The Preparation of Ground Blast Furnace Slag-Steel Slag Pavement Concrete Using Different Activators and Its Performance Investigation
Abstract
1. Introduction
2. Raw Materials and Methods
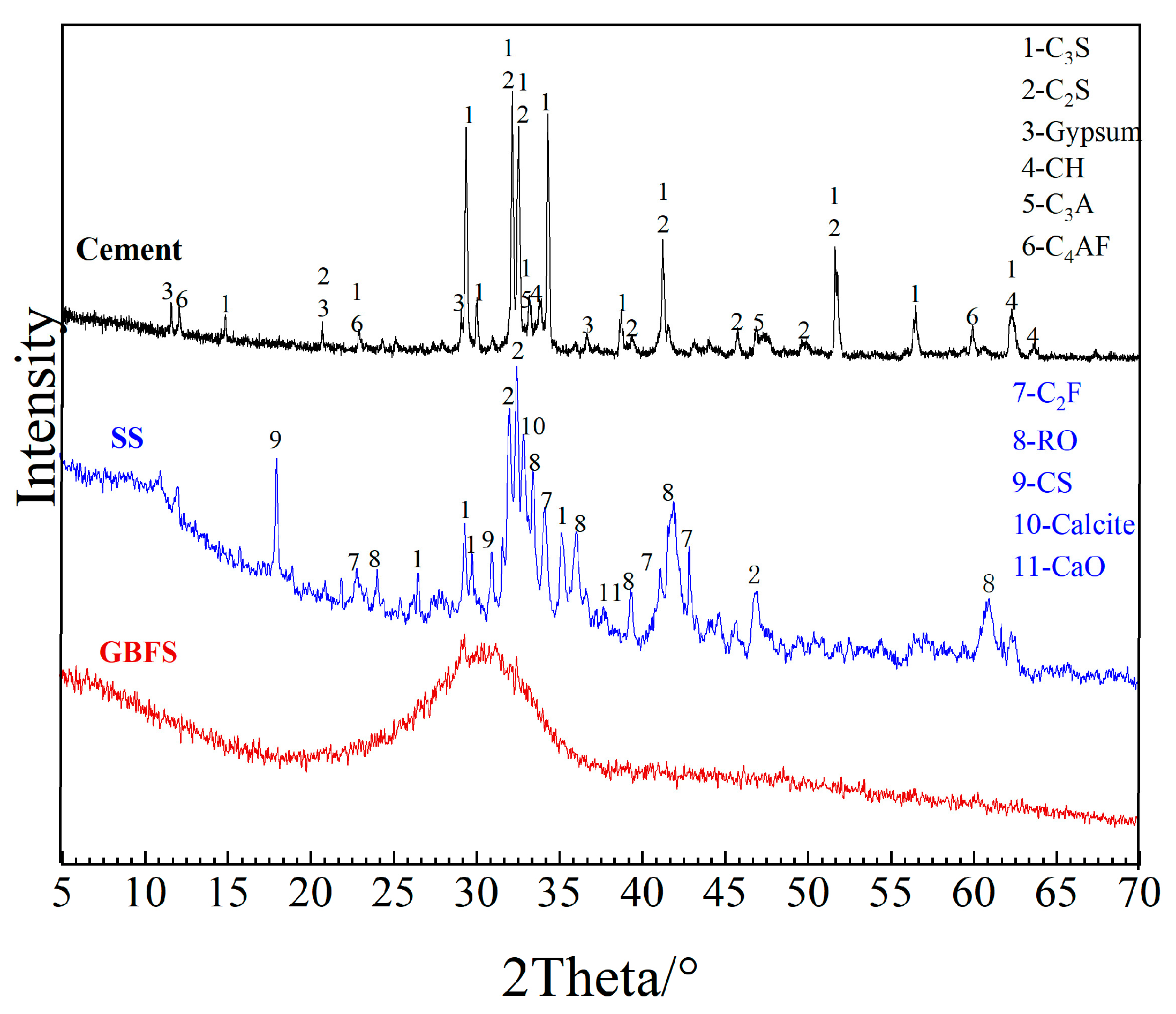
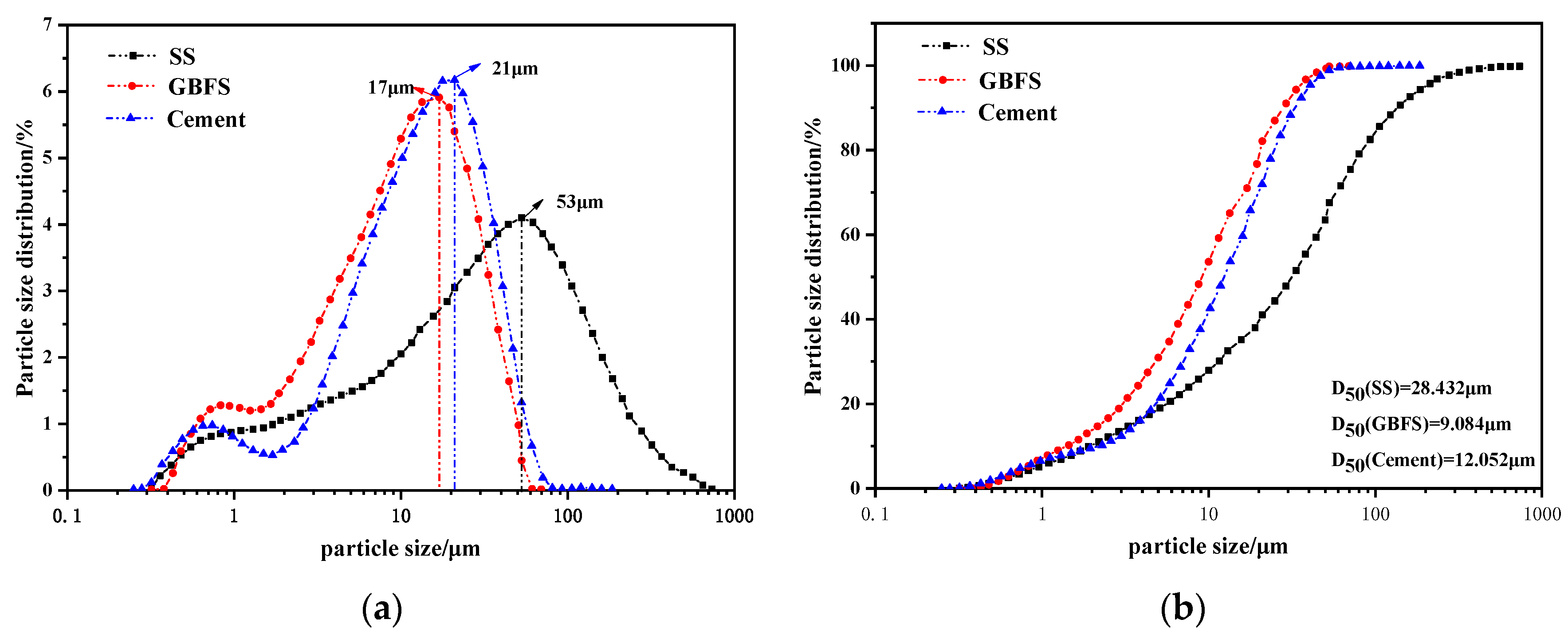
2.1. Raw Materials
2.2. Mixing Proportion
2.3. Testing Methods
2.3.1. Dry Shrinkage and Interconnected Porosity
2.3.2. Abrasion Performance
