Effect of Mix Design Parameters on the Properties of Dam Sediment/Slag-Based Geopolymer Mortars
Abstract
1. Introduction
2. Materials and Test Methods
2.1. Materials
- The first component was NaOH granules with a purity of 98%, supplied by HEX-Static. These granules were dissolved in demineralized water to prepare NaOH solutions of different concentrations (6M, 8M, 10M, 12M, and 14M).
- The second solution was a mixture of sodium silicate and sodium hydroxide, with a concentration of 12 M (Na2SiO3 + NaOH 12 M) and a mass ratio (silicate/hydroxide) equal to 2 (S/H = 2).
- The third one was composed only of sodium silicate (Na2SiO3), which was supplied by the company Sarl Ginie Chimie, with the following proportions: SiO2 = 28.3%, Na2O = 14.85%, and H2O = 56.75% (% by weight).
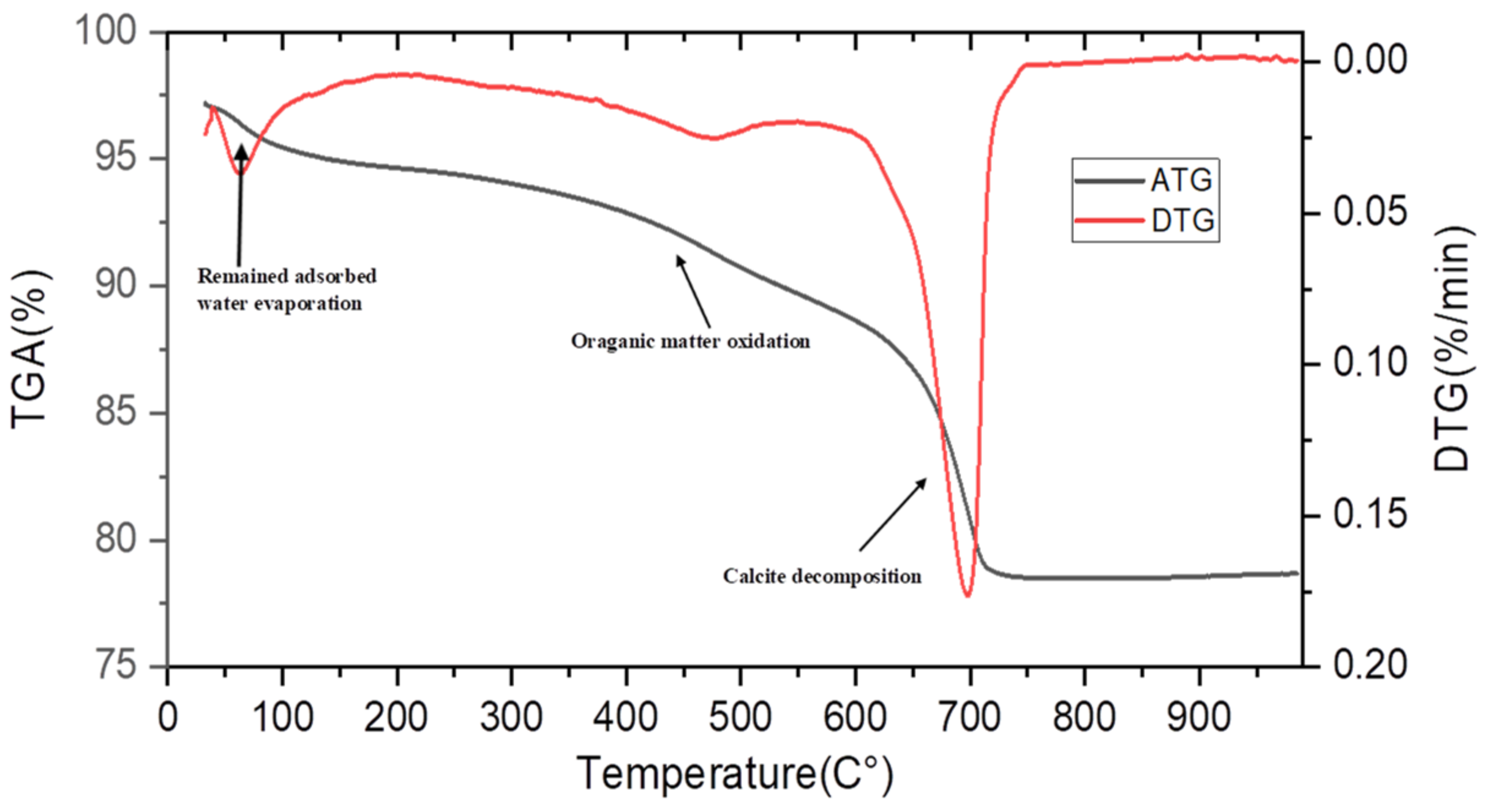
2.1.1. Specific Surface Area and Density of Raw and Calcined Sediment, and GGBFS
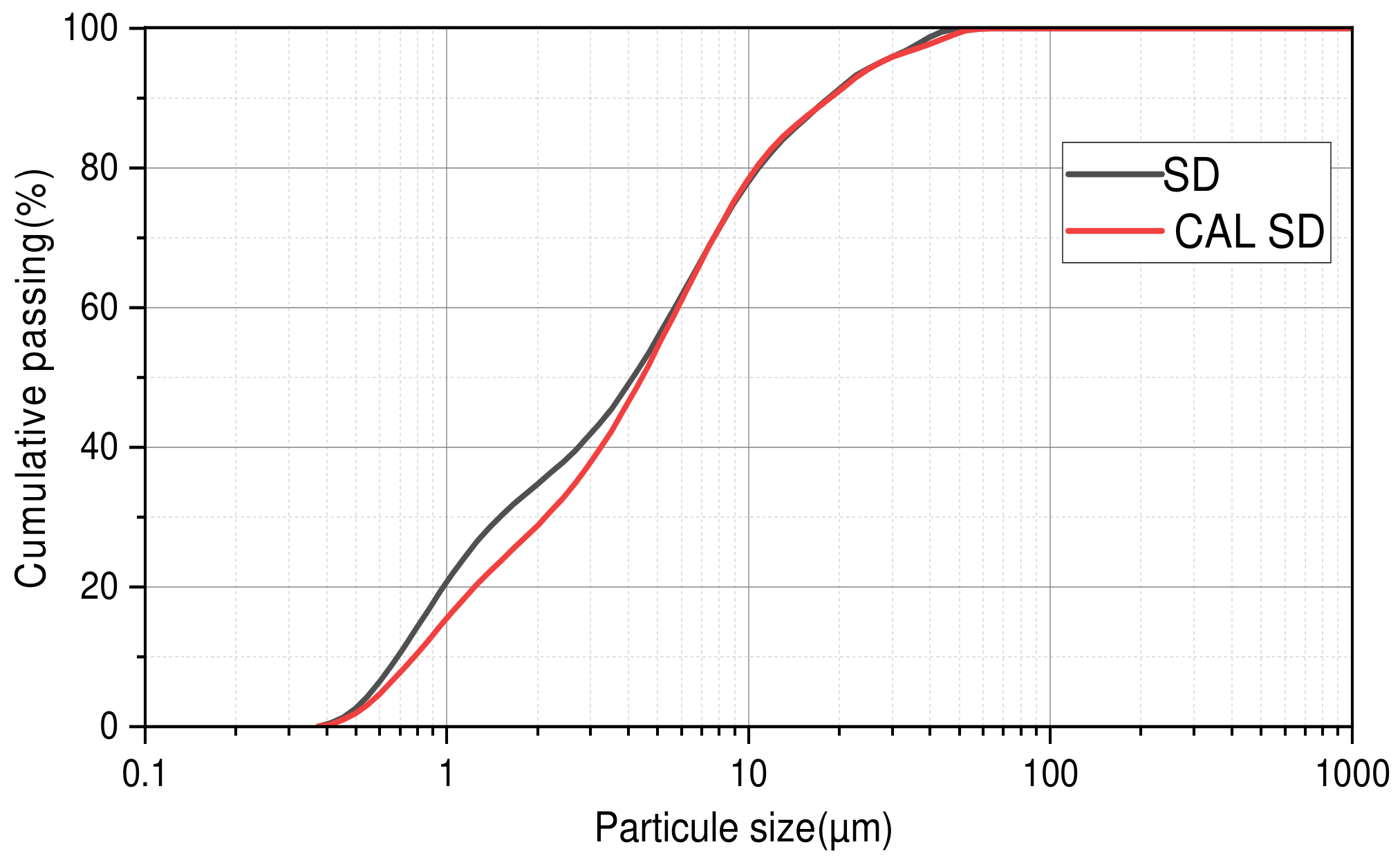
2.1.2. Particle Size Distribution of the Materials
2.1.3. Chemical Composition of Solid Materials
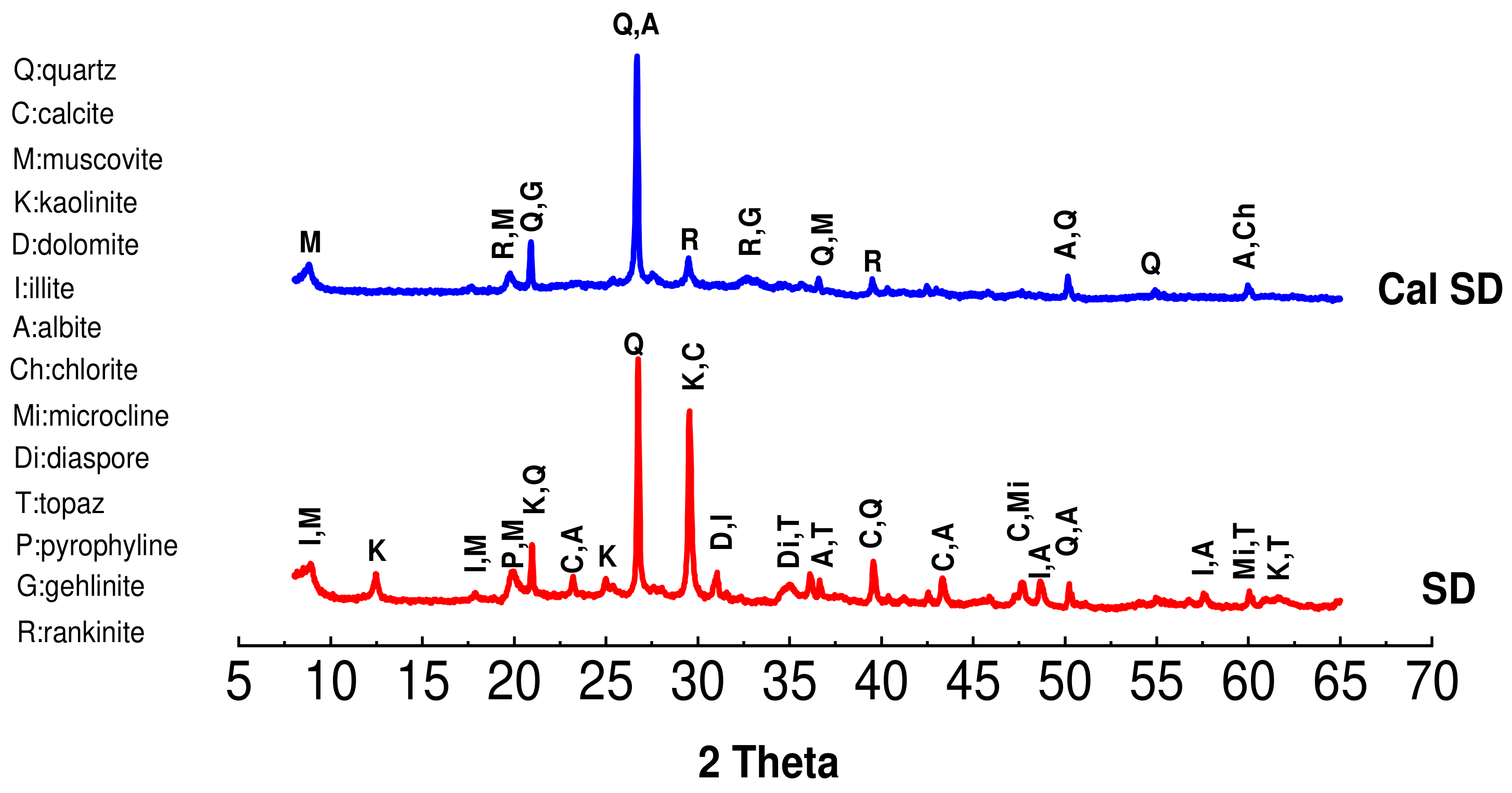
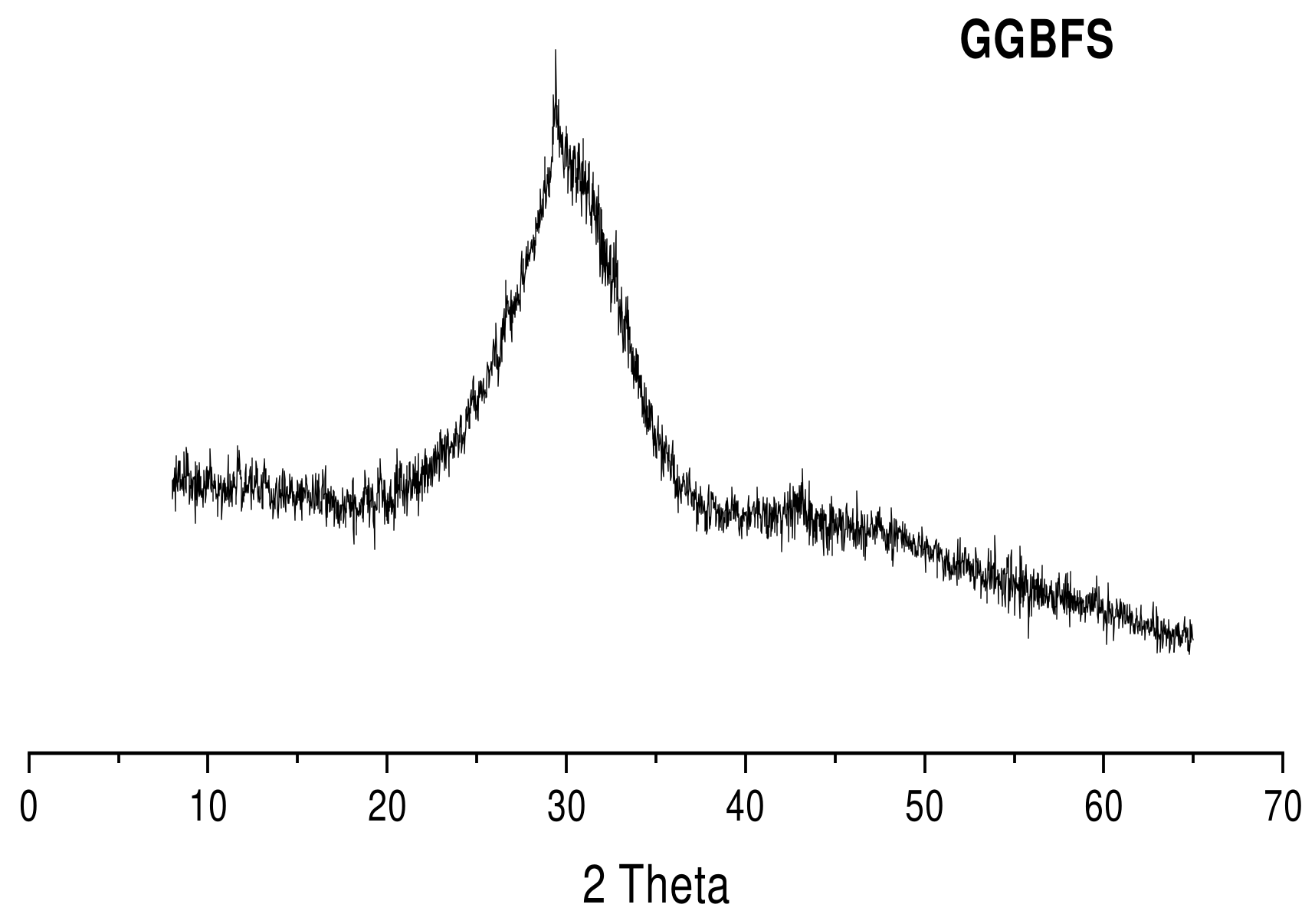
2.1.4. X-Ray Diffractometry
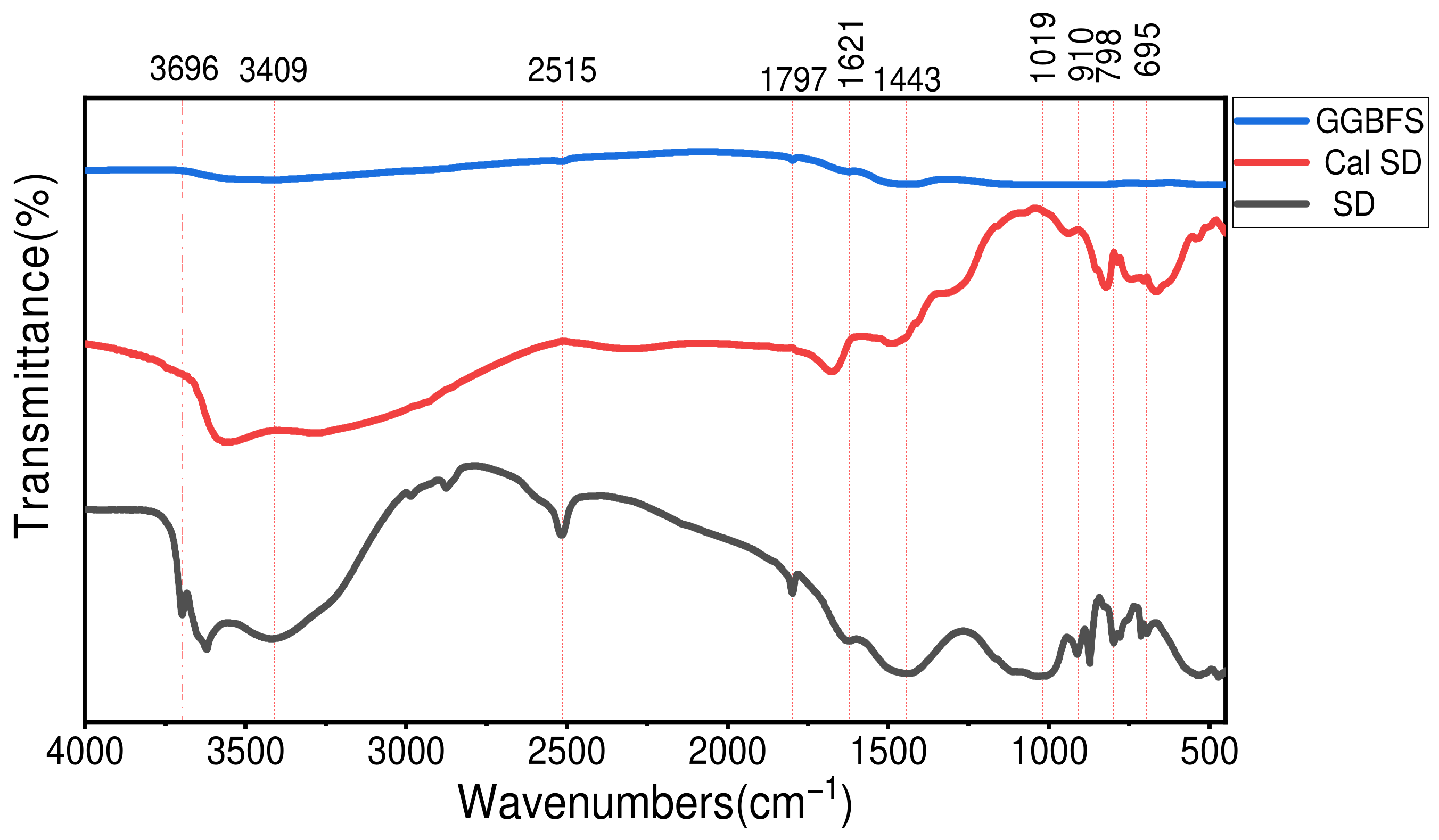
2.1.5. Fourier Transform Infrared Spectroscopy
