along with P-zwitterions-(Cl)-Fe and Zwitterions-(Cl)-Fe complexes, tertiary iron phosphate (FePO
4) could form once the corrosion initiation started and provide corrosion protection [
16,
24].
The Bode phase angle plot of RC specimens mixed with hybrid inhibitor after 15 cycles of wet–dry test is demonstrated in
Figure 12b. The phase angle value of HI-0 is found to be around −5° at 0.01 Hz, divulging that the embedded steel rebar is severely corroded and the unstable oxides/hydroxides film could form onto the steel rebar. There is a possibility that the corrosion products are heterogeneous and unstable. Moreover, the phase angle maxima of hybrid inhibitor-containing specimens are higher than HI-0. This result suggests that the passive film formed onto the inhibitor-containing specimens is protective and stable even after 15 cycles of wet–dry test. The longer duration of accelerated test perturbs the passive film and leads to the corrosion reaction. Therefore, the 15 cycles of wet–dry and immersion in 10 wt. % NaCl solution led to the defect formation in the passive film. Therefore, all the specimens exhibited a reduction in phase angle maxima at low frequency. Moreover, the higher amount of inhibitor exhibited greater protection.