2.3.3. Freeze–Thaw Test
2.3.4. Microstructure Tests
3. Results and Discussion
3.1. Effect of Activators on the Strength of Blended Paste
3.1.1. Effect of Na2SO4 Content on the Strength
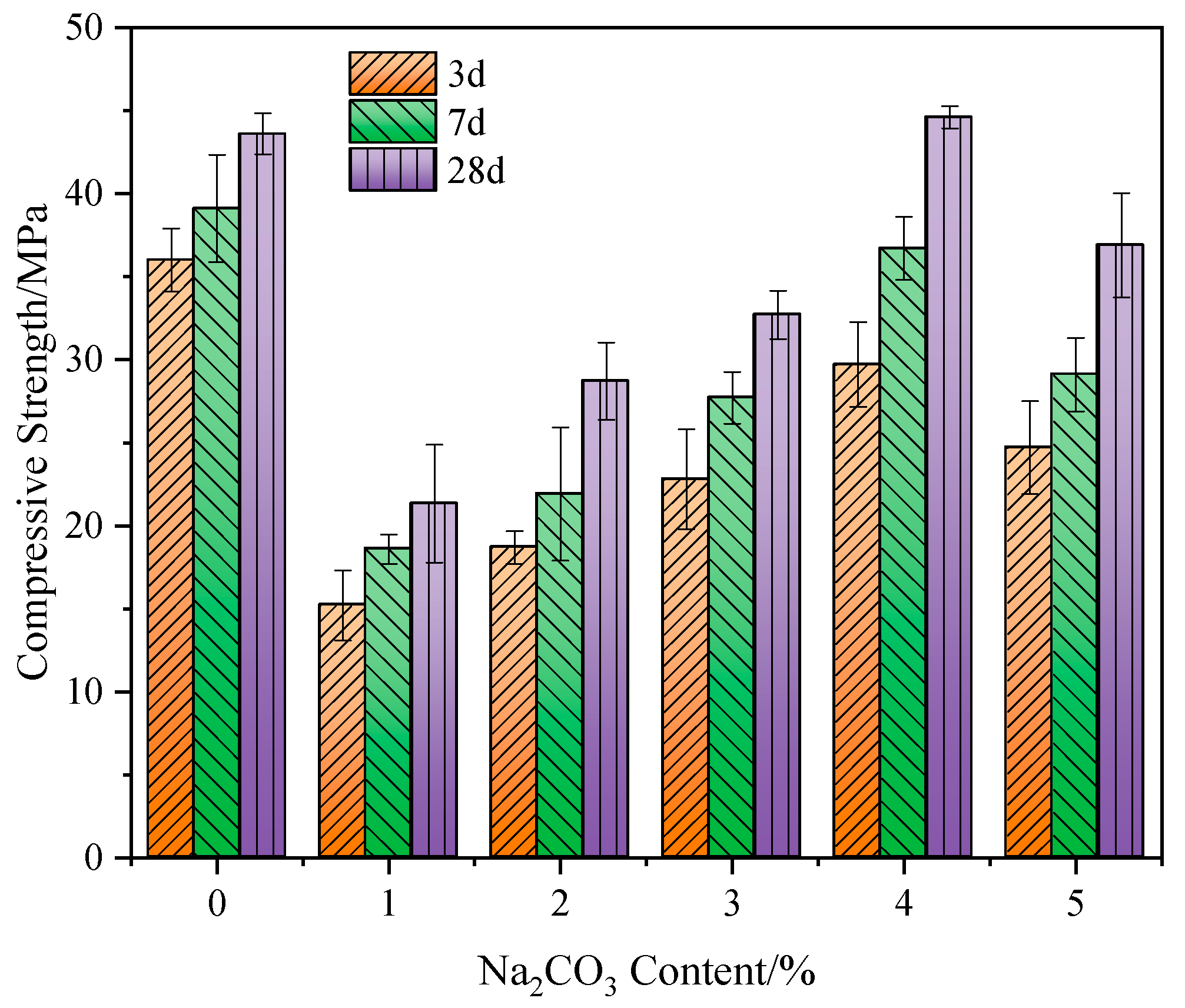
3.1.2. Effect of Na2CO3 Content on the Strength
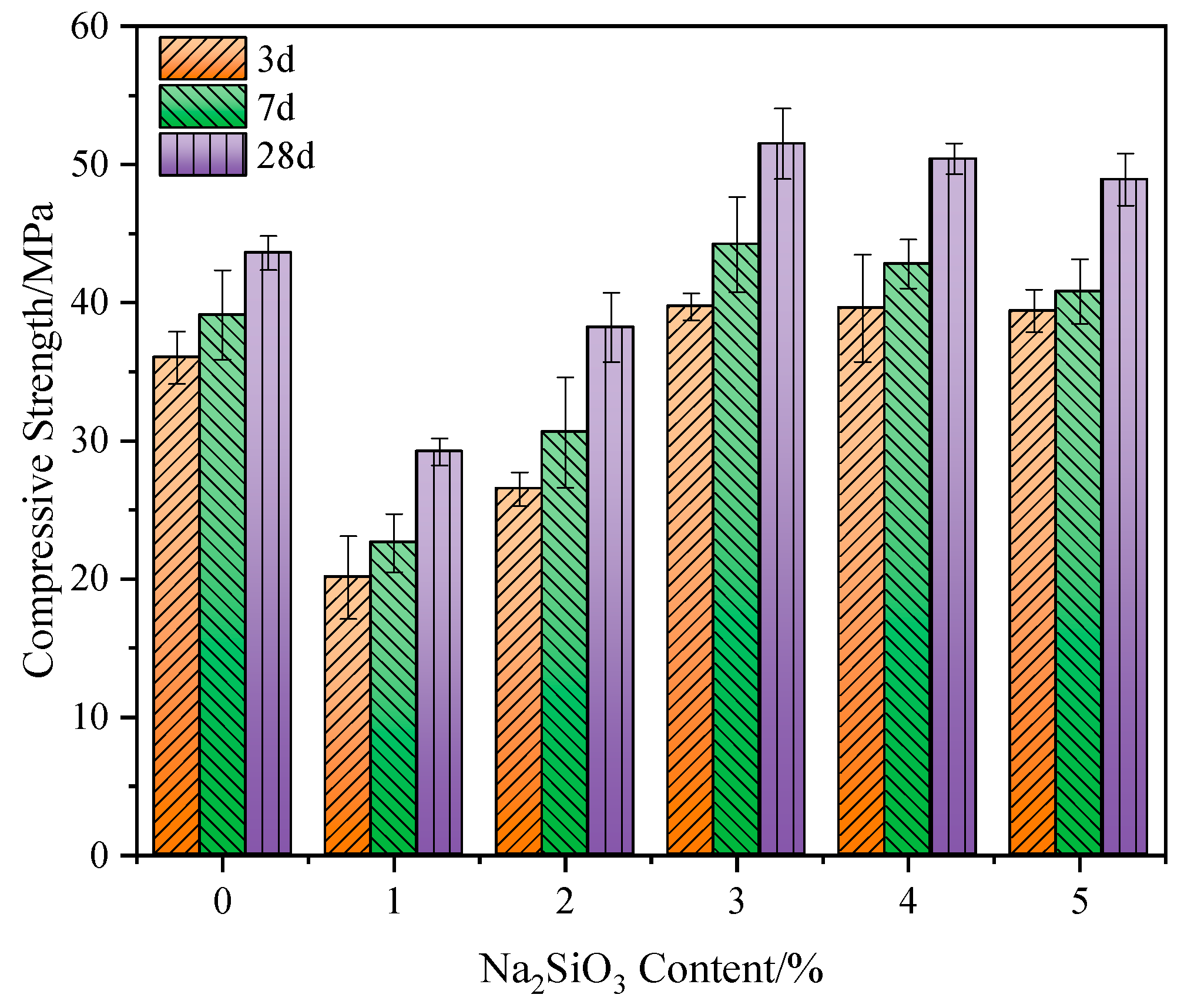
3.1.3. Effect of Na2SiO3 Content on the Strength
3.1.4. Effect of Na2SO4 and Na2SiO3 Combination on the Strength
