Effects of Hydraulic Materials on the Performance Evolution of Carbonated High-Volume Magnesium Slag Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Test Methods
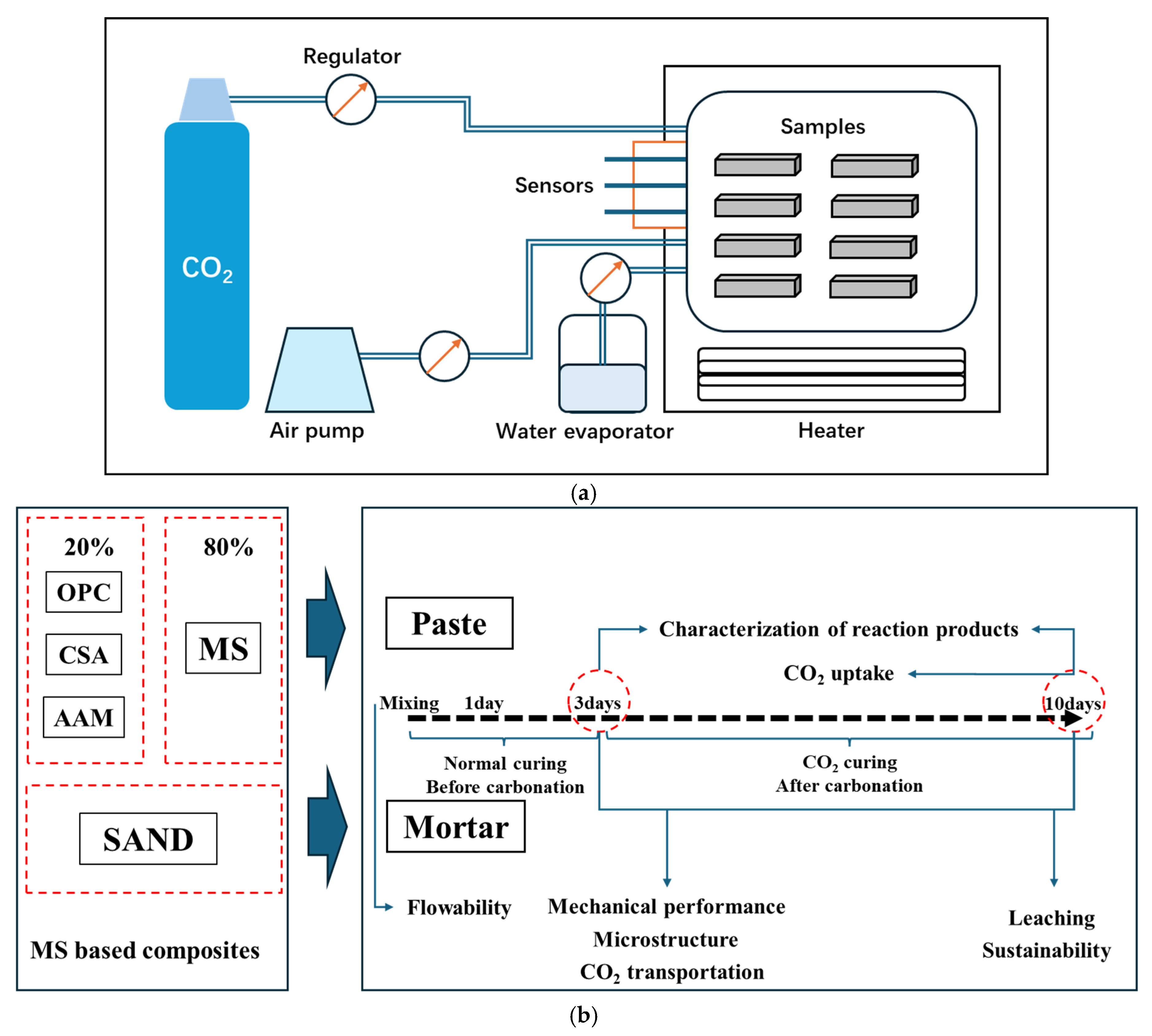
2.2.1. Sample Preparation
2.2.2. Characterization of Reaction Products
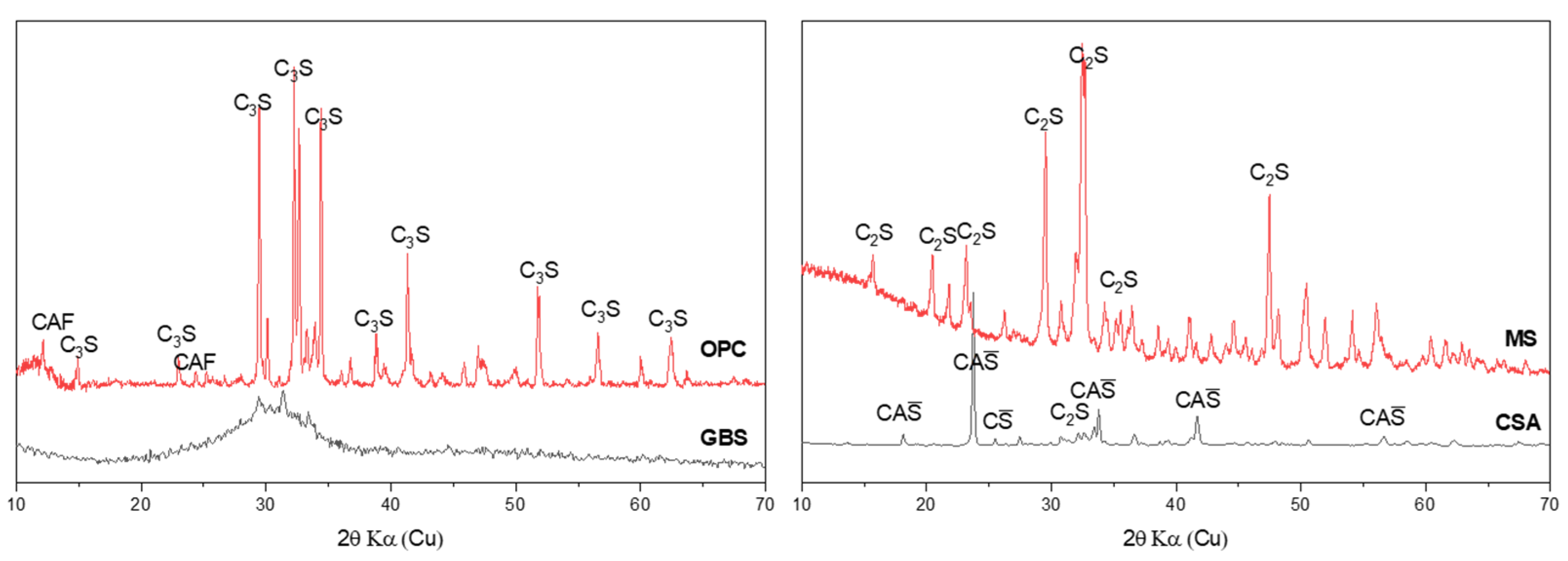
- Mineral phases identification: To investigate the mineral phase development of MS composites before and after carbonation, crushed paste samples after 3 days’ standard curing and 7 days’ carbonation curing were tested with Bruker D8 ADVANCE (Cu tube); the scanning range was from 5° to 70°, the scanning speed was set as 0.2 s/step, and the voltage and current during scanning were 40 KV and 40 A, respectively. The mineral phases were identified using Highscore Plus 3.0.