2.2. Geopolymer Mix Design
- The molar concentration of NaOH;
- The amount of added GGBFS and the (SD/GGBFS) ratio;
- The (SiO2/Al2O3) ratio (in the presence of sodium silicate);
- The curing temperature of geopolymer mortars at early age.
- SD-NaXM with (X = 6, 8, 10, 12, 14): geopolymer based on (100% sediment), with X representing the concentration of the NaOH activator solution and the 40 °C/48 H cure.
- SDnGGBFSm with n = 90, 80, 70, 60, 50 and m = 10, 20, 30, 40, 50: geopolymer based on sediment and slag, with n and m representing the percentage of sediment and slag, respectively, the NaOH activator solution with a concentration of 12M, and the 40 °C/48 h cure.
- SDnGGBFSm-1 with n = 100, 90, 80, 70, 60, 50 and m = 0, 10, 20, 30, 40, 50: geopolymer based on sediment and slag, with n and m representing the percentages of sediment and slag, respectively, the activator solution based on (Na2SiO3 + NaOH 12M), and the cure 40 °C/48 h.
- SDnGGBFSm-2 with n = 100, 90, 80, 70, 60, 50 and m = 0, 10, 20, 30, 40, 50: geopolymer based on sediment and slag, with n and m representing the percentages of sediment and slag, respectively, the activator solution based on Na2SiO3, and the cure 40 °C/48 H.