3.2. Effect of Na2SO4 and Na2SiO3 Combination on the Microstructure of GBFS-SS Composite Paste
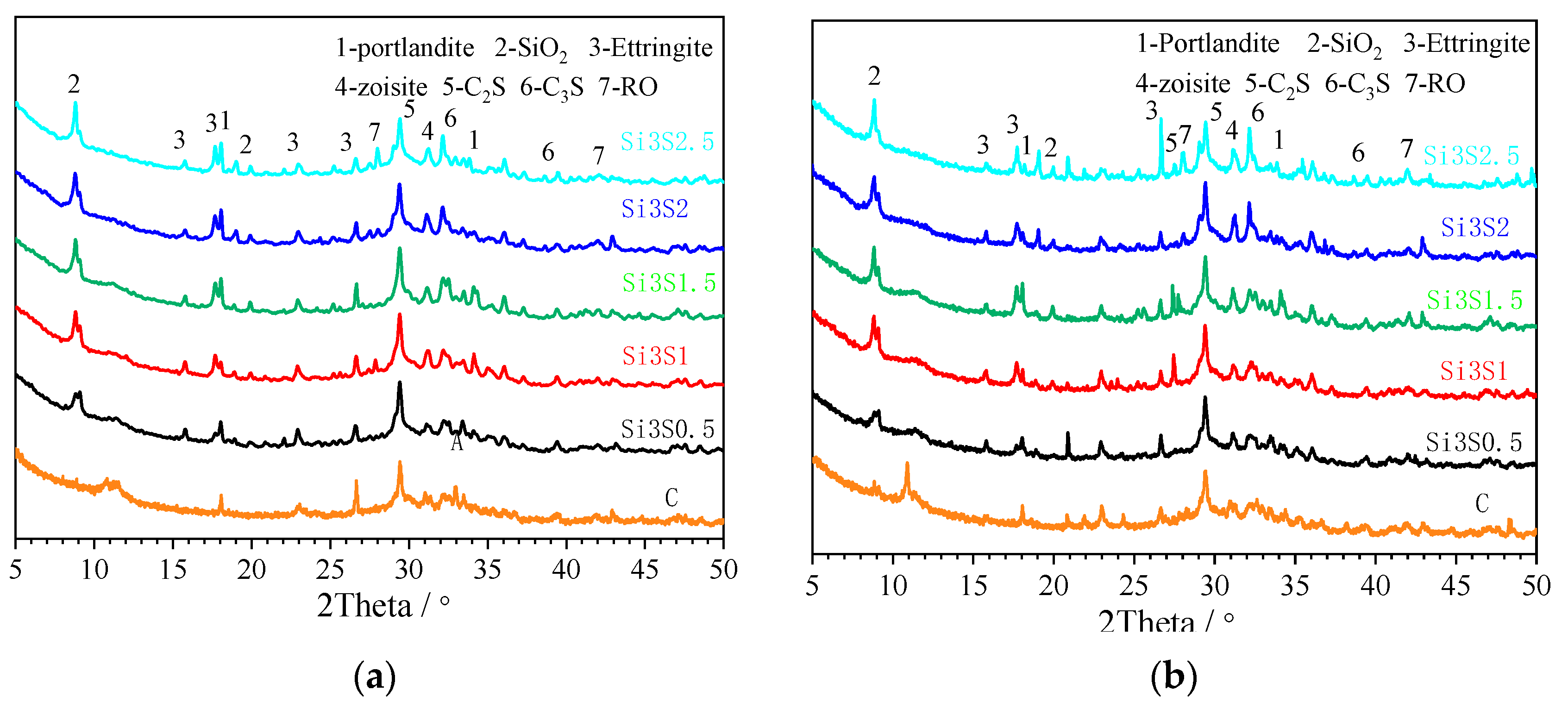
3.2.1. X-ray Diffraction
