Development of a Low-Density Waste-Based Geopolymer Construction Material
Abstract
:1. Introduction
1.1. Background, Aims, and Contributions
- How can waste fly ash and polystyrene be effectively incorporated into geopolymers to enhance their environmental and economic benefits?
- What are the optimal mix ratios and activator concentrations that result in low-density geopolymers with desirable mechanical properties?
- How do variations in curing conditions, such as temperature, impact the structure and performance of waste-based geopolymers?
- What are the key characteristics and properties of the developed geopolymers?
1.2. Literature Review
1.2.1. Global Perspectives
1.2.2. Fly Ash
1.2.3. Polystyrene
1.2.4. Fly Ash/Polystyrene Geopolymers
2. Material and Methods
2.1. Materials
2.2. Characterization
2.2.1. Laser Diffraction
2.2.2. X-ray Fluorescence (XRF) and Loss on Ignition (LOI)
2.2.3. Specific Gravity
2.2.4. Atterberg Limits Test
2.3. Geopolymer Production
2.4. Testing of Produced Geopolymers
2.4.1. X-ray Diffraction (XRD)
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Unconfined Compressive Strength (UCS)
2.4.4. Density
3. Results and Discussion
3.1. Fly Ash Characterization
3.1.1. XRF Analysis of Fly Ash
3.1.2. Atterberg Limits of Fly Ash
3.2. Geopolymer Testing