- Thermal gravimetric test: To evaluate the bound water and calcium carbonate formation in the paste samples after 3 days’ standard curing and 7 days’ carbonation curing, the powdered paste samples were tested using STA 449 F1. The test temperature range was from ambient temperature to 1000 °C, and the heating rate was 10 °C/min. Nitrogen was applied as the carrier gas during the test.
2.2.3. Microstructure Identification
- Pore structure of mortars: To evaluate the pore size distribution of mortars after 3 days’ standard curing and 7 days’ carbonation curing, crushed mortar fractions with size of 2–8 mm were prepared and tested using MicroActive AutoPore V 9600 (Micromertics).
- Morphology of reaction products: To identify the morphology of reaction products in mortars before and after carbonation, crushed MS-blended mortars with AAM, CSA, and OPC were prepared. The morphology images of reaction products were collected using a scanning electron microscope (Regulus 8100, HITACHI).
2.2.4. Evaluation of Mortar Performance
- Compressive strength test: To evaluate the strength performance evolution of mortars during standard curing and carbonation curing, the compressions of MS-blended mortar cubes were tested using a strength test bench. The cube samples (40 mm × 40 mm × 40 mm) were prepared for compressive strength test according to GB/T 17671-2021 [25]. The average result was collected by testing 6 parts. Mortars after 1 day and 3 days of standard curing and 1, 3, 5, and 7 days of carbonation curing were tested and recorded.
- Mini-slump flow test: The slump-flow of ternary mortars was conducted using the flow table test, according to GB/T 2419-2005 [26]. An average value of two tested diameters was recorded using a standard conical ring.
2.2.5. Development of Carbonation Front
2.2.6. Evaluation of Sustainability
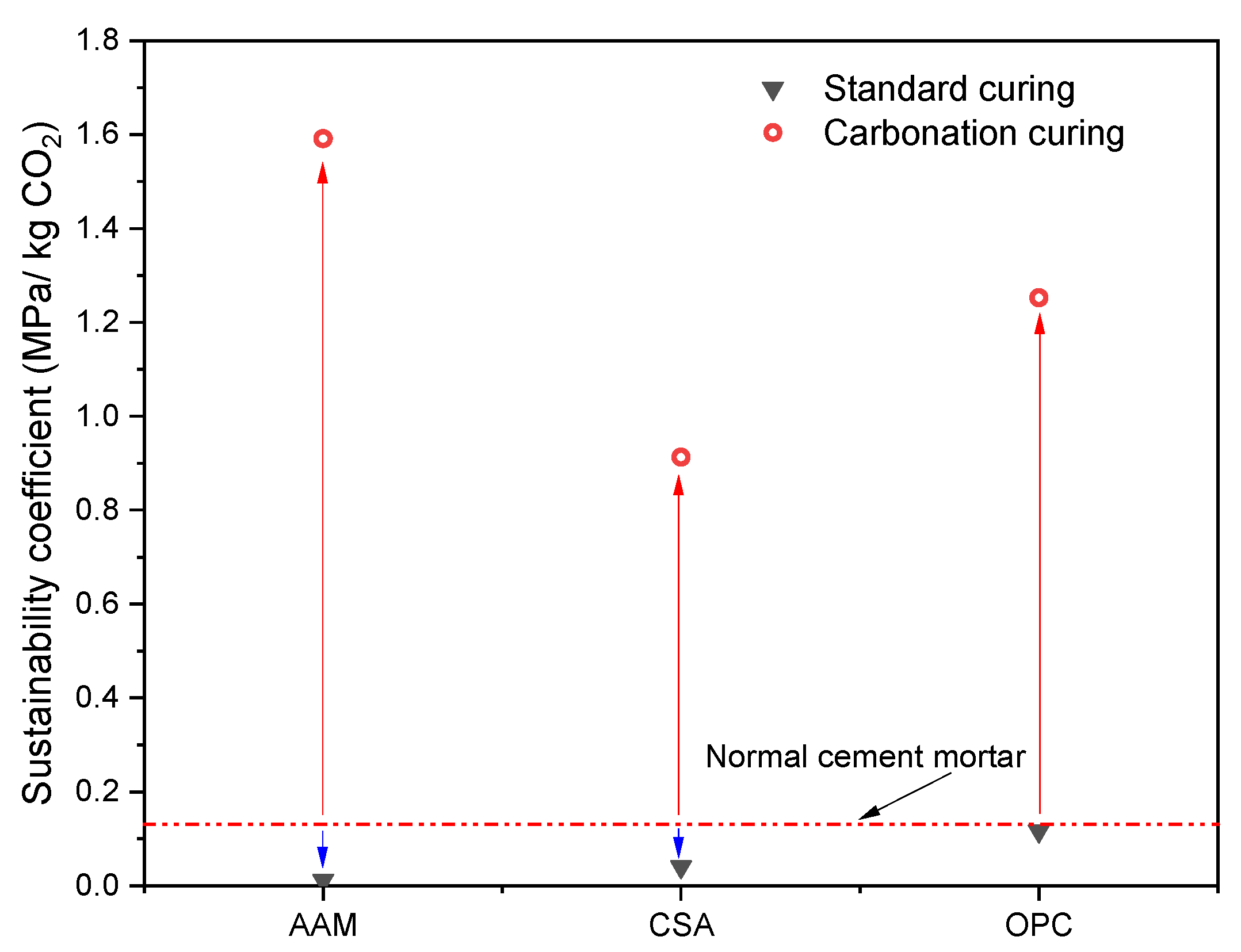
- Sustainability coefficient: The sustainability coefficient (SC) of mortars after carbonation curing was evaluated using the calculation according to [28]. The CO2 emissions per kilogram of OPC, CSA, GBS, sand, and water are 0.85 kg, 0.54 kg, 0.035 kg, 0.004 kg, and 0.0006 kg, respectively [29]. The CO2 emission of MS was calculated as natural limestone; the value was selected as 0.0245 kg.
2.2.7. Leaching Properties
- One batch leaching test: Mortars after 3 days of standard curing and 7 days of carbonation curing were crushed into fractions (<4 mm) for a dynamic leaching process. The distilled water was mixed with mortar fractions with a mass ratio of 10:1, the leaching duration was 24 h, and the shaking frequency was 250 rpm (EN 12457-2) [30]. After the leaching, the leachates were collected using the filter (0.45 μm). Afterward, the filtrates were acidified and tested using ICP-OES.