- SDnGGBFSm-3 with n = 100, 90, 80, 70, 60, 50 and m = 0, 10, 20, 30, 40, 50: geopolymer based on sediment and slag, with n and m representing the percentages of sediment and slag, respectively, the Na2SiO3-based activator solution, and the 20 °C/48 H cure.
2.2.1. Synthesis of Geopolymers with Different NaOH Concentrations (SD-NaXM)
2.2.2. Synthesis of Geopolymers with the Addition of GGBFS and Optimization of the (SD/GGBFS) Ratio
2.2.3. Synthesis of Geopolymers with an Optimized (SiO2/Al2O3) Ratio, with the Presence of Sodium Silicate in the Activator Solution
2.2.4. Influence of Curing Temperatures on Geopolymer Synthesis
2.3. Test Method
2.3.1. Compressive Strength
2.3.2. Mercury Porosity
2.3.3. Geopolymer Microstructure
3. Results and Discussion
3.1. Compressive Strength
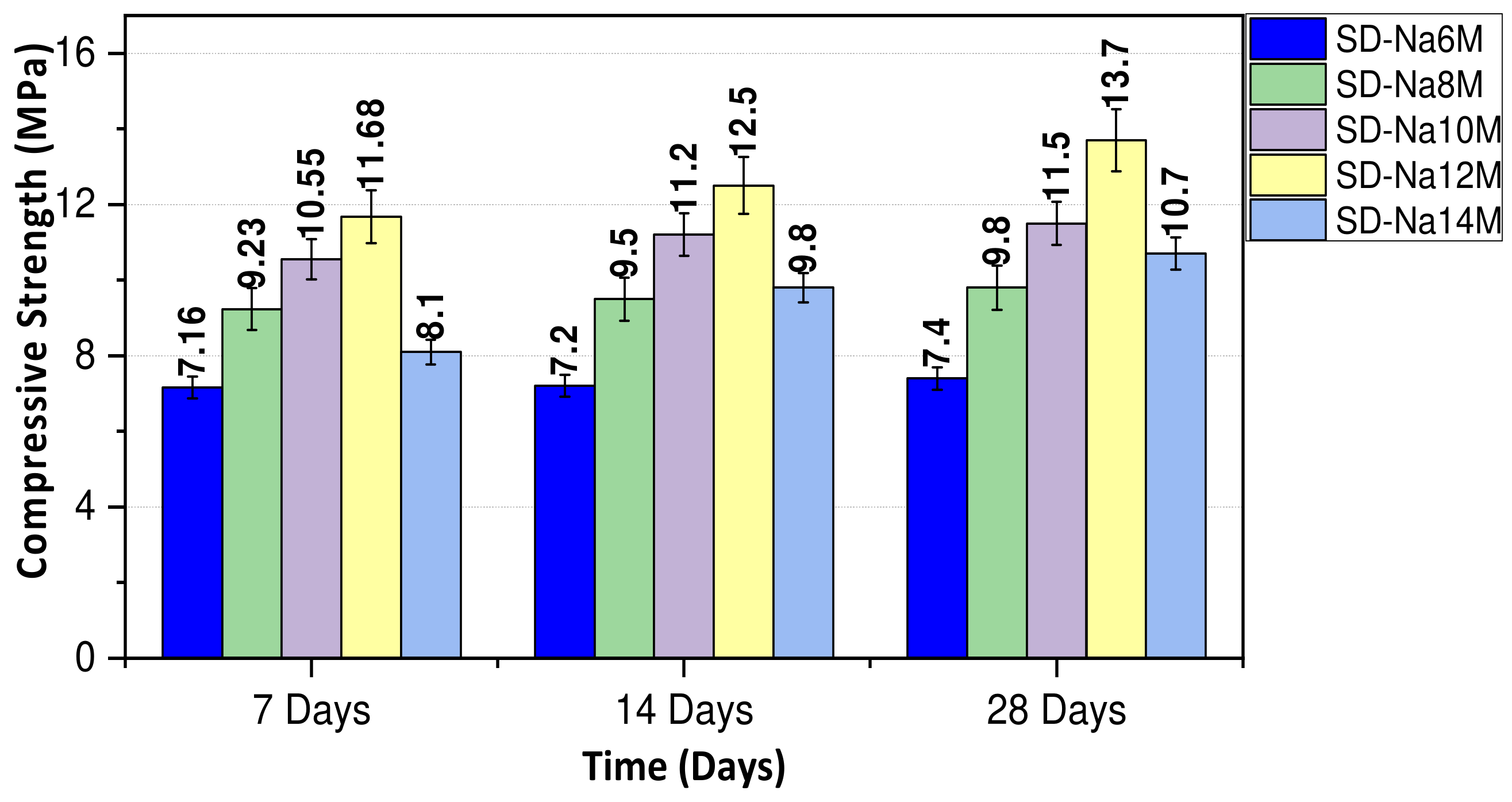
3.1.1. Effect of NaOH Molarity on the Compressive Strength of Geopolymers