3.2.1. Unconfined Compressive Strength (UCS) Test
Varied Sodium Hydroxide Concentrations and Curing Temperature
Varied Polystyrene Concentrations and Curing Temperature
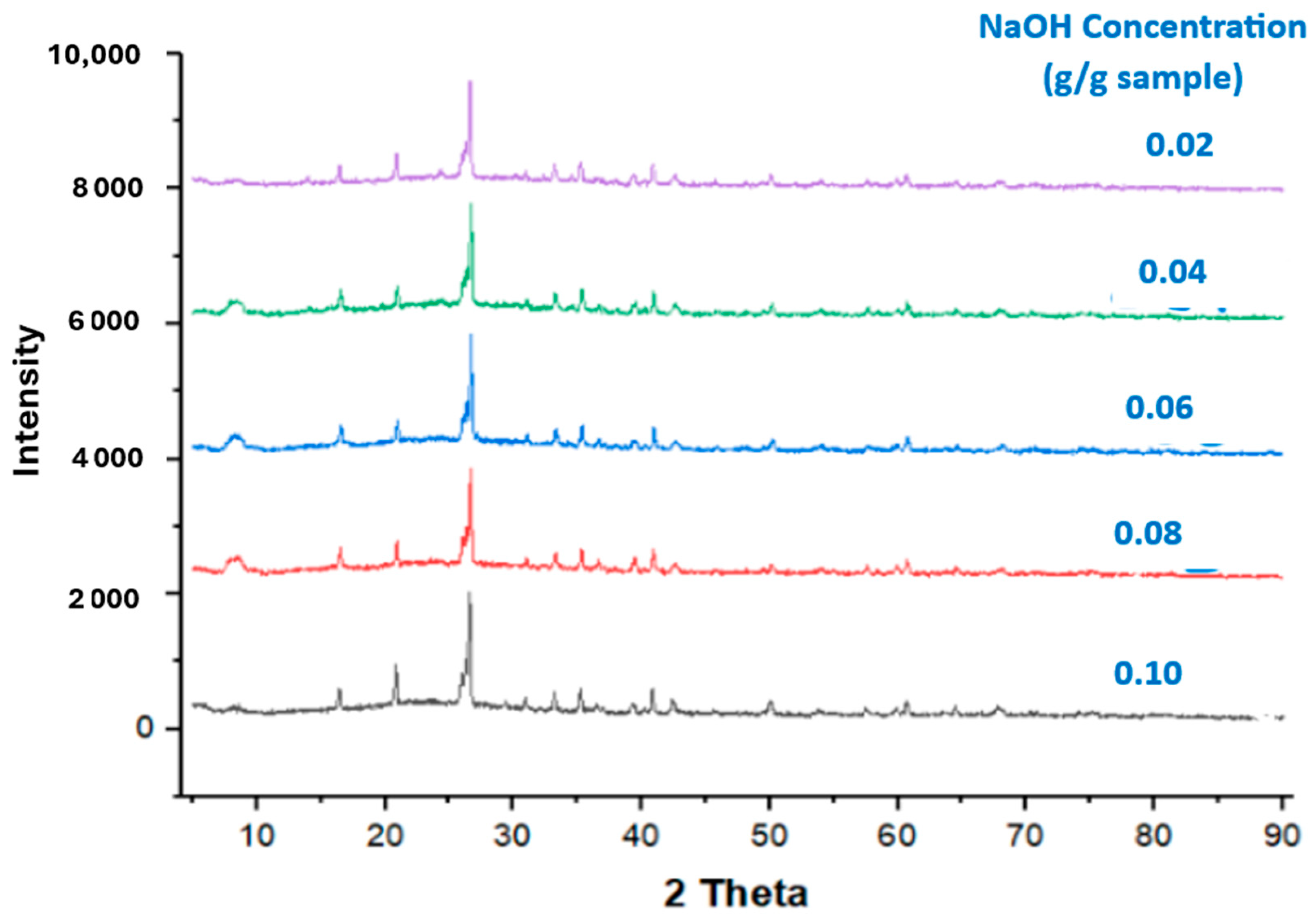
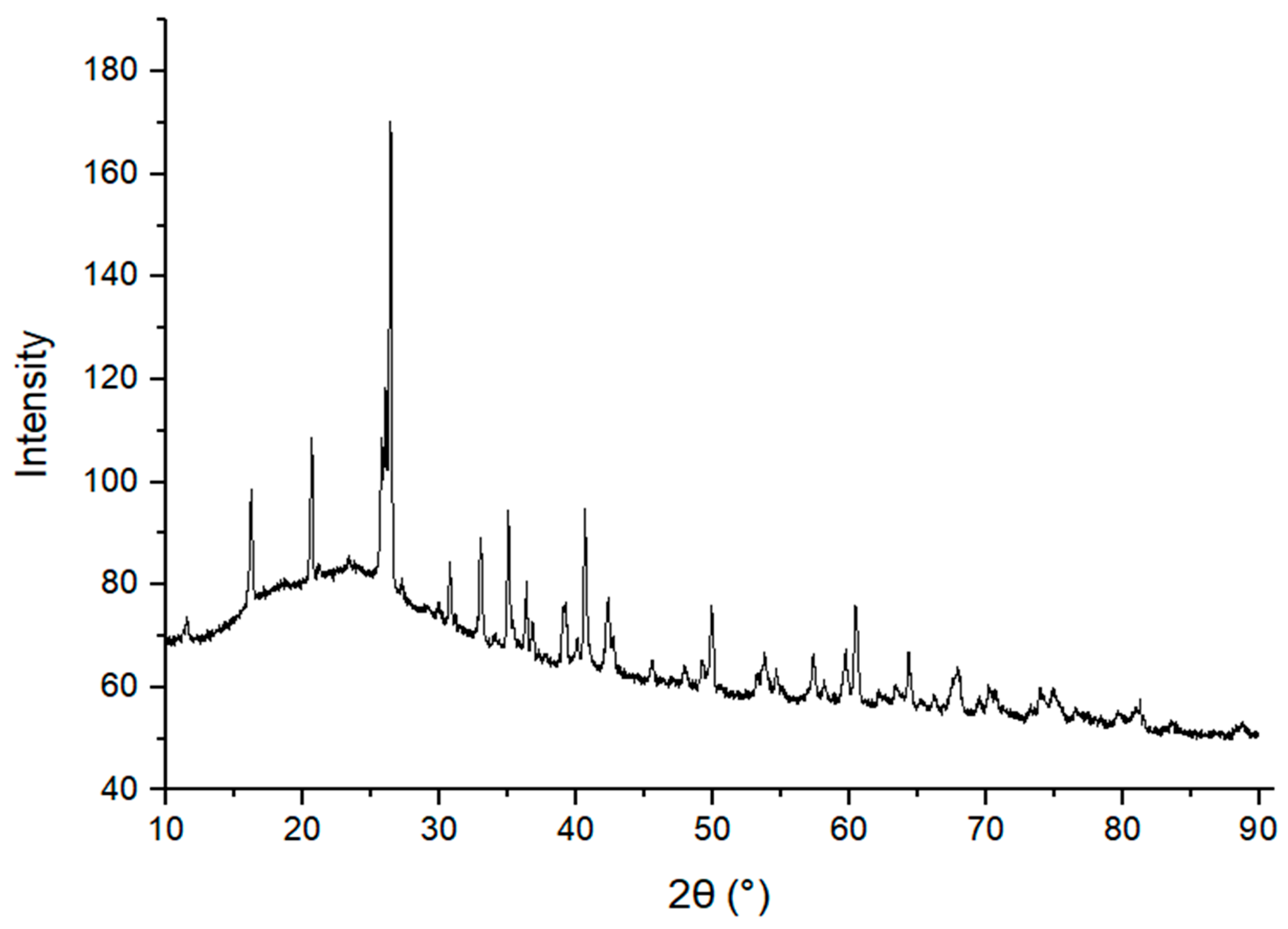
3.2.2. XRD Analysis
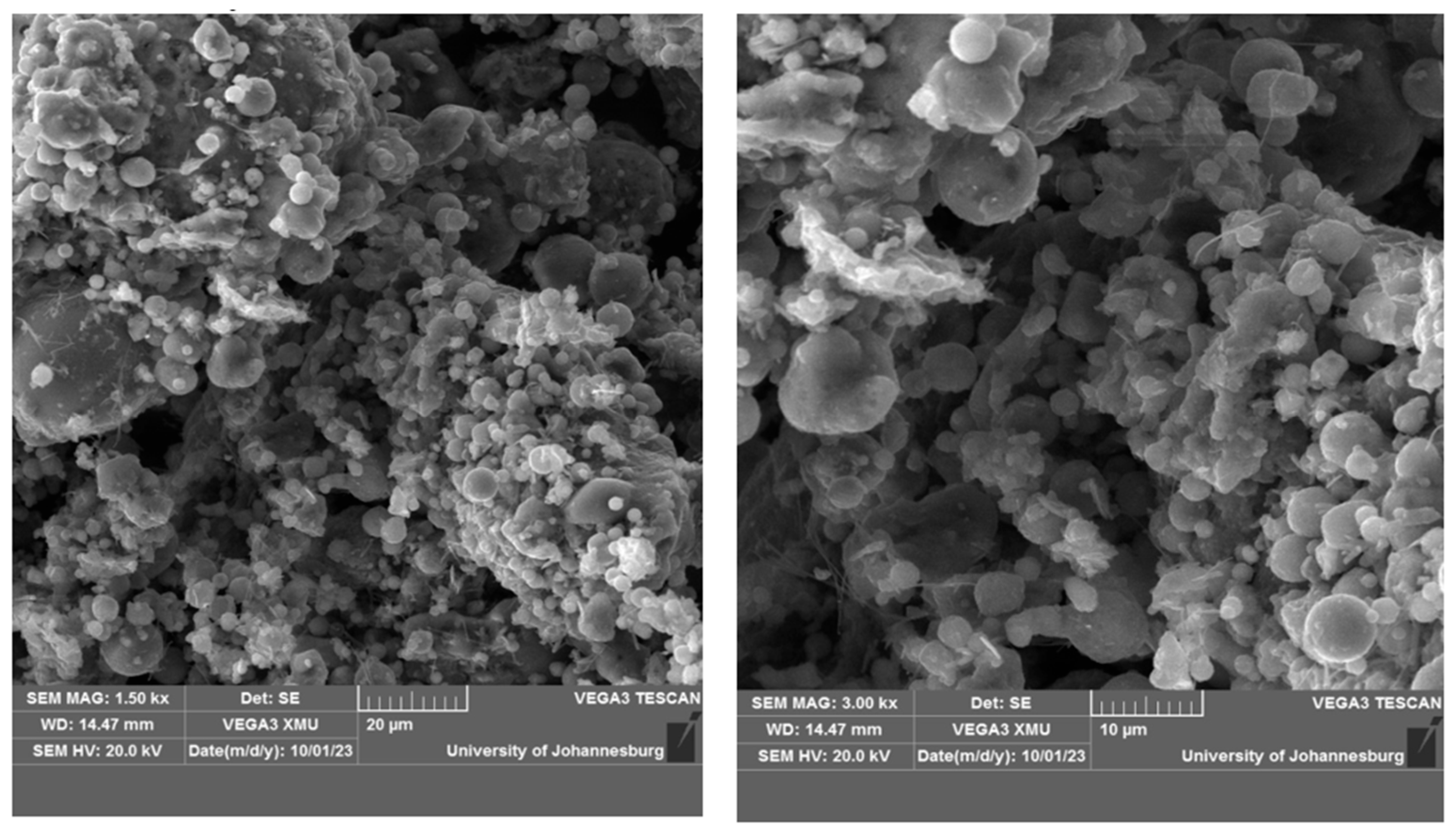
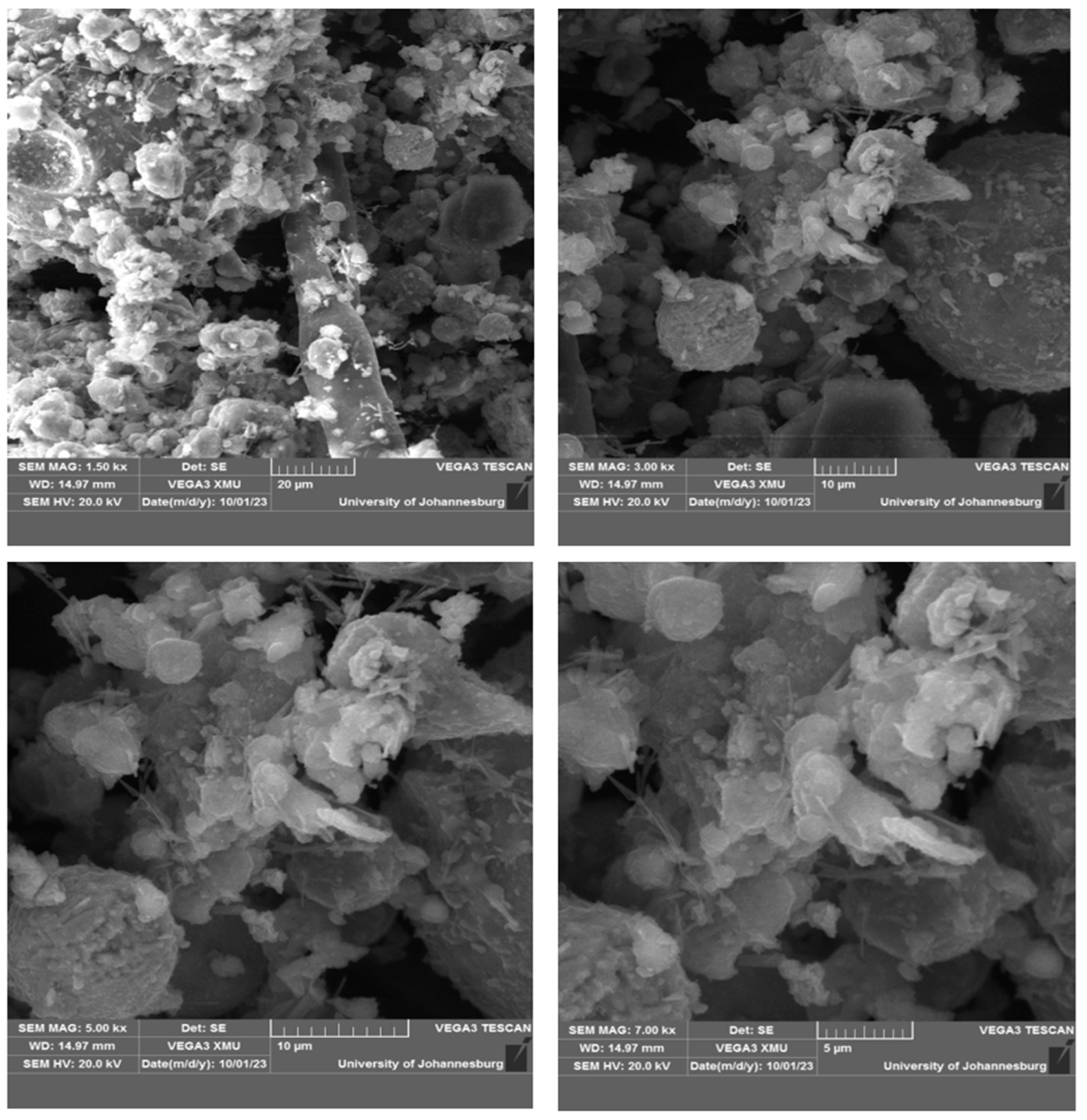
3.2.3. Scanning Electron Microscope (SEM)
3.2.4. FTIR
3.2.5. Density Analysis
3.2.6. Water Absorption Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Komnitsas, K.A. Potential of geopolymer technology towards green buildings and sustainable cities. Procedia Eng. 2011, 21, 1023–1032. [Google Scholar] [CrossRef]
- Roopchund, R.; Andrew, J.; Sithole, B. Using cellulose nanocrystals to improve the mechanical properties of fly ash-based geopolymer construction materials. Eng. Sci. Technol. Int. J. 2022, 25, 100989. [Google Scholar] [CrossRef]
- Henderson, K.; Loreau, M. A model of Sustainable Development Goals: Challenges and opportunities in promoting human well-being and environmental sustainability. Ecol. Model. 2023, 475, 110164. [Google Scholar] [CrossRef]
- Crompton, T.; Kasser, T.; World Wildlife Fund UK (WWF-UK). Meeting Environmental Challenges: The Role of Human Identity; World Wildlife Fund UK (WWF-UK): Surrey, UK, 2009. [Google Scholar]