3. Results
3.1. The Fresh Behavior and Mechanical Performance Evolution of MS-Blended Mortars with Various Hydraulic Materials
3.1.1. Flowability of MS-Blended Mortars with OPC, CSA, and AAM
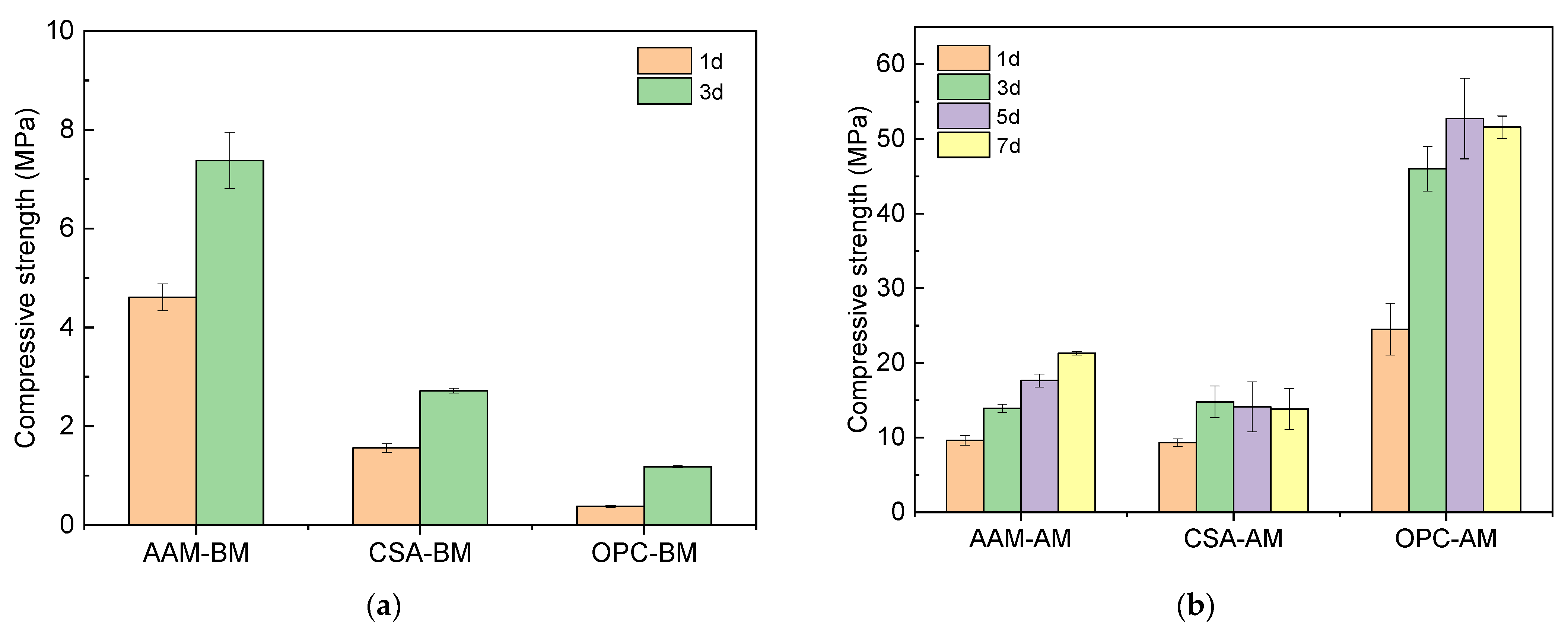
3.1.2. Mechanical Performance of MS-Blended Mortars with OPC, CSA, and AAM After Carbonation Curing
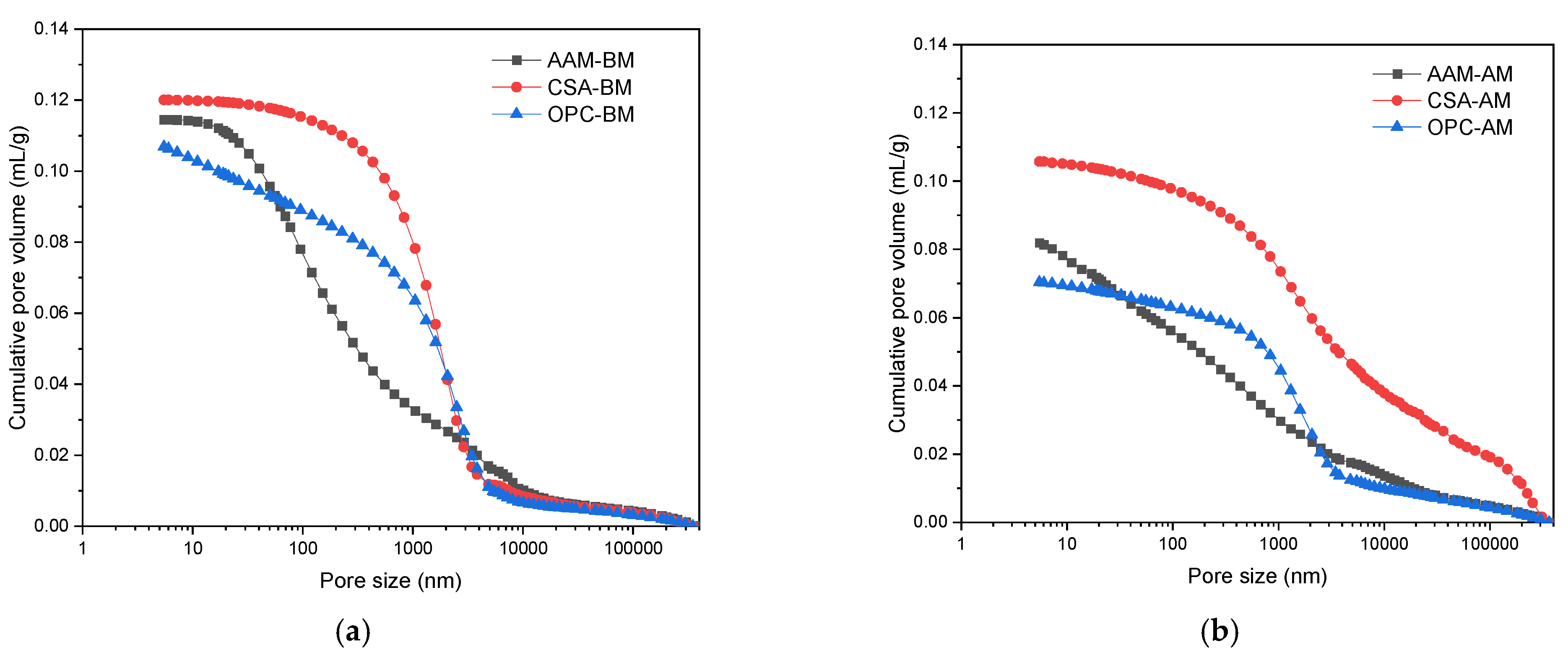
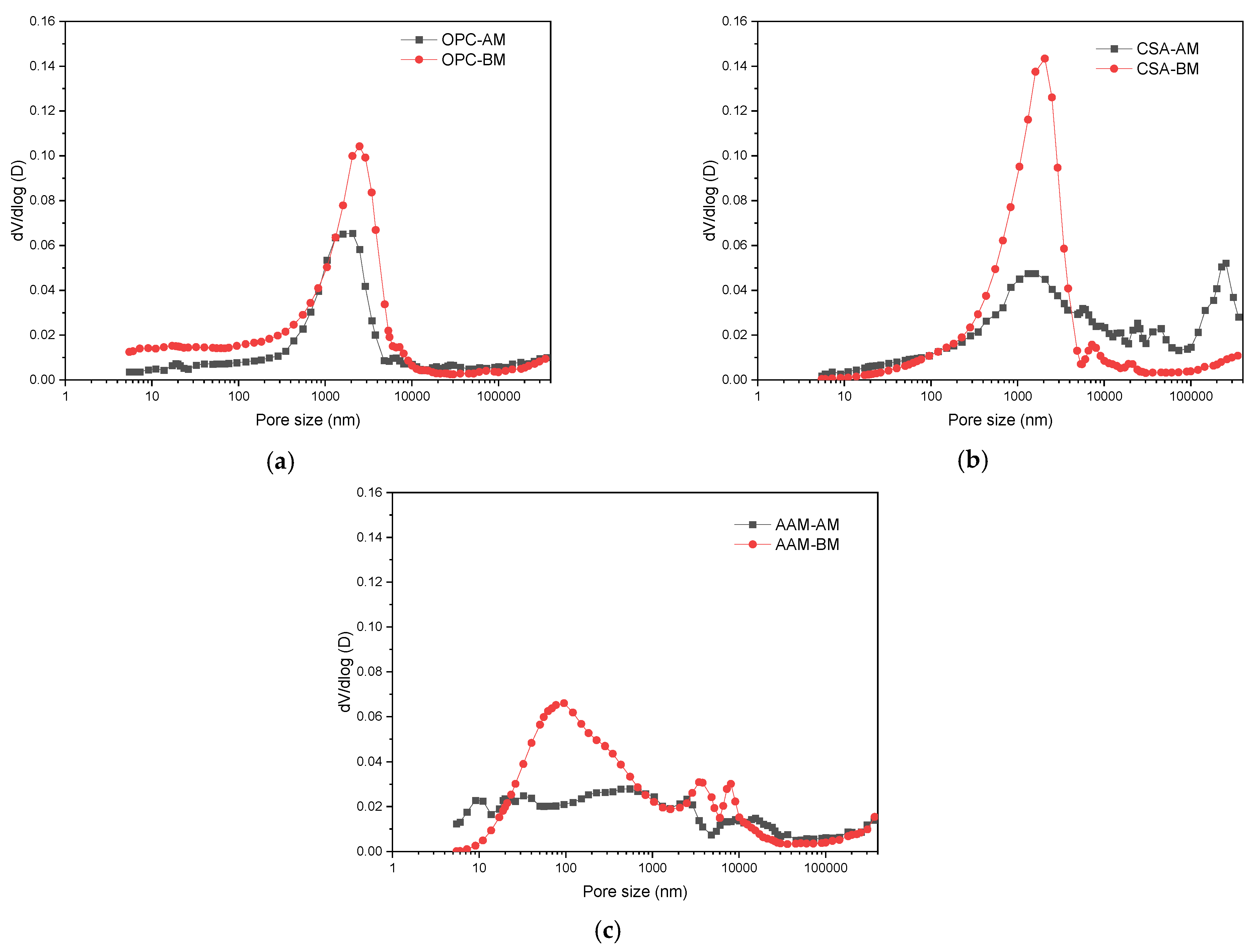
3.1.3. Pore Structure Evolution of MS Mortars with OPC, CSA, and AAM After Carbonation Curing
3.2. The Carbonation Coefficient of CO2-Cured MS-Blended Mortars
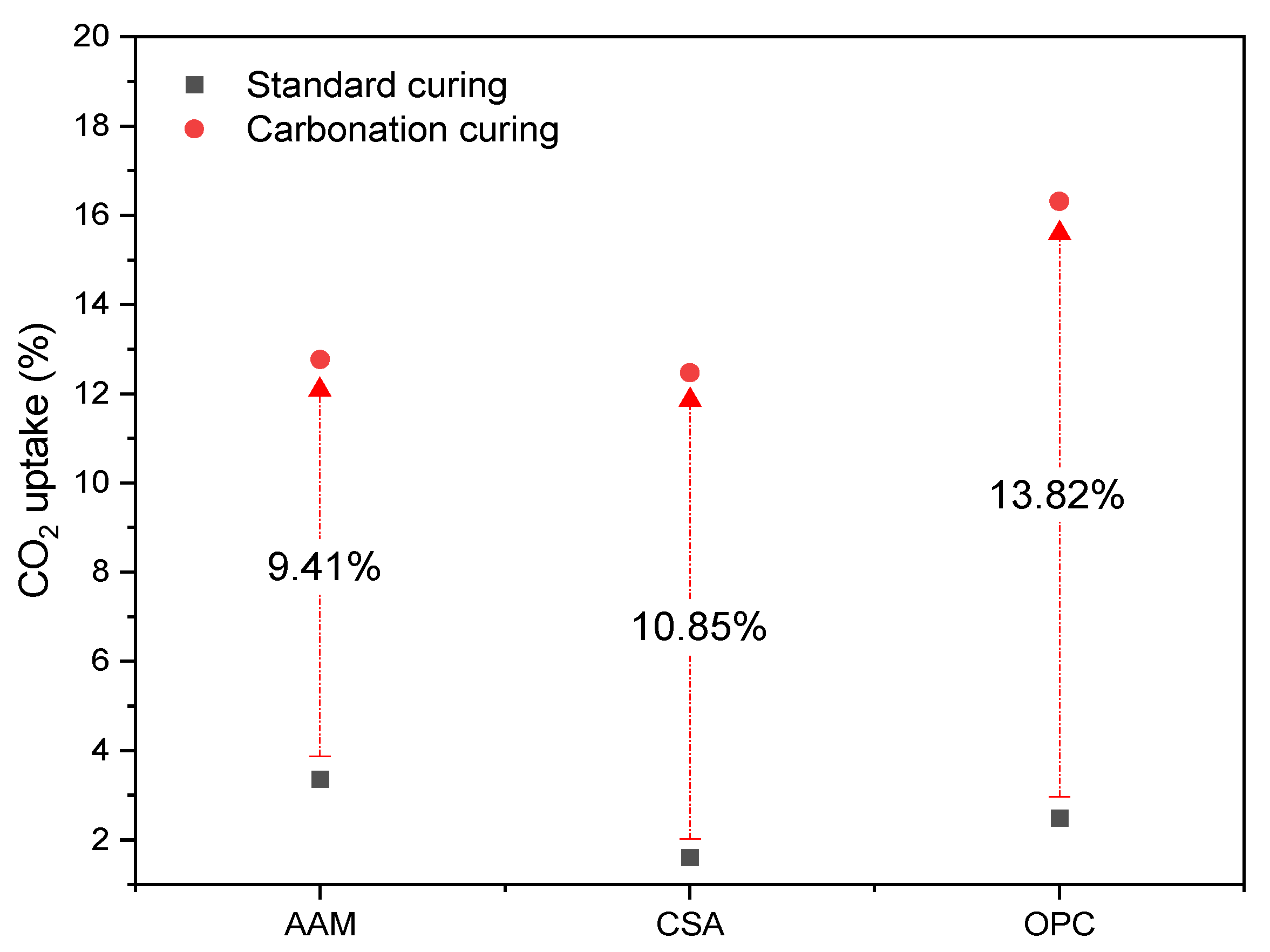
3.2.1. The CO2 Uptake Ability of MS-Blended Binder with OPC, CSA, and AAM
3.2.2. Carbonation Front Development in MS-Blended Mortars with OPC, CSA, and AAM
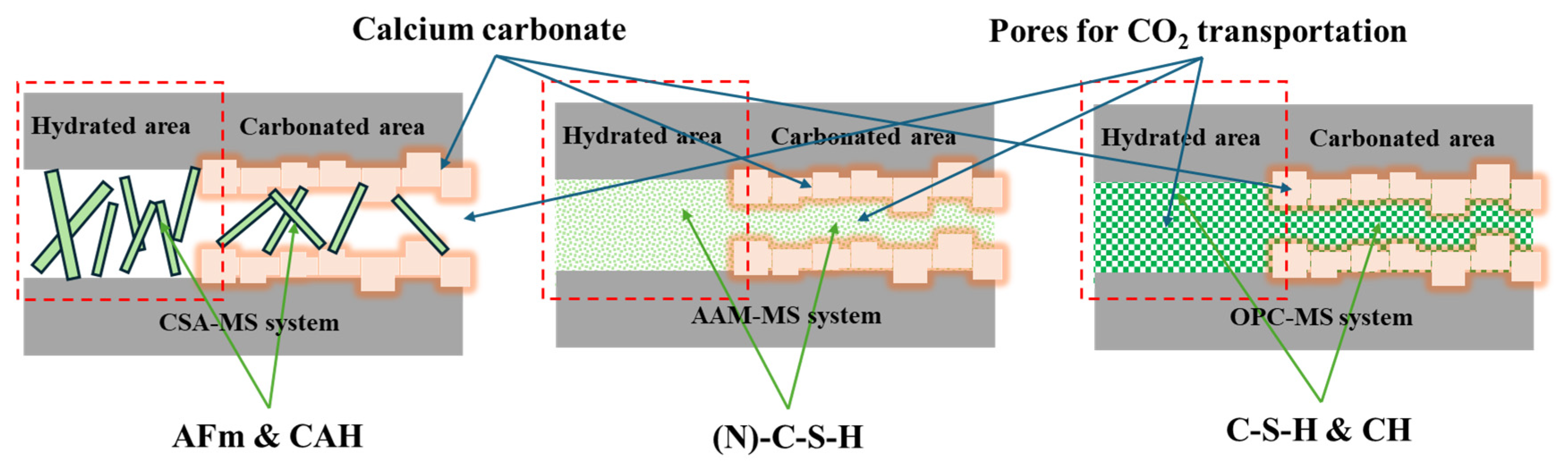
3.3. The Reaction Products of MS-Based Composites Before and After Carbonation
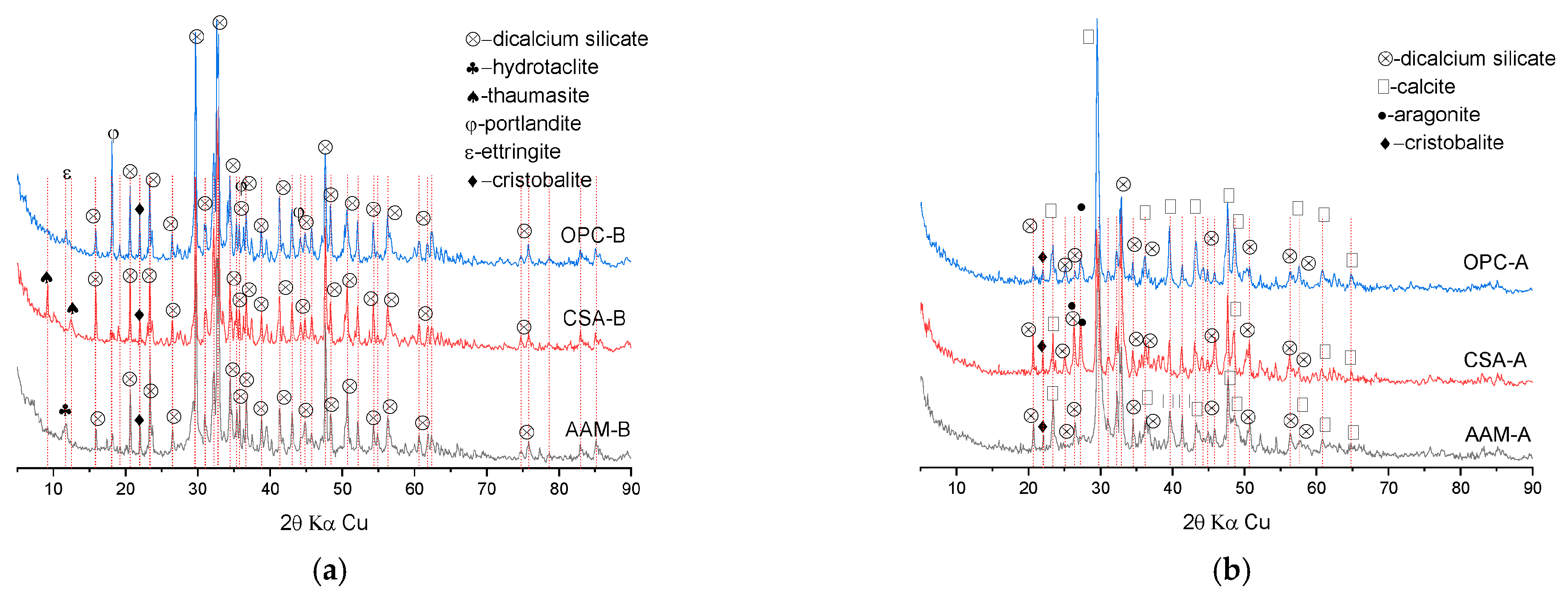
3.3.1. Mineral Phases Identification of Reaction Products
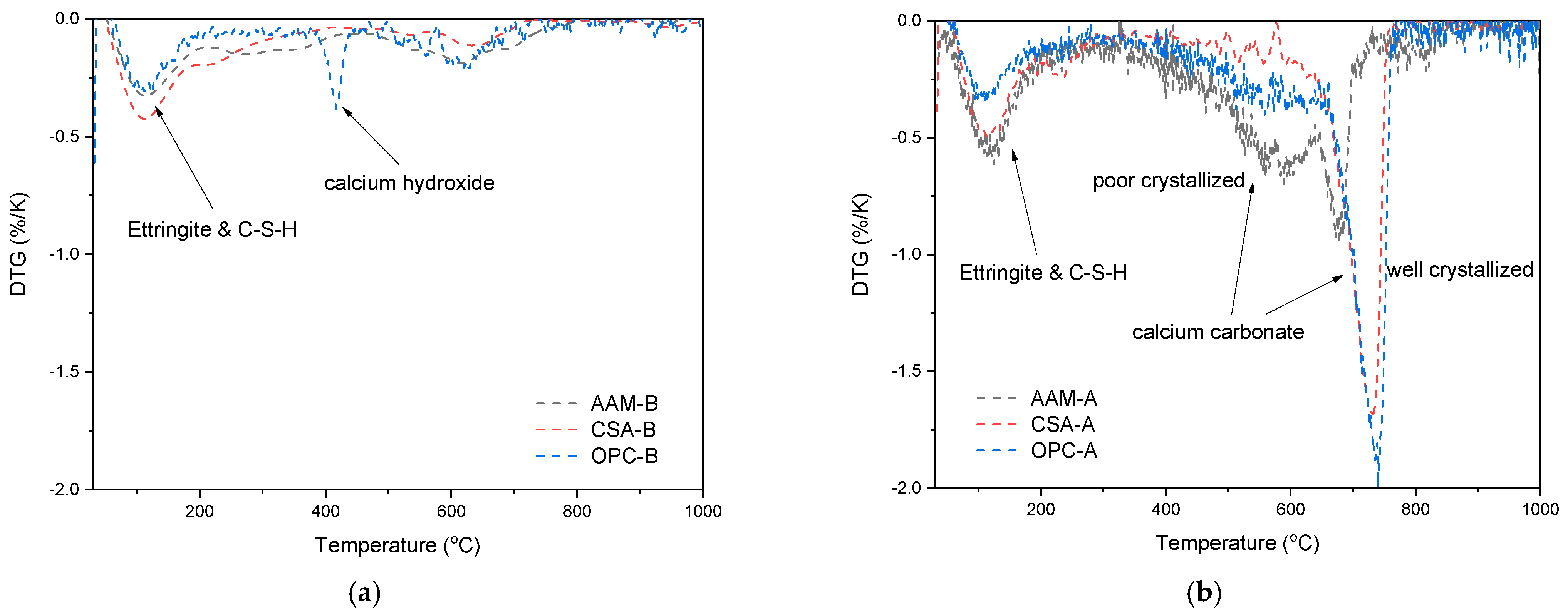
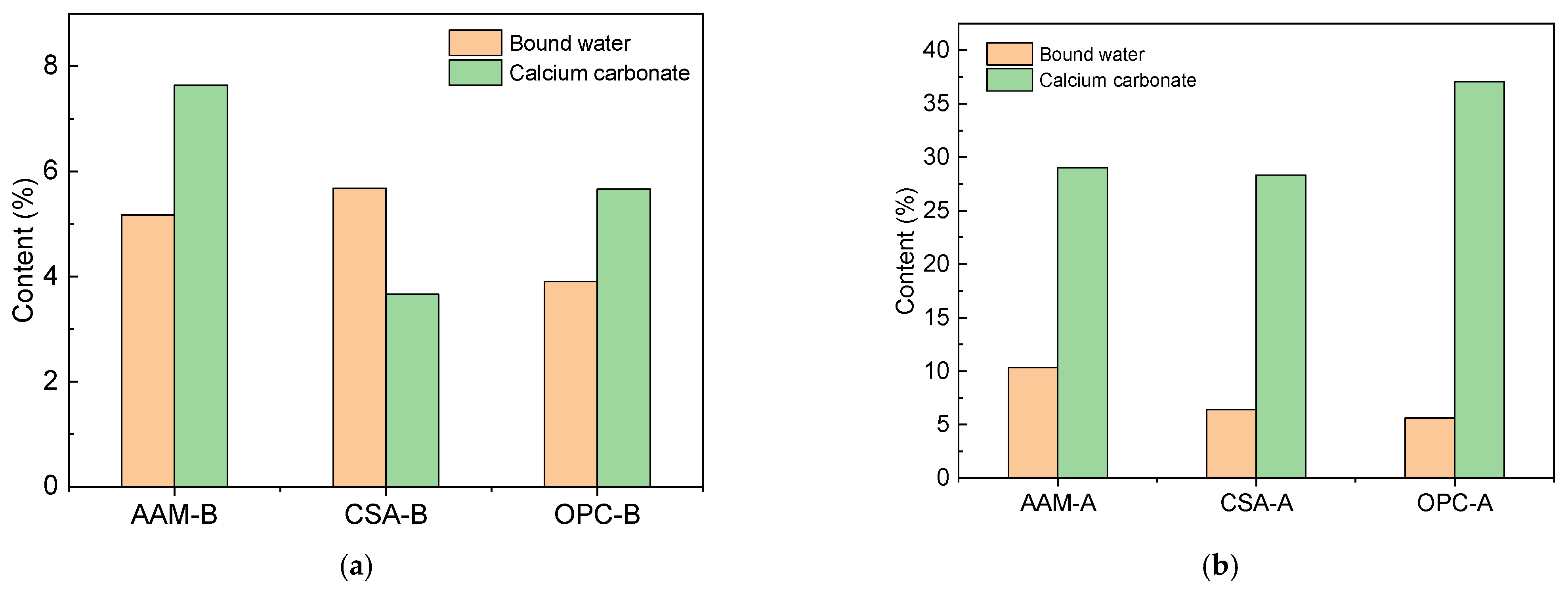
3.3.2. Thermal Gravimetric Analysis of Reaction Products
3.3.3. Morphology of Reaction Products
3.4. The Sustainability of MS-Based Composites