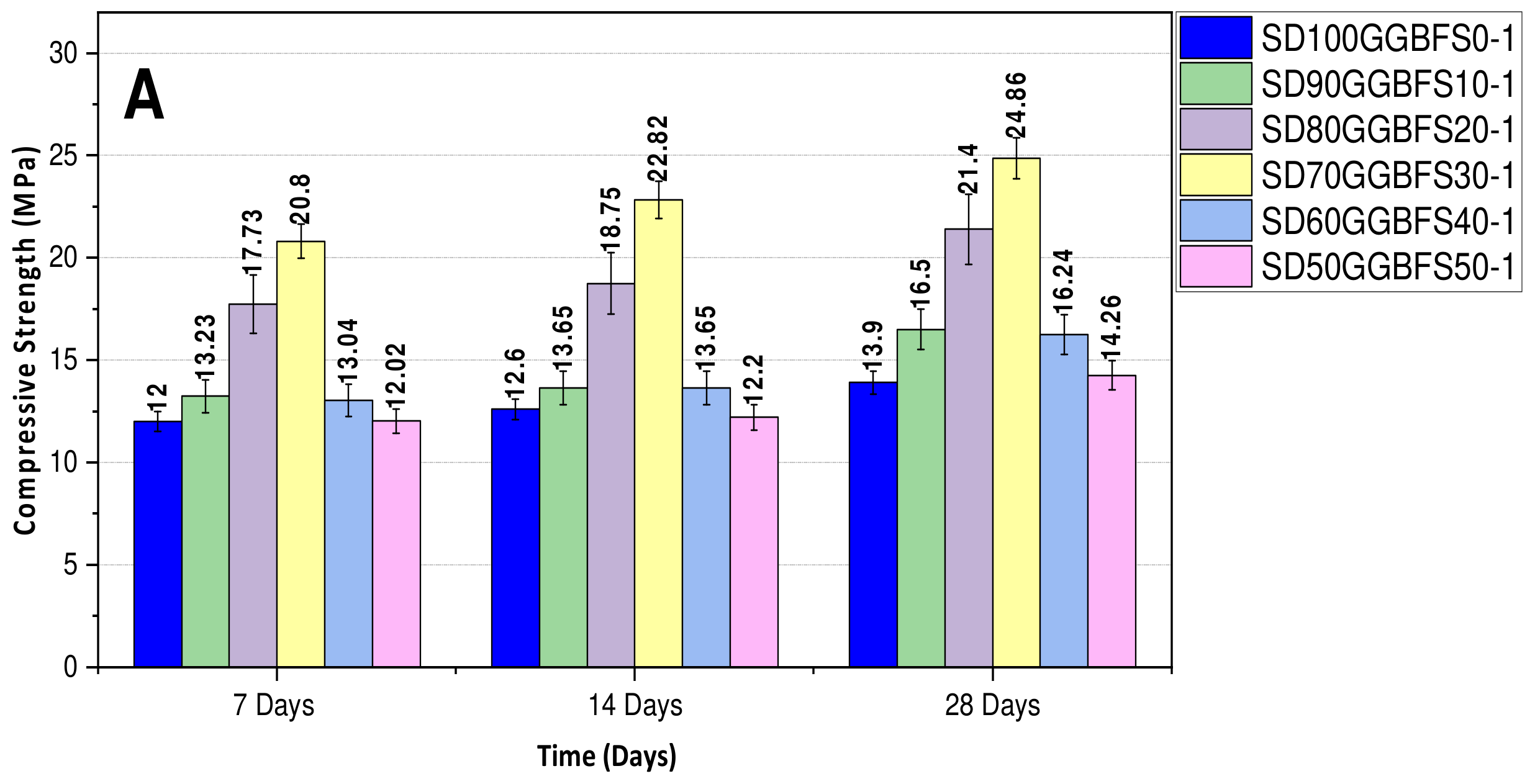
3.1.2. Effect of the (SD/GGBFS) Ratio
3.1.3. Effect of the (SiO2/Al2O3) Ratio, in the Presence of Sodium Silicate
3.1.4. Curing Temperature of Geopolymer Mortars
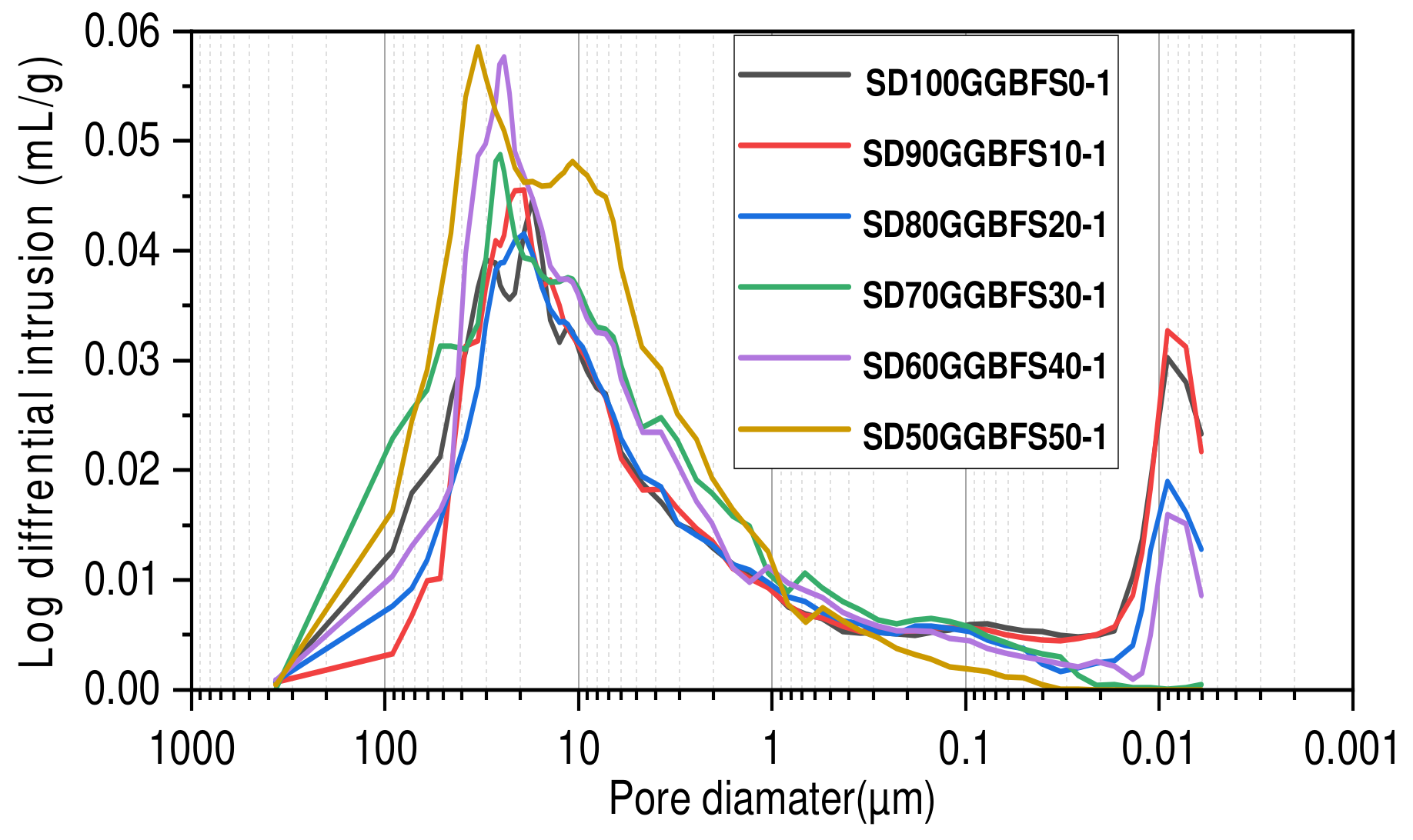
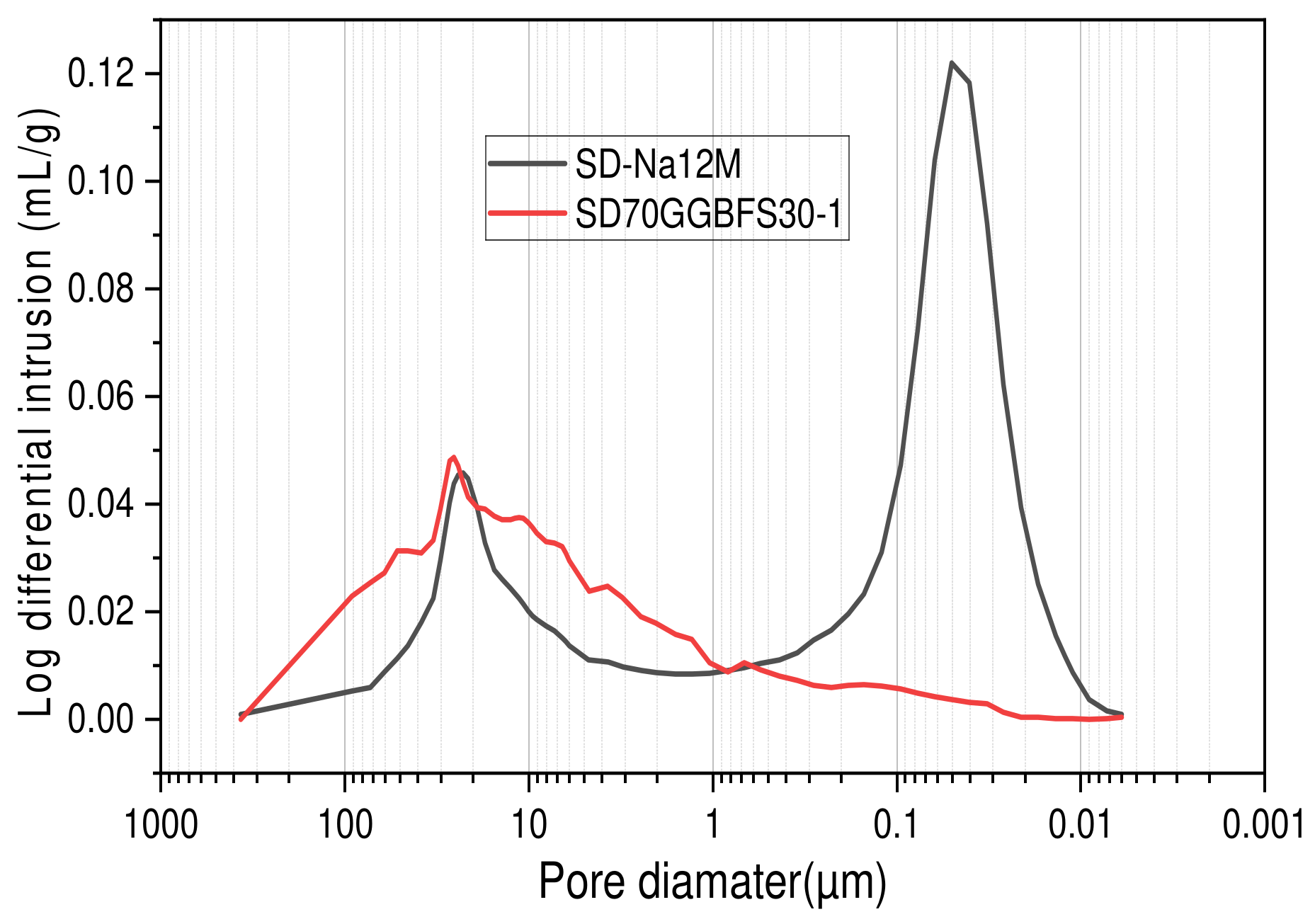
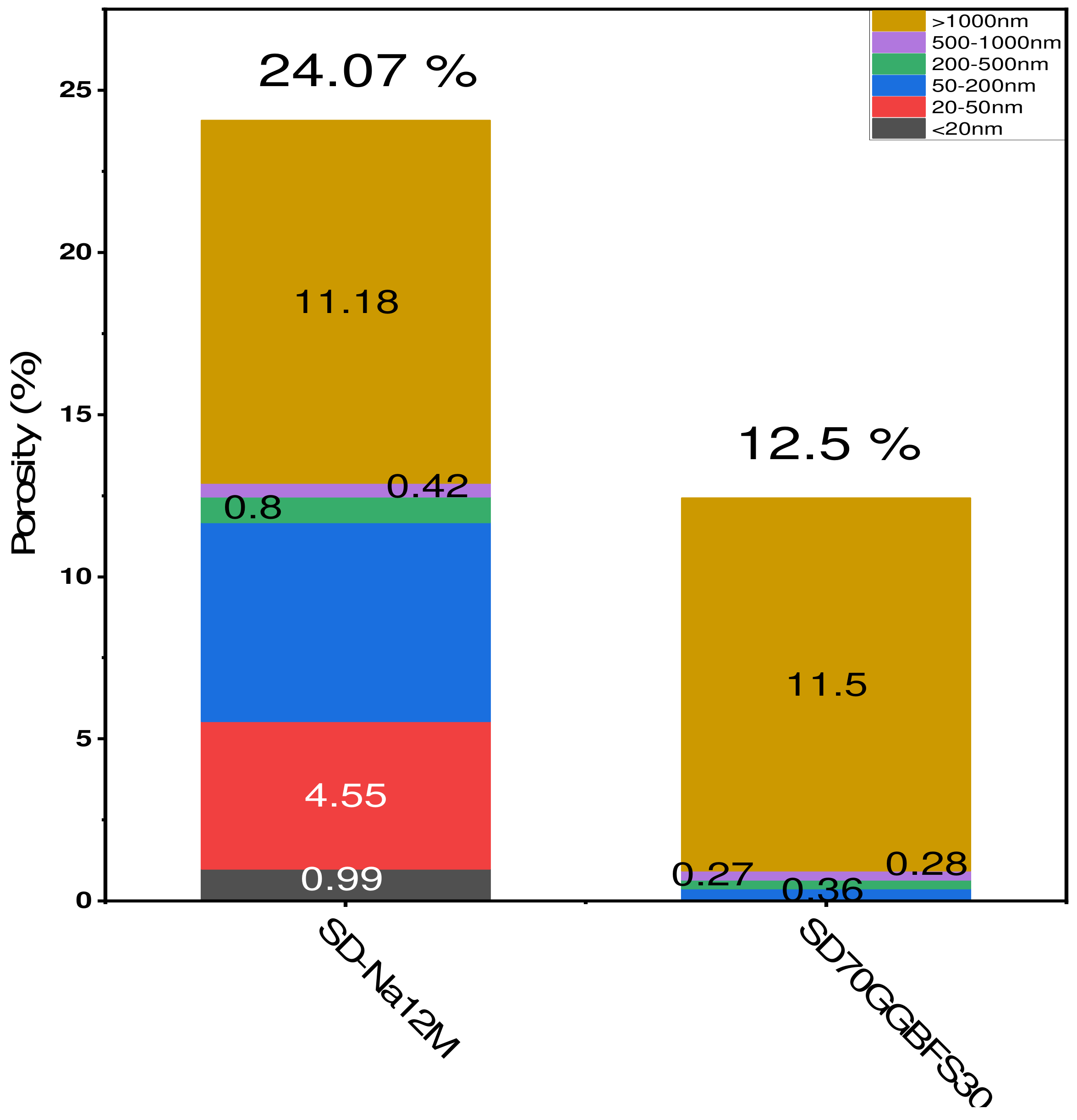
3.2. Mercury Intrusion Porosimetry
- Formulations with activation solution (Na2SiO3 + NaOH 12M). A mass ratio (silicate/hydroxide) equal to 2 was employed for the formulations (SD100GGBFS0-1), (SD90GGBFS10-1), (SD80GGBFS20-1), (SD70GGBFS30-1), (SD60GGBFS40-1), and (SD50GGBFS50-1). The total porosity percentages were measured and found equal to 14.00% for (SD100-1), 13.2% for (SD90GGBFS10-1), 14.40% for (SD80GGBFS20-1), 12.50% for (SD70GGBFS30-1), 14.60% for (SD60GGBFS40-1), and 16.2% for (SD50GGBFS50-1), as shown in Figure 10.