- Khan, S.; Anjum, R.; Raza, S.T.; Bazai, N.A.; Ihtisham, M. Technologies for municipal solid waste management: Current status, challenges, and future perspectives. Chemosphere 2022, 288, 132403. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.; Varjani, S.; Singhania, R.R.; Patel, A.K.; Awasthi, M.K.; Sindhu, R.; Zhang, Z.; Binod, P.; Awasthi, S.K.; Chaturvedi, P. Critical review on technological advancements for effective waste management of municipal solid waste—Updates and way forward. Environ. Technol. Innov. 2021, 23, 101749. [Google Scholar] [CrossRef]
- Bradu, P.; Biswas, A.; Nair, C.; Sreevalsakumar, S.; Patil, M.; Kannampuzha, S.; Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; et al. Recent advances in green technology and Industrial Revolution 4.0 for a sustainable future. Environ. Sci. Pollut. Res. 2022, 30, 124488–124519. [Google Scholar] [CrossRef] [PubMed]
- Meesala, C.R.; Verma, N.K.; Kumar, S. Critical review on fly-ash based geopolymer concrete. Struct. Concr. 2020, 21, 1013–1028. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Heyns, M.W.; Hassan, M.M. South Africa Class F Fly Ash for roads: Physical and chemical analysis. Interim Interdiscip. J. 2013, 12, 28–41. [Google Scholar]