3.5. Leaching of Hazardous Elements
4. Discussion
5. Conclusions
- (1)
- The application of alkali-activated GBS in the preparation of MS mortars resulted in the highest compressive strength of 7.38 MPa compared to CSA (2.72 MPa) and OPC (1.18 MPa) after the hydration stage. After carbonation, the compressive strength of AAM-MS mortar, OPC-MS mortar, and CSA-MS mortar achieved 21.28 MPa, 9.3 MPa, and 51.58 MPa, respectively.
- (2)
- The duration of full carbonation of blended mortar was 1 day, 3 days, and more than 7 days for CSA-, OPC-, and AAM-blended samples, respectively. The CO2 uptake ability achieved 13.82%, 10.85%, and 9.51% by the OPC-, CSA-, and AAM-blended binders, respectively.
- (3)
- The application of CSA in MS-blended mortar contributed to the highest porosity and volume of large pores compared to AAM and OPC. In the carbonated CSA-blended binder, well-crystallized calcite and aragonite were identified. Calcite was the only calcium carbonate polymorph in the carbonated OPC-blended binder, while an amount of poorly crystallized calcite existed in AAM-blended sample.
- (4)
- The leaching of Cd, Cr, Cu, Mn, Ni, and Pb from carbonated MS mortars satisfied the requirement of National Class I solid wastes. The Hg leaching was slightly higher than the requirement (<50 µg/L). AAM-based binders exhibited a higher ability on the leaching inhibition of Cr, Cu, and Pb.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Qin, D.; Hu, Y.; Ahmad, W.; Ahmad, A.; Aslam, F.; Joyklad, P. A Systematic Review of Waste Materials in Cement-Based Composites for Construction Applications. J. Build. Eng. 2022, 45, 103447. [Google Scholar] [CrossRef]
- Wu, S.; Shao, Z.; Andrew, R.M.; Bing, L.; Wang, J.; Niu, L.; Liu, Z.; Xi, F. Global CO2 Uptake by Cement Materials Accounts 1930–2023. Sci. Data 2024, 11, 1–11. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, M.; Deetman, S.; Steubing, B.; Lin, H.X.; Hernandez, G.A.; Harpprecht, C.; Zhang, C.; Tukker, A.; Behrens, P. Global Greenhouse Gas Emissions from Residential and Commercial Building Materials and Mitigation Strategies to 2060. Nat. Commun. 2021, 12, 6126. [Google Scholar] [CrossRef]
- Perez-Cortes, P.; Escalante-Garcia, J.I. Design and Optimization of Alkaline Binders of Limestone-Metakaolin—A Comparison of Strength, Microstructure and Sustainability with Portland Cement and Geopolymers. J. Clean. Prod. 2020, 273, 123118. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, S.; Sun, Q.; Li, B.; Cai, C.; Xia, Y.; Wei, X.; Mu, Q. Preparation and Microstructure of Fly Ash Geopolymer Paste Backfill Material. J. Clean. Prod. 2019, 225, 376–390. [Google Scholar] [CrossRef]
- Liu, G.; Florea, M.V.A.; Brouwers, H.J.H. Performance Evaluation of Sustainable High Strength Mortars Incorporating High Volume Waste Glass as Binder. Constr. Build. Mater. 2019, 202, 574–588. [Google Scholar] [CrossRef]
- Liang, C.; Li, B.; Guo, M.-Z.; Hou, S.; Wang, S.; Gao, Y.; Wang, X. Effects of Early-Age Carbonation Curing on the Properties of Cement-Based Materials: A Review. J. Build. Eng. 2024, 84, 108495. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical Modeling of CO2 Uptake by Concrete during Accelerated Carbonation Curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, W. Fundamental Understanding of Carbonation Curing and Durability of Carbonation-Cured Cement-Based Composites: A Review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Mechanisms of Carbonation Hydration Hardening in Portland Cements—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0008884621003367 (accessed on 29 October 2024).