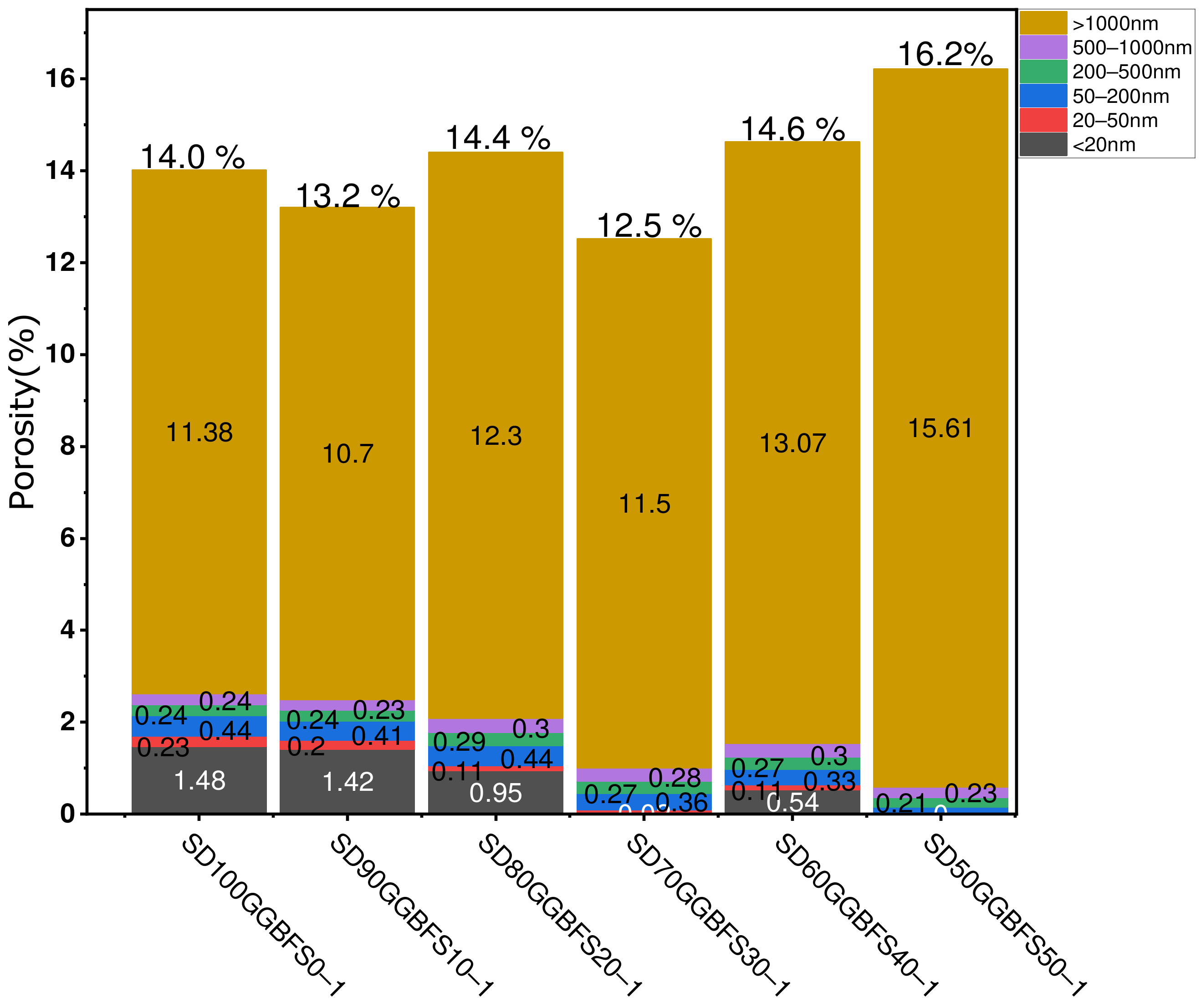
- Formulations with various NaOH molarities. The formulations studied include (SD-Na6M), (SD-Na8M), (SD-Na10M), (SD-Na12M), and (SD-Na14M). The total porosity percentages for the different formulations are 29.7% for (SD-Na6M), 24.3% for (SD-Na8M), 24.5% for (SD-Na10M), 24.03% for (SD-Na12M), and 24.60% for (SD-Na14M), as shown in Figure 11. It is worth noting that for the formulations with different NaOH molarities, the total porosity remains relatively stable for concentrations ranging from 8M to 14M (Figure 11). In contrast, formulations using the activating solution (Na2SiO3) exhibit some variation in porosity of approximately (±2%) between formulations, with a minimum of 12.5% for (SD70GGBFS30-1), which corresponds to an optimum compressive strength of 24.86 MPa, after 28 days (Figure 10).

3.3. Microstructure and Morphology
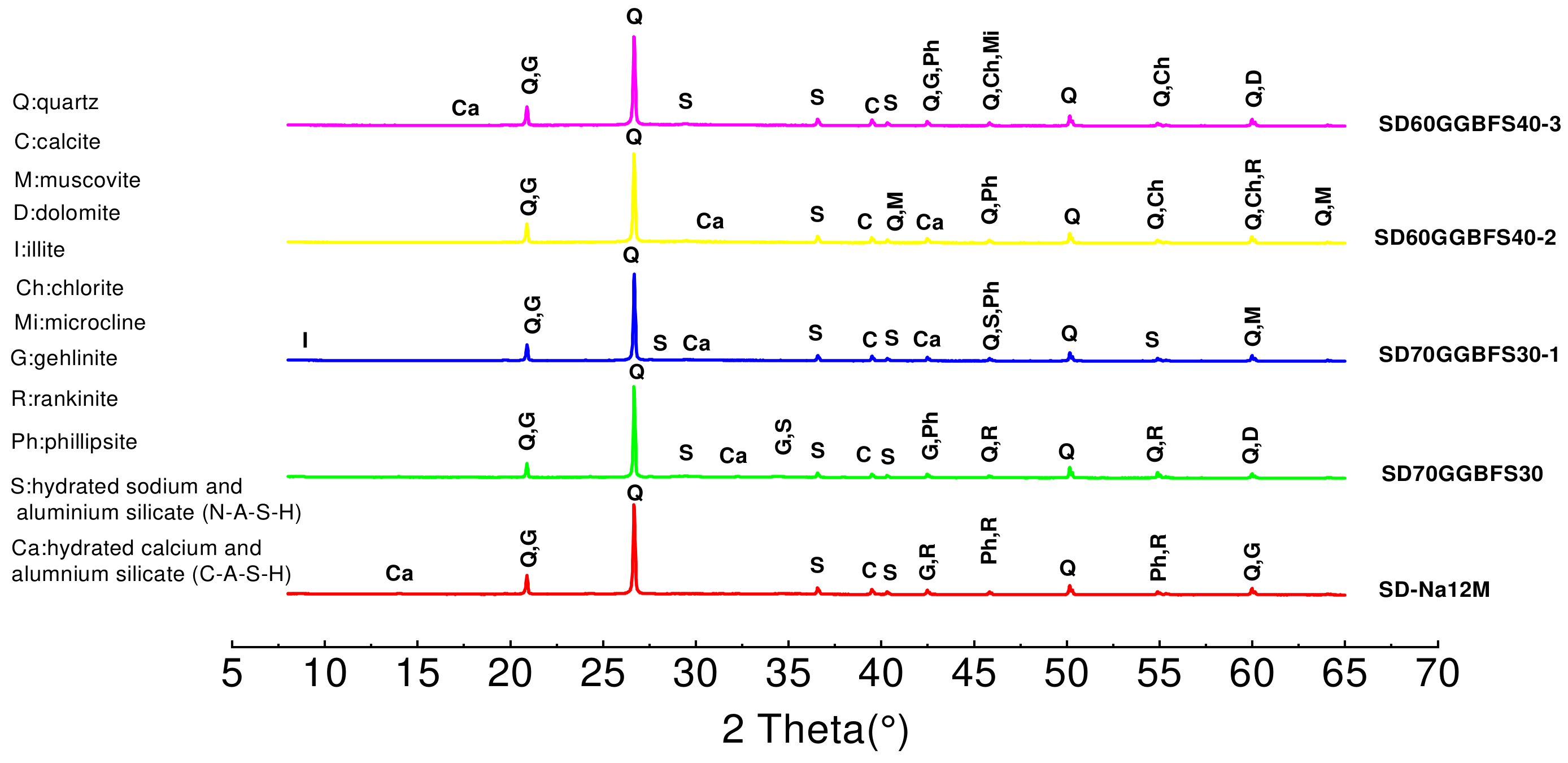
3.3.1. X-Ray Diffraction
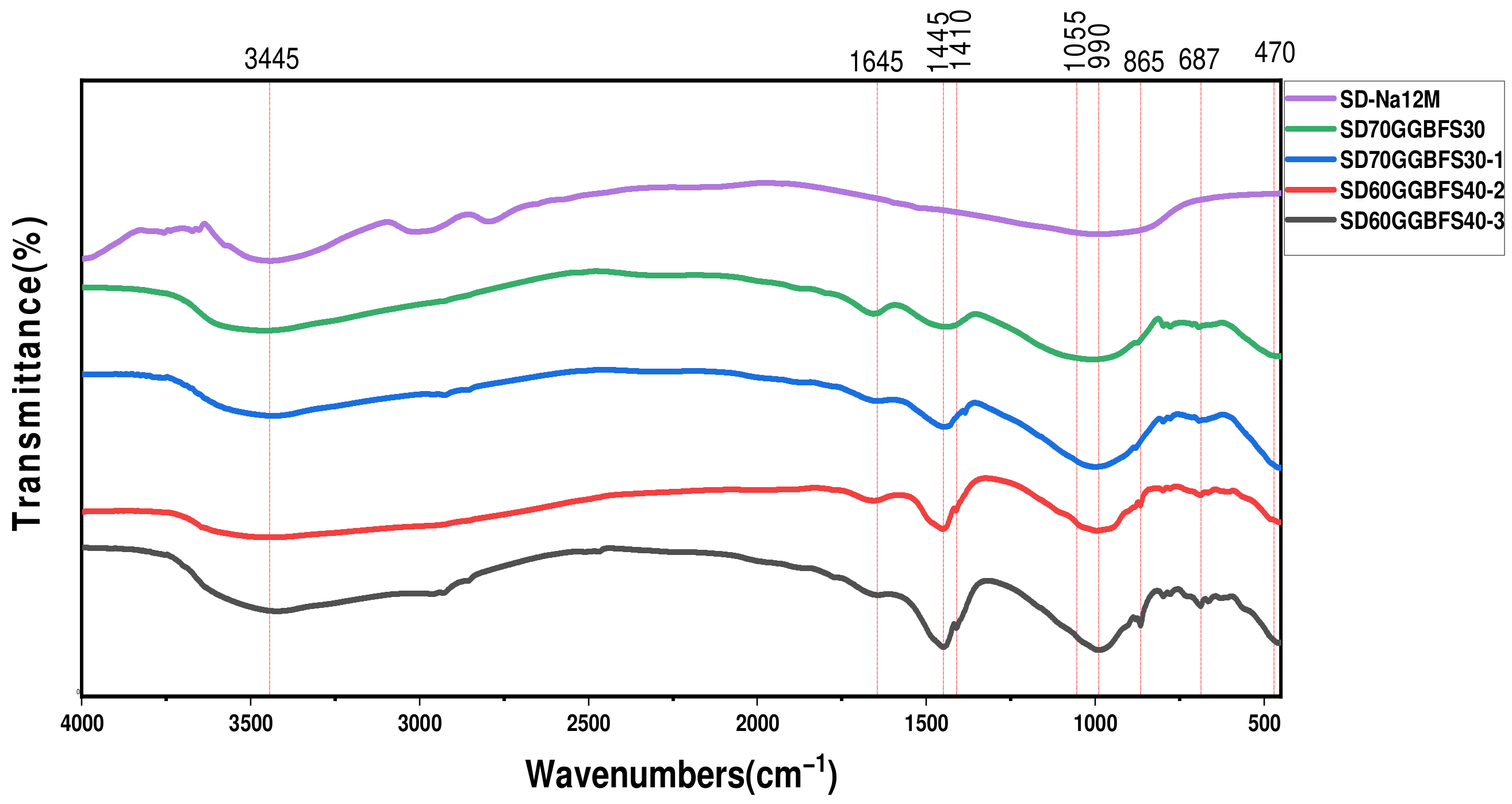
3.3.2. Fourier Transform Infrared Spectroscopy
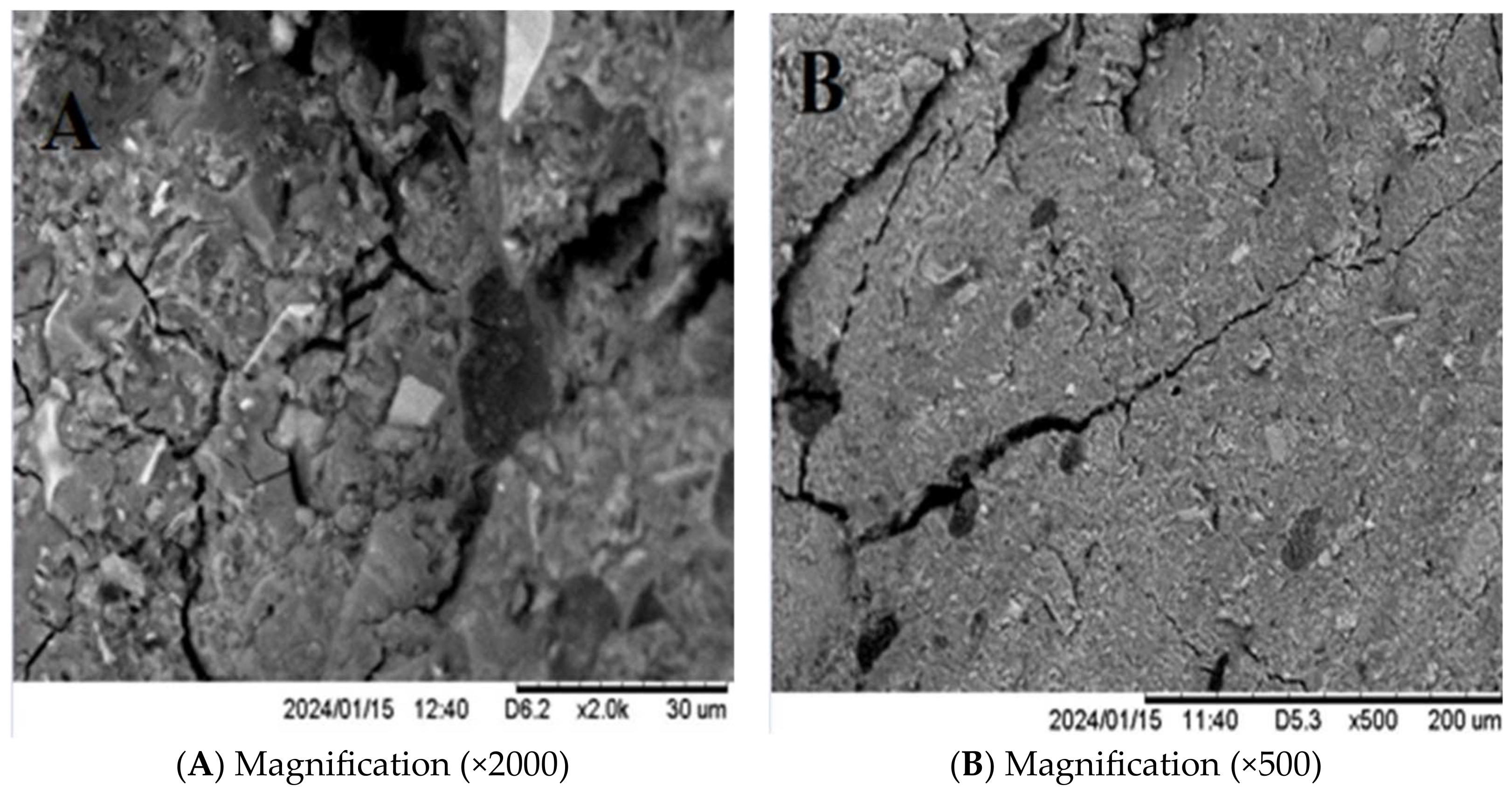
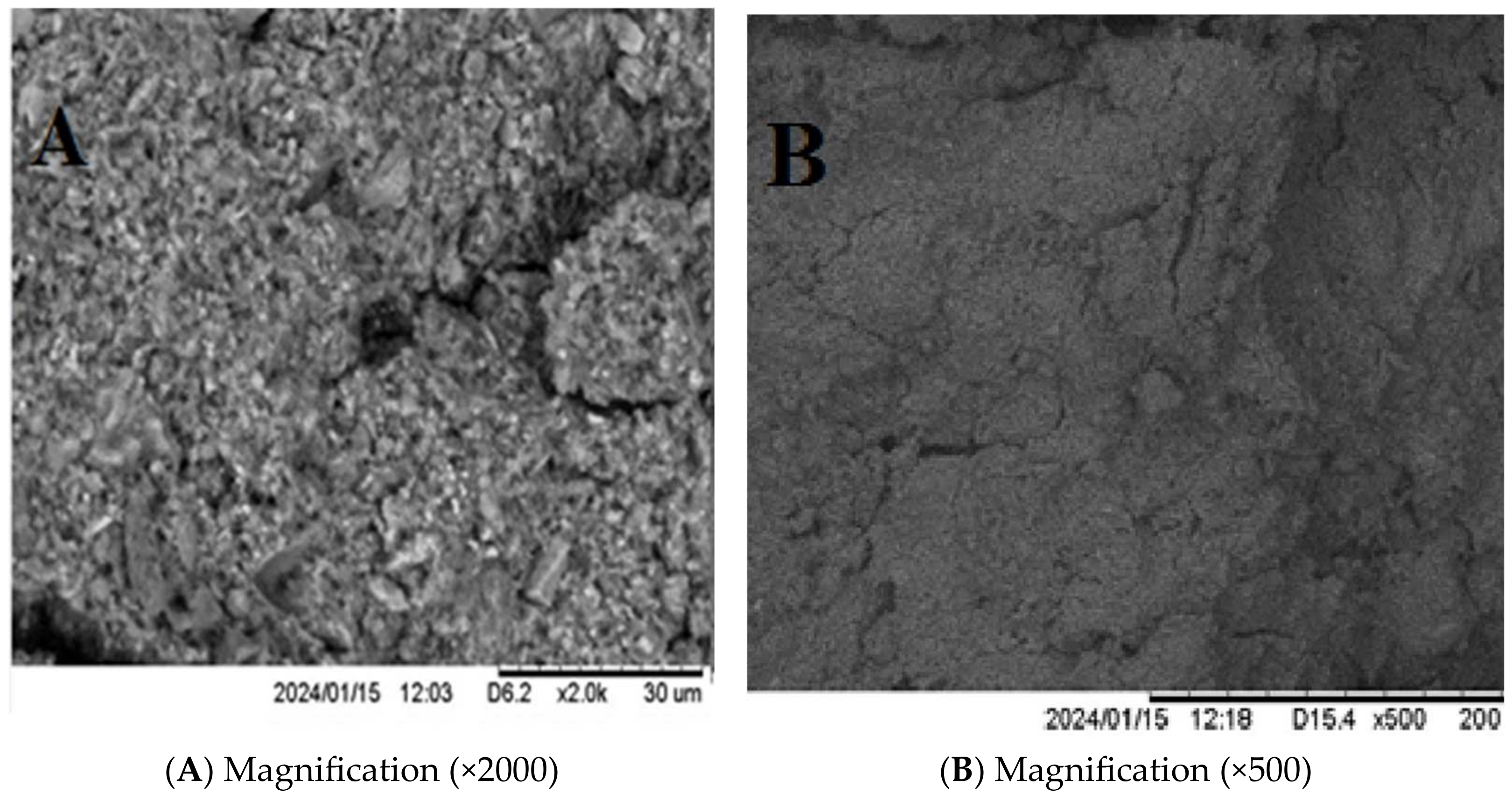
3.3.3. Scanning Electron Microscopy
4. Conclusions
- The molarity of NaOH in the activator solution plays a crucial role in the mechanical strength of geopolymers. Moreover, the NaOH concentration of 12 M turned out to be optimal, as it provided the best compressive strength of the formulations under study. This can be explained by the maximum dissolution of aluminosilicate minerals in the NaOH solution, thus favoring the formation of geopolymers with higher mechanical strength.
- The substitution of sediment with GGBFS improves the mechanical strength of geopolymers. Moreover, the optimal SD-to-GGBFS ratio in the geopolymer formulation was determined to be 70/30. The improvement in the mechanical performance of geopolymers was essentially due to the presence of calcium oxide which promotes the geopolymerization reaction and strengthens the geopolymer binder.
- It turned out that, by varying the ratio (SiO2/Al2O3), some specific optimal ratios were found for different types of binders. Indeed, the (Si/Al) ratio of 4.45 was found for the formulation of geopolymer composed of 70% sediment and 30% slag, activated with a Na2SiO3 + NaOH 12M solution, and cured at 40 °C for 48 h, with a compressive strength of 24.86 MPa and a (Ca/Si) ratio of 0.26. Both ratios were optimal for this geopolymer.
- The curing temperature of geopolymers also has a significant impact on their mechanical strength. In addition, the thermal curing at 40 °C for 48 h produced geopolymers with compressive strength values greater than those of geopolymers cured at room temperature (20 °C), highlighting that the curing temperature accelerates the geopolymerization process and improves the mechanical performance as well.