- Fauzi, A.; Nuruddin, M.F.; Malkawi, A.B.; Abdullah, M.M.A.B. Study of Fly Ash Characterization as a Cementitious Material. Procedia Eng. 2016, 148, 487–493. [Google Scholar] [CrossRef]
- Gulizia, A.M.; Patel, K.; Philippa, B.; Motti, C.A.; van Herwerden, L.; Vamvounis, G. Understanding plasticiser leaching from polystyrene microplastics. Sci. Total Environ. 2023, 857, 159099. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, W.-Y.; Lee, S.-B.; Kim, S.-B.; Choi, M.-J. Degradation of polystyrene waste over base promoted Fe catalysts. Catal. Today 2003, 87, 59–68. [Google Scholar] [CrossRef]
- Veiseh, S.; Yousefi, A.A. The Use of Polystyrene in Lightweight Brick Production polystyrene foam; lightweight bricks; thermal insulation; pore-forming. Iran. Polym. J. 2003, 12, 323–329. Available online: www.sid.ir (accessed on 23 June 2023).
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. (1980–2015) 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; MacKenzie, K. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Williams, R.P.; van Riessen, A. Determination of the reactive component of fly ashes for geopolymer production using XRF and XRD. Fuel 2010, 89, 3683–3692. [Google Scholar] [CrossRef]
- Arun, N.; Singh, P.; Gupta, S. Utilisation of ground bottom ash in concrete. Mater. Today Proc. 2020, 32, 663–669. [Google Scholar] [CrossRef]