- Bagchi, A.K. Historical Perspectives on Development. Int. Handb. Dev. Econ. 2008, 1, 16–31. [Google Scholar]
- Jin, Y.; Xiong, Z.; Feng, W.; Zheng, D.; Ng, S.; Wang, Y. Semi-Dry Carbonated Recycled Concrete Paste as Alternative to Limestone and Its Reactivity in LC3. J. Therm. Anal. Calorim. 2024, 149, 215–7224. [Google Scholar] [CrossRef]
- Poon, C.S.; Shen, P.; Jiang, Y.; Ma, Z.; Xuan, D. Total Recycling of Concrete Waste Using Accelerated Carbonation: A Review. Cem. Concr. Res. 2023, 173, 107284. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M.; Jin, F.; Al-Tabbaa, A.; Wang, A. Accelerated Carbonation and Performance of Concrete Made with Steel Slag as Binding Materials and Aggregates. Cem. Concr. Compos. 2017, 83, 138–145. [Google Scholar] [CrossRef]
- Liu, P.; Mo, L.; Zhang, Z. Effects of Carbonation Degree on the Hydration Reactivity of Steel Slag in Cement-Based Materials. Constr. Build. Mater. 2023, 370, 130653. [Google Scholar] [CrossRef]
- Liu, G.; Schollbach, K.; van der Laan, S.; Tang, P.; Florea, M.V.A.; Brouwers, H.J.H. Recycling and Utilization of High Volume Converter Steel Slag into CO2 Activated Mortars—The Role of Slag Particle Size. Resour. Conserv. Recycl. 2020, 160, 104883. [Google Scholar] [CrossRef]
- Liu, G.; Rong, H.; Wang, J. Valorization of Converter Steel Slag in Sustainable Mortars by a Combined Alkali and Carbonation Activation. J. Clean. Prod. 2022, 370, 133519. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Hu, X. Utilization of Accelerated Carbonation to Enhance the Application of Steel Slag: A Review. J. Sustain. Cem.-Based Mater. 2023, 12, 471–486. [Google Scholar] [CrossRef]
- Song, Q.; Guo, M.-Z.; Wang, L.; Ling, T.-C. Use of Steel Slag as Sustainable Construction Materials: A Review of Accelerated Carbonation Treatment. Resour. Conserv. Recycl. 2021, 173, 105740. [Google Scholar] [CrossRef]
- Yang, X.; Dong, F.; Zhang, X.; Li, C.; Gao, Q. Review on Comprehensive Utilization of Magnesium Slag and Development Prospect of Preparing Backfilling Materials. Minerals 2022, 12, 1415. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Li, X. Environmental Risks for Application of Magnesium Slag to Soils in China. J. Integr. Agric. 2020, 19, 1671–1679. [Google Scholar] [CrossRef]
- Amini, O.; Ghasemi, M. Laboratory Study of the Effects of Using Magnesium Slag on the Geotechnical Properties of Cement Stabilized Soil. Constr. Build. Mater. 2019, 223, 409–420. [Google Scholar] [CrossRef]
- GB 8076-2008; National Standard for Concrete Admixtures. Standards Press of China (SPC): Beijing, China, 2008.
- Sasui, S.; Kim, G.; Nam, J.; van Riessen, A.; Eu, H.; Chansomsak, S.; Alam, S.F.; Cho, C.H. Incorporation of Waste Glass as an Activator in Class-C Fly Ash/GGBS Based Alkali Activated Material. Materials 2020, 13, 3906. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength. Standards Press of China (SPC): Beijing, China, 2021.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. Standards Press of China (SPC): Beijing, China, 2005.
- JGJT23-2011; Technical Specification for Inspection of Concrete Compressive Strength by Rebound Method. Standards Press of China (SPC): Beijing, China, 2011.
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the Eco-Efficiency of Cement Use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service Life and Life Cycle Assessment of Reinforced Concrete Systems with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]
- EN 12457-2:2002; Characterisation of Waste—Leaching, 2022nd ed. CEN: Milan, Italy, 2004.