- The mercury intrusion porosimetry (MIP) results show that the optimal formulations exhibit low total porosity, especially when using NaOH at high molar concentrations with a GGBFS percentage of 30%. A decrease in total porosity is associated with an increase in mechanical strength.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zawrah, M.F.; Gado, R.A.; Feltin, N.; Ducourtieux, S.; Devoille, L. Recycling and Utilization Assessment of Waste Fired Clay Bricks (Grog) with Granulated Blast-Furnace Slag for Geopolymer Production. Process Saf. Environ. Prot. 2016, 103, 237–251. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Pastor, J.Y.; Martín, A. New Cementitious Materials Based on Alkali-Activated Fly Ash: Performance at High Temperatures. J. Am. Ceram. Soc. 2008, 91, 3308–3314. [Google Scholar] [CrossRef]
- Xie, J.F.; Huang, Y.X.; Li, W.W.; Song, X.N.; Xiong, L.; Yu, H.Q. Efficient Electrochemical CO2 Reduction on a Unique Chrysanthemum-like Cu Nanoflower Electrode and Direct Observation of Carbon Deposite. Electrochim. Acta 2014, 139, 137–144. [Google Scholar] [CrossRef]
- Kazi Aoual-Benslafa, F.; Ameur, M.; Mekerta, B.; Semcha, A. Caractérisation des Sédiments de Dragage du Barrage de Bouhanifia Pour Une Réutilisation; Editions Paralia CFL; Centre Francais du Littoral: Nantes, France, 2014; pp. 999–1006. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020; ISBN 9782954453118. [Google Scholar]
- Dimas, D.; Giannopoulou, I.; Panias, D. Polymerization in Sodium Silicate Solutions: A Fundamental Process in Geopolymerization Technology. J. Mater. Sci. 2009, 44, 3719–3730. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Mehsas, B.; Siline, M.; Zeghichi, L. The Effect of Using Low Reactive Metakaolin on Performances of Geopolymer Binder. Innov. Infrastruct. Solut. 2022, 7, 233. [Google Scholar] [CrossRef]
- Youssef, N.; Rabenantoandro, A.Z.; Dakhli, Z.; Chapiseau, C.; Waendendries, F.; Hage Chehade, F.; Lafhaj, Z. Reuse of Waste Bricks: A New Generation of Geopolymer Bricks. SN Appl. Sci. 2019, 1, 1252. [Google Scholar] [CrossRef]
- Youssef, N.; Zaid Rabenantoandro, A.; Dakhli, Z.; Hage Chehade, F.; Lafhaj, Z. Environmental Evaluation of Geopolymer Bricks. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2019. [Google Scholar]
- Youssef, N.; Lafhaj, Z.; Chapiseau, C. Economic Analysis of Geopolymer Brick Manufacturing: A French Case Study. Sustainability 2020, 12, 7403. [Google Scholar] [CrossRef]
- Youssef, N.; Rabenantoandro, A.Z.; Lafhaj, Z.; Dakhli, Z.; Hage Chehade, F.; Ducoulombier, L. A Novel Approach of Geopolymer Formulation Based on Clay for Additive Manufacturing. Constr. Robot. 2021, 5, 175–190. [Google Scholar] [CrossRef]
- Beleuk à Moungam, L.M.; Mohamed, H.; Kamseu, E.; Billong, N.; Melo, U.C. Properties of Geopolymers Made from Fired Clay Bricks Wastes and Rice Husk Ash (RHA)-Sodium Hydroxide (NaOH) Activator. Mater. Sci. Appl. 2017, 08, 537–552. [Google Scholar] [CrossRef]
- Kejkar, R.B.; Wanjari, S.P. Development and Optimisation of Curing Temperature of Energy-Efficient Geopolymer Bricks. Gradjevinar 2020, 72, 411–420. [Google Scholar] [CrossRef]
- Rezzoug, A.; Ayed, K.; Leklou, N. Thermal, mechanical and microstructural properties of geopolymer mortars derived from ceramic sanitary-ware wastes: Pathway to net zero emission. Ceram. Int. 2024, 50, 55535–55545. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Use of Reservoir Clay Sediments as Raw Materials for Geopolymer Binders. Adv. Appl. Ceram. 2013, 112, 184–189. [Google Scholar] [CrossRef]
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioffi, R.; Cappelletto, E.; Di Maggio, R. Thermally Treated Clay Sediments as Geopolymer Source Material. Appl. Clay Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- Molino, B.; De Vincenzo, A.; Ferone, C.; Messina, F.; Colangelo, F.; Cioffi, R. Recycling of Clay Sediments for Geopolymer Binder Production. A New Perspective for Reservoir Management in the Framework of Italian Legislation: The Occhito Reservoir Case Study. Materials 2014, 7, 5603–5616. [Google Scholar] [CrossRef]
- Merabtene, M.; Kacimi, L.; Clastres, P. Elaboration of Geopolymer Binders from Poor Kaolin and Dam Sludge Waste. Heliyon 2019, 5, e01938. [Google Scholar] [CrossRef]
- Mostefa, F.; Bouhamou, N.E.; Mesbah, H.A.; Aggoune, S.; Mekhatria, D. Sedimentary Clays as Geopolymer Precursor. Int. J. Eng. Res. Afr. 2018, 39, 97–111. [Google Scholar] [CrossRef]
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and Chemical Properties of Composite Materials Made of Dredged Sediments in a Fly-Ash Based Geopolymer. J. Environ. Manag. 2017, 191, 1–7. [Google Scholar] [CrossRef]
- Karam, R.; Paris, M.; Deneele, D.; Wattez, T.; Cyr, M.; Bulteel, D. Effect of Sediment Incorporation on the Reactivity of Alkali-Activated GGBFS Systems. Mater. Struct. Constr. 2021, 54, 118. [Google Scholar] [CrossRef]
- Hosseini, S.; Brake, N.A.; Nikookar, M.; Günaydın-Şen, Ö.; Snyder, H.A. Enhanced Strength and Microstructure of Dredged Clay Sediment-Fly Ash Geopolymer by Mechanochemical Activation. Constr. Build. Mater. 2021, 301, 123984. [Google Scholar] [CrossRef]
- Mahfoud, E.; Maherzi, W.; Ndiaye, K.; Benzerzour, M.; Aggoun, S.; Abriak, N.-E. Mechanical and Microstructural Properties of Just Add Water Geopolymer Cement Comprised of Thermo-Mechanicalsynthesis Sediments-Fly Ash Mix. Constr. Build. Mater. 2023, 400, 132626. [Google Scholar] [CrossRef]
- Mahfoud, E.; Ndiaye, K.; Maherzi, W.; Aggoun, S.; Abriak, N.; Benzerzour, M. Carbonation in Just Add Water Geopolymer Based on Fly Ash and Dredged Sediments Mix. Constr. Build. Mater. 2024, 452, 138959. [Google Scholar] [CrossRef]
- Zerzouri, M.; Bouchenafa, O.; Hamzaoui, R.; Ziyani, L.; Zerzouri, M.; Bouchenafa, O.; Hamzaoui, R.; Ziyani, L.; Physico, S.A. Physico-Chemical and Mechanical Properties of Fly Ash Based-Geopolymer Pastes Produced from Pre-Geopolymer Powders Obtained by Mechanosynthesis. Constr. Build. Mater. 2021, 288, 123135. [Google Scholar] [CrossRef]
- Zerzouri, M.; Hamzaoui, R.; Ziyani, L.; Alehyen, S. Influence of Slag Based Pre-Geopolymer Powders Produced by Mechanosynthesis on Structure, Microstructure and Mechanical Performance of Geopolymer Pastes. Constr. Build. Mater. 2022, 361, 129637. [Google Scholar] [CrossRef]
- AFNOR NF EN 196-1; Méthodes D’essais Des Ciments. AFNOR: La Plaine Saint-Denis, France, 1995; pp. 1–27.
- AFNOR P94-054; Détermination de la Masse Volumique des Particules Solides des Sols. AFNOR: La Plaine Saint-Denis, France, 1991; pp. 1–8.
- AFNOR EN 196-6; Détermination de la Finesse. AFNOR: La Plaine Saint-Denis, France, 1990; pp. 1–20.
- Kirschner, A.V.; Harmuth, H. Investigation of Geopolymer Binders with Respect to Their Application for Building Materials. Ceram. Silik. 2004, 48, 117–120. [Google Scholar]
- Bellara, S.; Hidjeb, M.; Maherzi, W.; Mezazigh, S.; Senouci, A. Optimization of an Eco-Friendly Hydraulic Road Binders Comprising Clayey Dam Sediments and Ground Granulated Blast-Furnace Slag. Buildings 2021, 11, 443. [Google Scholar] [CrossRef]
- Bellara, S.; Maherzi, W.; Mezazigh, S.; Senouci, A. Mineral Waste Valorization in Road Subgrade Construction: Algerian Case Study Based on Technical and Environmental Features. Case Stud. Constr. Mater. 2024, 20, e02764. [Google Scholar] [CrossRef]
- Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A.A. Cement & Concrete Composites Effect of Type, Form, and Dosage of Activators on Strength of Alkali-Activated Natural Pozzolans. Cem. Concr. Compos. 2011, 33, 251–260. [Google Scholar] [CrossRef]
- Durmus, H.; Erdemir, M. Microstructural Alteration of Alkali Activated Slag Mortars Depend on Exposed High Temperature Level. Constr. Build. Mater. 2016, 104, 169–180. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Mohsen, Q. Characterization of Low-Purity Clays for Geopolymer Binder Formulation. Int. J. Miner. Metall. Mater. 2014, 21, 609–619. [Google Scholar] [CrossRef]
- Nadeau, P.H. A Handbook of Determinative Methods in Clay Mineralogy: Methods; Chapman and Hall: London, UK, 1987; Available online: https://www.researchgate.net/publication/327594658 (accessed on 2 February 2025).
- Davidovits, J.; Sawyer, J.L. Early High-Strength Mineral Polymer. Patent Number 4509985, 9 April 1985. [Google Scholar]
- Firdous, R.; Stephan, D. Effect of Silica Modulus on the Geopolymerization Activity of Natural Pozzolans. Constr. Build. Mater. 2019, 219, 31–43. [Google Scholar] [CrossRef]
- Mccarter, W.J.; Chrisp, T.M.; Starrs, G. The Early Hydration of Alkali-Activated Slag: Developments in Monitoring Techniques. Cem. Concr. Compos. 1999, 21, 277–283. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on Setting, Workability and Early Strength Properties of Fly Ash Geopolymer Concrete Cured in Ambient Condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S.J. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al Ratio on the Structure and Properties of Metakaolin Based Geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; Mackenzie, K.J.D.; Thaumaturgo, C. Synthesis and Characterisation of Materials Based on Inorganic Polymers of Alumina and Silica: Sodium Polysialate Polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Fadhil, M.; Bashar, S.; Orcid, I. Methods of Curing Geopolymer Concrete: A Review. Int. J. Adv. Appl. Sci. 2018, 5, 31–36. [Google Scholar]
- ISO 15901-1; Evaluation of Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption. ISO: Geneva, Switzerland, 2016; pp. 1–11.