- Andavan, S.; Pagadala, V.K. Experimental study on addition of lime and fly ash for the soil stabilization. Mater. Today Proc. 2020, 22, 1065–1069. [Google Scholar] [CrossRef]
- Nurruddin, E.A. Methods of curing geopolymer concrete: A review. Int. J. Adv. Appl. Sci. 2018, 5, 31–36. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ramaswamy, A. A review on fly ash characteristics—Towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 546–559. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Mádai, F.; Debreczeni, Á. Control of geopolymer properties by grinding of land filled fly ash. Int. J. Miner. Process. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Waijarean, N.; Asavapisit, S.; Sombatsompop, K.; MacKenzie, K.J.D. The Effect of SiO2/Al2O3 Ratios on the Properties of Geopolymers Prepared from Water Treatment Residue (WTR) in the Presence of Heavy Metals. GMSARN Int. J. 2014, 8, 97–102. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Prusty, J.K.; Pradhan, B. Effect of GGBS and chloride on compressive strength and corrosion performance of steel in fly ash-GGBS based geopolymer concrete. Mater. Today Proc. 2020, 32, 850–855. [Google Scholar] [CrossRef]
- Rashid, H.M.A.; Sardar, A.; Ismail, A. Geotechnical characterization of bentonite-fly ash mixtures for their application as landfill liner in Pakistan. Arab. J. Geosci. 2021, 14, 1307. [Google Scholar] [CrossRef]