- Cortes, D.; Kim, H.-K.; Palomino, A.; Santamarina, J. Rheological and Mechanical Properties of Mortars Prepared with Natural and Manufactured Sands. Cem. Concr. Res. 2008, 38, 1142–1147. [Google Scholar] [CrossRef]
- Yahia, A.; Tanimura, M.; Shimoyama, Y. Rheological Properties of Highly Flowable Mortar Containing Limestone Filler-Effect of Powder Content and W/C Ratio. Cem. Concr. Res. 2005, 35, 532–539. [Google Scholar] [CrossRef]
- Mohan, M.K.; Rahul, A.V.; De Schutter, G.; Van Tittelboom, K. Early Age Hydration, Rheology and Pumping Characteristics of CSA Cement-Based 3D Printable Concrete. Constr. Build. Mater. 2021, 275, 122136. [Google Scholar] [CrossRef]
- Chaunsali, P.; Mondal, P. Physico-Chemical Interaction between Mineral Admixtures and OPC–Calcium Sulfoaluminate (CSA) Cements and Its Influence on Early-Age Expansion. Cem. Concr. Res. 2016, 80, 10–20. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, H.; Liu, W.; Zhao, H.; Dong, D.; Zhao, P.; Lu, L. Improved Early-Age and Late-Age Performances of Calcium Sulphoaluminate Cement with the Presence of Calcium Nitrate. Constr. Build. Mater. 2022, 327, 126927. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, Z.; Duan, Z.; Zhang, C. Pore Structure Characteristics, Modulation and Its Effect on Concrete Properties: A Review. Constr. Build. Mater. 2023, 397, 132430. [Google Scholar] [CrossRef]
- Wang, F.; Sun, C. Effect of Pore Structure on Compressive Strength and Permeability of Planting Recycled Concrete. Constr. Build. Mater. 2023, 394, 132167. [Google Scholar] [CrossRef]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Kunhi Mohamed, A. Advances in Understanding Cement Hydration Mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Galan, I.; Steindl, F.; Grengg, C.; Dietzel, M.; Mittermayr, F. On the Hydration of Ternesite and the Formation of Thaumasite. Cem. Concr. Res. 2023, 172, 107212. [Google Scholar] [CrossRef]
- Thomas, J.J.; Ghazizadeh, S.; Masoero, E. Kinetic Mechanisms and Activation Energies for Hydration of Standard and Highly Reactive Forms of β-Dicalcium Silicate (C2S). Cem. Concr. Res. 2017, 100, 322–328. [Google Scholar] [CrossRef]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Carbonation of Calcium Sulfoaluminate Mortars. Cem. Concr. Compos. 2017, 80, 123–134. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Durdzinski, P.; Bullerjahn, F.; Skibsted, J.; Ben Haha, M. Effect of Carbonated Cement Paste on Composite Cement Hydration and Performance. Cem. Concr. Res. 2020, 134, 106090. [Google Scholar] [CrossRef]
- Liu, G.; Florea, M.; Brouwers, H. The Role of Recycled Waste Glass Incorporation on the Carbonation Behaviour of Sodium Carbonate Activated Slag Mortar. J. Clean. Prod. 2021, 292, 126050. [Google Scholar] [CrossRef]
- Kamath, M.; Prashant, S.; Kumar, M. Micro-Characterisation of Alkali Activated Paste with Fly Ash-GGBS-Metakaolin Binder System with Ambient Setting Characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A Hydration Study of Various Calcium Sulfoaluminate Cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Effects of Thin-Film Accelerated Carbonation on Steel Slag Leaching. J. Hazard. Mater. 2015, 286, 369–378. [Google Scholar] [CrossRef]
- Xu, J.; Kleja, D.B.; Biester, H.; Lagerkvist, A.; Kumpiene, J. Influence of Particle Size Distribution, Organic Carbon, pH and Chlorides on Washing of Mercury Contaminated Soil. Chemosphere 2014, 109, 99–105. [Google Scholar] [CrossRef]
- Orhan, G. Leaching and Cementation of Heavy Metals from Electric Arc Furnace Dust in Alkaline Medium. Hydrometallurgy 2005, 78, 236–245. [Google Scholar] [CrossRef]
- Zuo, W.; Zhao, X.; Luo, J.; He, Z.; Zhao, J. Investigating the Impact of Carbonation Curing Concentrations on the Mechanical Properties and Microstructure of Recycled Concrete. J. Build. Eng. 2024, 96, 110465. [Google Scholar] [CrossRef]
- Shen, P.; Lu, L.; He, Y.; Wang, F.; Hu, S. The Effect of Curing Regimes on the Mechanical Properties, Nano-Mechanical Properties and Microstructure of Ultra-High Performance Concrete. Cem. Concr. Res. 2019, 118, 1–13. [Google Scholar] [CrossRef]
- Park, S.; Jeong, Y.; Moon, J.; Lee, N. Hydration Characteristics of Calcium Sulfoaluminate (CSA) Cement/Portland Cement Blended Pastes. J. Build. Eng. 2021, 34, 101880. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration Products of Alkali Activated Slag Cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Tawfik, A.; Mohammed, M.S.; Abd-El-Raoof, F.; Aggor, F.; Ahmed, E.M. Heat-Treated Portland Cement Pastes Incorporating Superabsorbent Hydrogels for Precast Applications. Interceram-Int. Ceram. Rev. 2018, 67, 30–37. [Google Scholar] [CrossRef]
- Nishikawa, T.; Suzuki, K.; Ito, S.; Sato, K.; Takebe, T. Decomposition of Synthesized Ettringite by Carbonation. Cem. Concr. Res. 1992, 22, 6–14. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of Alkali Dosage and Silicate Modulus on Carbonation of Alkali-Activated Slag Mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Azar, P.; Patapy, C.; Samson, G.; Cussigh, F.; Frouin, L.; Cyr, M. Effect of Natural and Accelerated Carbonation on Microstructure and pH of Sodium Carbonate Alkali-Activated Slag. Cem. Concr. Res. 2024, 181, 107525. [Google Scholar] [CrossRef]
- Leemann, A.; Moro, F. Carbonation of Concrete: The Role of CO2 Concentration, Relative Humidity and CO2 Buffer Capacity. Mater. Struct. 2017, 50, 30. [Google Scholar] [CrossRef]
- Siddique, S.; Naqi, A.; Jang, J.G. Influence of Water to Cement Ratio on CO2 Uptake Capacity of Belite-Rich Cement upon Exposure to Carbonation Curing. Cem. Concr. Compos. 2020, 111, 103616. [Google Scholar] [CrossRef]





| Chemical Composition | OPC (%) | MS (%) | CSA (%) | GBS (%) |
|---|---|---|---|---|
| Na2O | / | 0.056 | 0.201 | 0.671 |
| MgO | 1.712 | 3.29 | 1.455 | 10.847 |
| Al2O3 | 3.793 | 1.29 | 38.631 | 19.312 |
| SiO2 | 16.188 | 31.35 | 8.178 | 32.526 |
| SO3 | 4.055 | 0.089 | 9.259 | 2.500 |
| K2O | 0.187 | / | 0.366 | 0.287 |
| CaO | 67.968 | 63.43 | 38.285 | 32.365 |
| TiO2 | 0.277 | 0.04 | 1.403 | 0.781 |
| Cr2O3 | 0.01 | / | 0.035 | 0.748 |
| MnO | 0.094 | / | 0.015 | 0.314 |
| Fe2O3 | 3.589 | 0.38 | 1.753 | 0.258 |
| ZnO | 0.1 | 0.004 | / | / |
| Cl | 0.041 | / | 0.067 | / |
| Sample ID | OPC (g) | CSA (g) | GBS (g) | MS (g) | Water (g) | Activator (g) | Sand (g) |
|---|---|---|---|---|---|---|---|
| OPC | 90 | 0 | 0 | 360 | 225 | 0 | 1350 |
| CSA | 0 | 90 | 0 | 360 | 225 | 0 | 1350 |
| AAM | 0 | 0 | 90 | 360 | 225 | 36 | 1350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Liu, S.; Yin, B.; Wang, J. Effects of Hydraulic Materials on the Performance Evolution of Carbonated High-Volume Magnesium Slag Mortars. Buildings 2025, 15, 3062. https://doi.org/10.3390/buildings15173062
Liu G, Liu S, Yin B, Wang J. Effects of Hydraulic Materials on the Performance Evolution of Carbonated High-Volume Magnesium Slag Mortars. Buildings. 2025; 15(17):3062. https://doi.org/10.3390/buildings15173062
Chicago/Turabian StyleLiu, Gang, Shichuang Liu, Bohao Yin, and Jianyun Wang. 2025. "Effects of Hydraulic Materials on the Performance Evolution of Carbonated High-Volume Magnesium Slag Mortars" Buildings 15, no. 17: 3062. https://doi.org/10.3390/buildings15173062
APA StyleLiu, G., Liu, S., Yin, B., & Wang, J. (2025). Effects of Hydraulic Materials on the Performance Evolution of Carbonated High-Volume Magnesium Slag Mortars. Buildings, 15(17), 3062. https://doi.org/10.3390/buildings15173062