- De Silva, P.; Sirivivatnanon, V. Kinetics of Geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Dehghani, A.; Aslani, F.; Ghaebi Panah, N. Effects of Initial SiO2/Al2O3 Molar Ratio and Slag on Fly Ash-Based Ambient Cured Geopolymer Properties. Constr. Build. Mater. 2021, 293, 123527. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The Effect of Alkali and Si/Al Ratio on the Development of Mechanical Properties of Metakaolin-Based Geopolymers. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Kioupis, D.; Skaropoulou, A.; Tsivilis, S.; Kakali, G. Valorization of Brick and Glass Cdws for the Development of Geopolymers Containing More than 80% of Wastes. Minerals 2020, 10, 672. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.; Delaqua, G.C.G.; Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F. Performance of Geopolymer Tiles in High Temperature and Saturation Conditions. Constr. Build. Mater. 2021, 286, 122994. [Google Scholar] [CrossRef]
- Davidovits, J. Mineral Polymer and Methods of Making Them. Patent Number 4349386, 14 September 1982. [Google Scholar]
- Zhao, S.; Xia, M.; Yu, L.; Huang, X.; Jiao, B.; Li, D. Optimization for the Preparation of Composite Geopolymer Using Response Surface Methodology and Its Application in Lead-Zinc Tailings Solidification. Constr. Build. Mater. 2021, 266, 120969. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C. Effect of Blast Furnace Slag Addition on Microstructure. Adv. Ceram. Matrix Compos. IX 2003, 153, 187–209. [Google Scholar]
- Yip, C.K.; Lukey, G.C.; Van Deventer, J.S.J. The Coexistence of Geopolymeric Gel and Calcium Silicate Hydrate at the Early Stage of Alkaline Activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of One-Part Geopolymer Binders Made from Fly Ash. Waste Biomass Valorization 2017, 8, 225–233. [Google Scholar] [CrossRef]
- Youse, S.; Chen, B.; Riaz, M.; Farasat, S.; Shah, A. Fresh and Hardened Properties of One-Part Fl y Ash-Based Geopolymer Binders Cured at Room Temperature: Effect of Slag and Alkali Activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Wahab, M.M.A.; Liew, M.S.; Haruna, A. Heliyon Mechanical and Microstructural Properties of High Calcium Fl y Ash One-Part Geopolymer Cement Made with Granular Activator. Heliyon 2019, 5, e02255. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Attenuated Total Reflectance Fourier Transform Infrared Analysis of Fly Ash Geopolymer Gel Aging. Langmuir 2023, 23, 8170–8179. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V. Cement & Concrete Composites Effects of NaOH Concentrations on Physical and Electrical Properties of High Calcium Fly Ash Geopolymer Paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.; Bansal, N. Effect of Blast Furnace Slag Addition on Microstructure and Properties of Metakaolinite Geopolymeric Materials. In Advances in Ceramic Matrix Composites IX 187 Advances in Ceramic Matrix Composites IX; Bansal, N.P., Singh, J.P., Kriven, W.M., Schneider, H., Eds.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mollah, M.Y.A.; Hess, T.R.; Tsai, Y. An FTIR and XPS investigations of the effects of carbonation on the solidification/stabilization of cement based systems-Portland type V with zinc. Cem. Concr. Res. 1993, 23, 773–784. [Google Scholar]
- Ma, C.; Zhao, B.; Guo, S.; Long, G.; Xie, Y. Properties and Characterization of Green One-Part Geopolymer Activated by Composite Activators. J. Clean. Prod. 2019, 220, 188–199. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Zhang, Z.; Li, Y. In-Situ Transition of Amorphous Gels to Na-P1 Zeolite in Geopolymer: Mechanical and Adsorption Properties. Constr. Build. Mater. 2019, 202, 851–860. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; Van Deventer, J.S.J. One-Part Geopolymer Mixes from Geothermal Silica and Sodium Aluminate. Ind. Eng. Chem. Res. 2008, 47, 9396–9405. [Google Scholar] [CrossRef]
- El Hafid, K.; Hajjaji, M. Geopolymerization of Glass- and Silicate-Containing Heated Clay. Constr. Build. Mater. 2018, 159, 598–609. [Google Scholar] [CrossRef]
- Poggetto, G.D.; Catauro, M.; Crescente, G.; Leonelli, C. Efficient Addition of Waste Glass in MK-Based Geopolymers. Polymers 2021, 13, 1493. [Google Scholar] [CrossRef]




| Density (g/cm3) | Specific Surface Area (m2/g) | |
|---|---|---|
| SD | 2.52 | 24.031 |
| Calcined SD | 2.78 | 24.755 |
| GGBFS | 2.79 | 4.863 |
| Compositions | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | TiO2 | Cl | L.O.I |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | 42.34 | 13.59 | 5.77 | 14 | 2.64 | 0.25 | 1.18 | 0.50 | - | 0.025 | 18.96 |
| Calcined SD | 46.23 | 16.18 | 5.68 | 15 | 4.06 | 0.18 | 3.04 | 0.41 | 0.68 | 0.013 | 6.3 |
| GGBFS | 41.30 | 11.80 | 4.3 | 18 | 5.7 | 0.10 | 0.83 | 0.25 | 1.66 | 0.001 | 4.7 |
| Mix | Sample | SD (%) | GGBFS (%) | AS | Cure Condition | SiO2/Al2O3 Weight Ratio | CaO/SiO2 Weight Ratio |
|---|---|---|---|---|---|---|---|
| 1 | SD-Na6M | 100 | 0 | NaOH 6M | 40 °C/48 h | 3.12 | 0.33 |
| 2 | SD-Na8M | 100 | 0 | NaOH 8M | 40 °C/48 h | 3.12 | 0.33 |
| 3 | SD-Na10M | 100 | 0 | NaOH 10M | 40 °C/48 h | 3.12 | 0.33 |
| 4 | SD-Na12M | 100 | 0 | NaOH 12M | 40 °C/48 h | 3.12 | 0.33 |
| 5 | SD-Na14M | 100 | 0 | NaOH 14M | 40 °C/48 h | 3.12 | 0.33 |
| 6 | SD90GGBFS10 | 90 | 10 | NaOH 12M | 40 °C/48 h | 3.15 | 0.34 |
| 7 | SD80GGBFS20 | 80 | 20 | NaOH 12M | 40 °C/48 h | 3.18 | 0.35 |
| 8 | SD70GGBFS30 | 70 | 30 | NaOH 12M | 40 °C/48 h | 3.22 | 0.36 |
| 9 | SD60GGBFS40 | 60 | 40 | NaOH 12M | 40 °C/48 h | 3.26 | 0.37 |
| 10 | SD50GGBFS50 | 50 | 50 | NaOH 12M | 40 °C/48 h | 3.29 | 0.38 |
| 11 | SD100GGBFS0-1 | 100 | 0 | (Na2SiO3 + NaOH 12M) | 40 °C/48 h | 4.29 | 0.23 |
| 12 | SD90GGBFS10-1 | 90 | 10 | (Na2SiO3 + NaOH 12M) | 40 °C/48 h | 4.34 | 0.24 |
| 13 | SD80GGBFS20-1 | 80 | 20 | (Na2SiO3 + NaOH 12M) | 40 °C/48 h | 4.39 | 0.25 |
| 14 | SD70GGBFS30-1 | 70 | 30 | (Na2SiO3 + NaOH 12M) | 40 °C/48 h | 4.45 | 0.26 |
| 15 | SD60GGBFS40-1 | 60 | 40 | (Na2SiO3 + NaOH 12M) | 40 °C/48 h | 4.5 | 0.27 |
| 16 | SD50GGBFS50-1 | 50 | 50 | (Na2SiO3 + NaOH 12M) | 40 °C/48 h | 4.56 | 0.28 |
| 17 | SD100GGBFS0-2 | 100 | 0 | Na2SiO3 | 40 °C/48 h | 4.89 | 0.21 |
| 18 | SD90GGBFS10-2 | 90 | 10 | Na2SiO3 | 40 °C/48 h | 4.94 | 0.22 |
| 19 | SD80GGBFS20-2 | 80 | 20 | Na2SiO3 | 40° C/48 h | 5 | 0.22 |
| 20 | SD70GGBFS30-2 | 70 | 30 | Na2SiO3 | 40° C/48 h | 5.06 | 0.23 |
| 21 | SD60GGBFS40-2 | 60 | 40 | Na2SiO3 | 40° C/48 h | 5.12 | 0.24 |
| 22 | SD50GGBFS50-2 | 50 | 50 | Na2SiO3 | 40 °C/48 h | 5.19 | 0.24 |
| 23 | SD100GGBFS0-3 | 100 | 0 | Na2SiO3 | 20 °C/48 h | 4.89 | 0.21 |
| 24 | SD90GGBFS10-3 | 90 | 10 | Na2SiO3 | 20 °C/48 h | 4.94 | 0.22 |
| 25 | SD80GGBFS20-3 | 80 | 20 | Na2SiO3 | 20 °C/48 h | 5 | 0.22 |
| 26 | SD70GGBFS30-3 | 70 | 30 | Na2SiO3 | 20 °C/48 h | 5.06 | 0.23 |
| 27 | SD60GGBFS40-3 | 60 | 40 | Na2SiO3 | 20 °C/48 h | 5.12 | 0.24 |
| 28 | SD50GGBFS50-3 | 50 | 50 | Na2SiO3 | 20 °C/48 h | 5.19 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouaissa, M.S.; Marouf, H.; Ali-Dahmane, T.; Benosman, A.S.; Maherzi, W. Effect of Mix Design Parameters on the Properties of Dam Sediment/Slag-Based Geopolymer Mortars. Buildings 2025, 15, 886. https://doi.org/10.3390/buildings15060886
Mouaissa MS, Marouf H, Ali-Dahmane T, Benosman AS, Maherzi W. Effect of Mix Design Parameters on the Properties of Dam Sediment/Slag-Based Geopolymer Mortars. Buildings. 2025; 15(6):886. https://doi.org/10.3390/buildings15060886
Chicago/Turabian StyleMouaissa, Mohamed Salah, Hafida Marouf, Tewfik Ali-Dahmane, Ahmed Soufiane Benosman, and Walid Maherzi. 2025. "Effect of Mix Design Parameters on the Properties of Dam Sediment/Slag-Based Geopolymer Mortars" Buildings 15, no. 6: 886. https://doi.org/10.3390/buildings15060886
APA StyleMouaissa, M. S., Marouf, H., Ali-Dahmane, T., Benosman, A. S., & Maherzi, W. (2025). Effect of Mix Design Parameters on the Properties of Dam Sediment/Slag-Based Geopolymer Mortars. Buildings, 15(6), 886. https://doi.org/10.3390/buildings15060886