- Deepak, M.; Rohini, S.; Harini, B.; Ananthi, G.B.G. Influence of fly-ash on the engineering characteristics of stabilised clay soil. Mater. Today Proc. 2021, 37 Pt 2, 2014–2018. [Google Scholar] [CrossRef]
- Alehyen, S.; Achouri, M.E.L.; Taibi, M. Characterization, microstructure and properties of fly ash-based geopolymer. J. Mater. Environ. Sci. 2017, 8, 1783–1796. [Google Scholar]
- Titova, I.; Vladimir, V. The history and development of the classification system for the construction industry. Constr. Unique Build. Struct. 2020, 87, 20–29. (In Russian) [Google Scholar]
- Rambabu, D.; Sharma, S.K.; Akbar, M.A. Properties Exhibited by Nanomaterial Based Geopolymers: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1081–1118. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Sajan, P.; Jiang, T.; Lau, C.; Tan, G.; Ng, K. Combined effect of curing temperature, curing period and alkaline concentration on the mechanical properties of fly ash-based geopolymer. Clean. Mater. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. In Situ ATR-FTIR Study of the Early Stages Geopolymer Gel Formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Ahmad, M.I. Chemical reactions in the geopolymerisation process using fly ash–based geopolymer: A review. Aust. J. Basic Appl. Sci. 2011, 5, 1199–1203. Available online: https://www.researchgate.net/publication/287952700 (accessed on 15 June 2023).
- Thokchom, S.; Ghosh, S.; Ghosh, P. Effect of water absorption, porosity and sorptivity on durability of geopolymer mortars. ARPN J. Eng. Appl. Sci. 2009, 4, 28–32. Available online: https://www.researchgate.net/publication/237215851 (accessed on 18 June 2023).







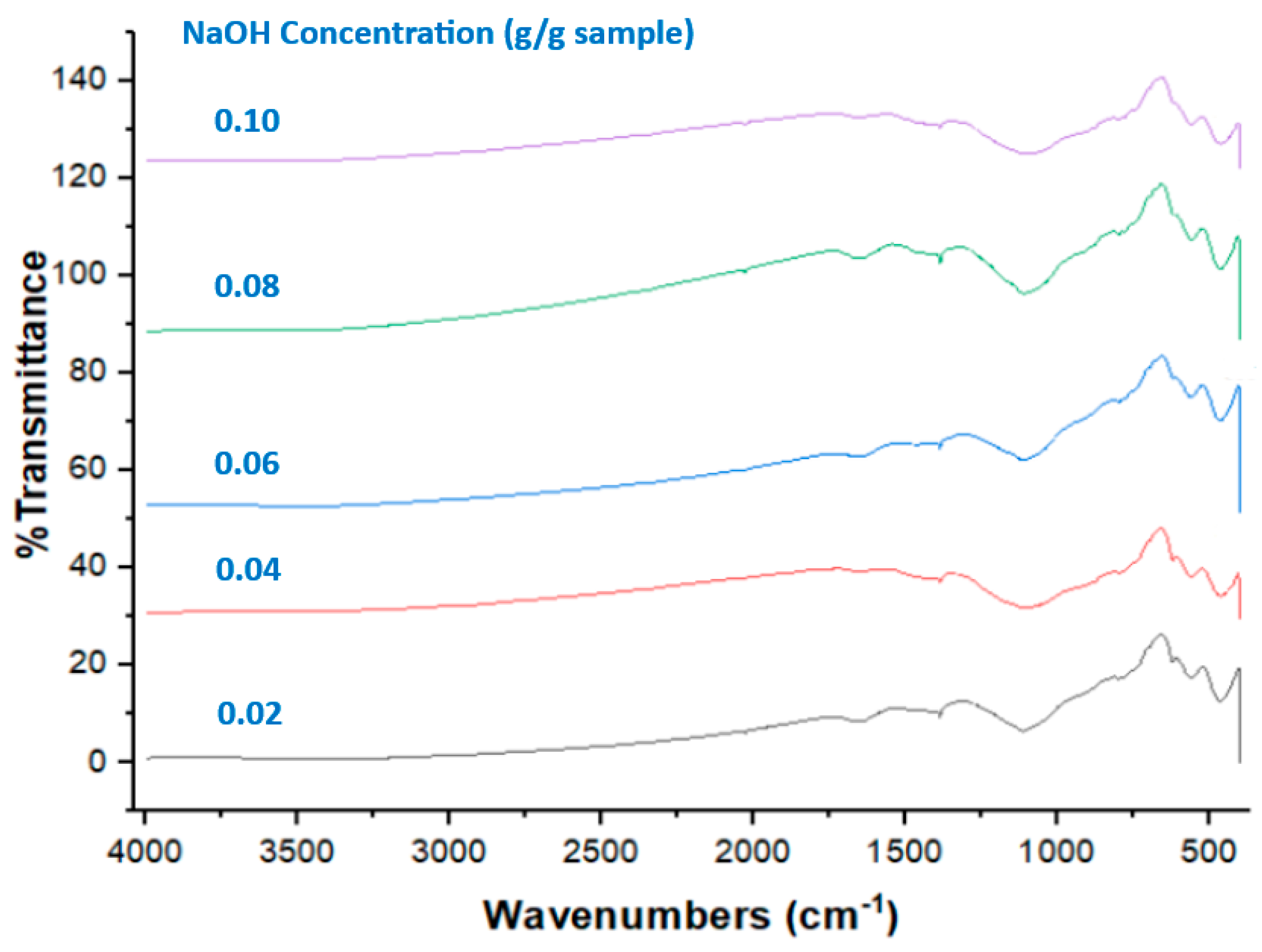

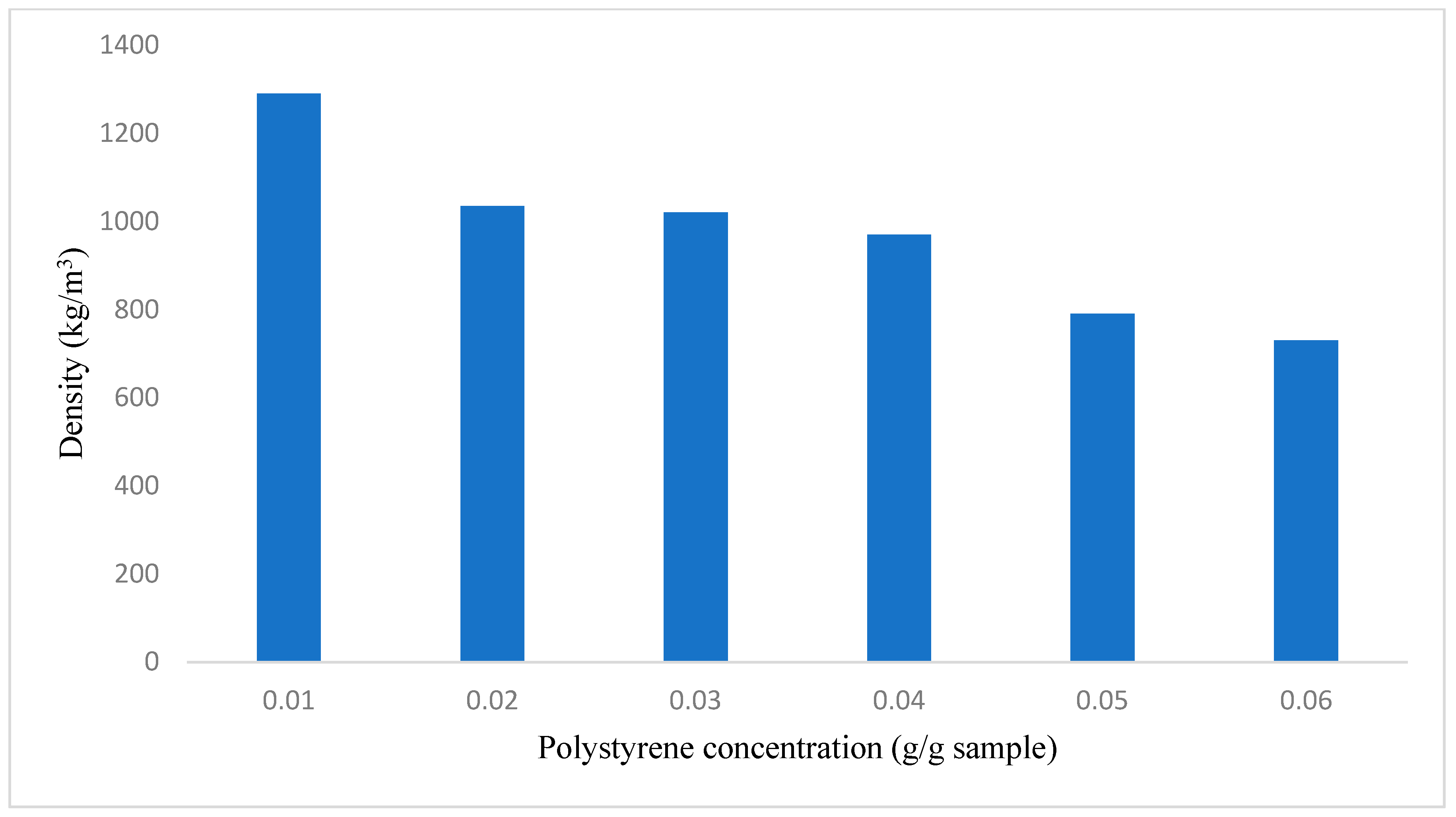
| NaOH Concentration (g/g Sample) | Fly Ash (g) | Water (g) | NaOH (g) | Polystyrene Concentration (g/g Sample) | Fly Ash (g) | Water (g) | NaOH (g) | Polystyrene (g) |
|---|---|---|---|---|---|---|---|---|
| 0.02 | 300 | 110 | 8.5 | 0.01 | 400 | 200 | 52.5 | 6.7 |
| 0.04 | 300 | 110 | 17.2 | 0.02 | 400 | 200 | 52.5 | 13.5 |
| 0.06 | 300 | 110 | 26.5 | 0.03 | 400 | 200 | 52.5 | 20.5 |
| 0.08 | 300 | 110 | 36 | 0.04 | 400 | 200 | 52.5 | 27.5 |
| 0.10 | 300 | 110 | 47 | 0.05 | 400 | 200 | 52.5 | 34.5 |
| 0.06 | 400 | 200 | 52.5 | 42 |
| Component | Composition (%) |
|---|---|
| 0.2219 | |
| MgO | 9.6648 |
| 6.4166 | |
| 38.1873 | |
| 0.1350 | |
| 2.6967 | |
| Cl | 0.0555 |
| 1.0861 | |
| 9.5524 | |
| 1.1964 | |
| 6.5742 | |
| 0.2424 | |
| 23.2966 | |
| 0.3990 | |
| 0.1302 | |
| 0.0500 | |
| 0.0106 | |
| 0.0521 | |
| 0.0323 |
| Liquid Limit (LL) | Plastic Limit (PL) | Plastic Index (PI) |
|---|---|---|
| 49% | 26% | 23% |
| Sodium Hydroxide Concentration (g/g Sample) | (g) | (g) | Water Absorption (%) |
|---|---|---|---|
| 0.02 | 144.30 | 165.73 | 14 |
| 0.04 | 156.54 | 173.89 | 11 |
| 0.06 | 166.80 | 181.03 | 8.53 |
| 0.08 | 169.62 | 176.91 | 4.30 |
| 0.10 | 175.70 | 184.67 | 5.11 |
| Polystyrene Concentration (g/g Sample) | (g) | Water Absorption (%) | |
|---|---|---|---|
| 0.01 | 161.27 | 177.70 | 10 |
| 0.02 | 129.36 | 143.65 | 11.05 |
| 0.03 | 127.58 | 141.75 | 11.11 |
| 0.04 | 121.20 | 135.55 | 11.84 |
| 0.05 | 98.87 | 112.30 | 13.58 |
| 0.06 | 91.29 | 105.78 | 15.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ncube, B.; Roopchund, R. Development of a Low-Density Waste-Based Geopolymer Construction Material. Buildings 2024, 14, 684. https://doi.org/10.3390/buildings14030684
Ncube B, Roopchund R. Development of a Low-Density Waste-Based Geopolymer Construction Material. Buildings. 2024; 14(3):684. https://doi.org/10.3390/buildings14030684
Chicago/Turabian StyleNcube, Brian, and Rishen Roopchund. 2024. "Development of a Low-Density Waste-Based Geopolymer Construction Material" Buildings 14, no. 3: 684. https://doi.org/10.3390/buildings14030684