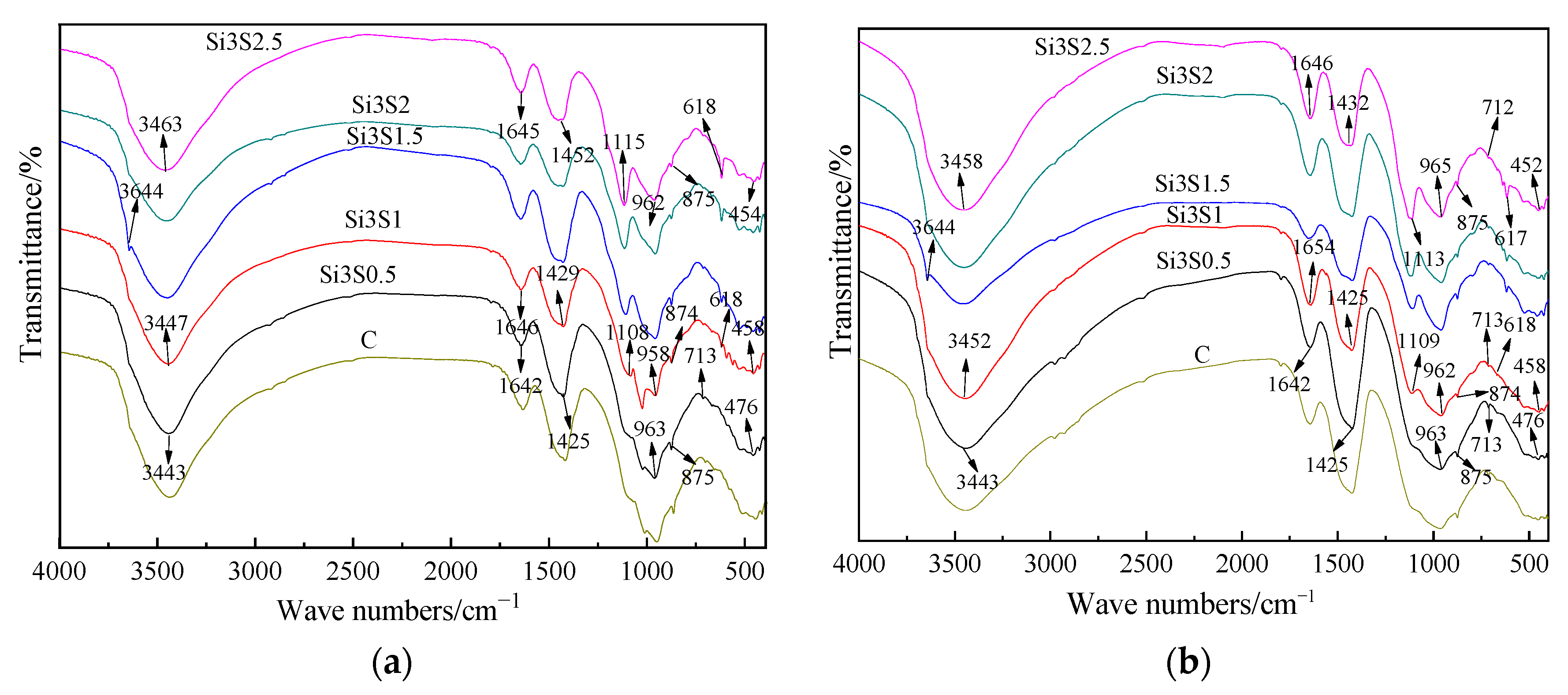
3.2.2. Fourier Transform Infrared
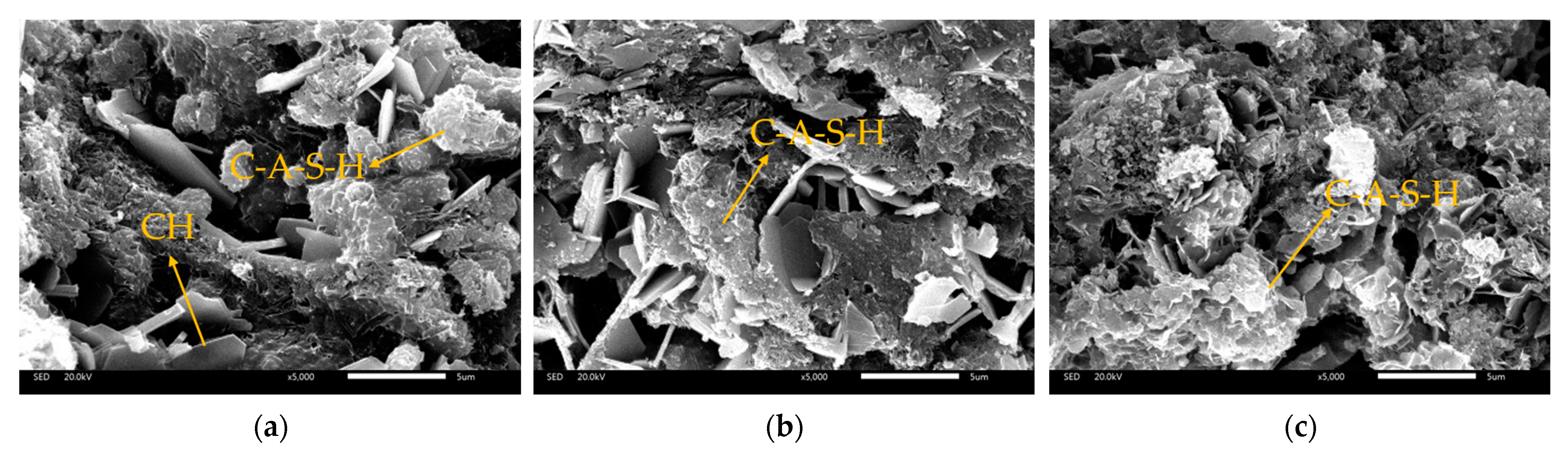
3.2.3. Scanning Electronic Microscopy
3.3. The Performance of GBFS-SS Composite Pavement Concrete
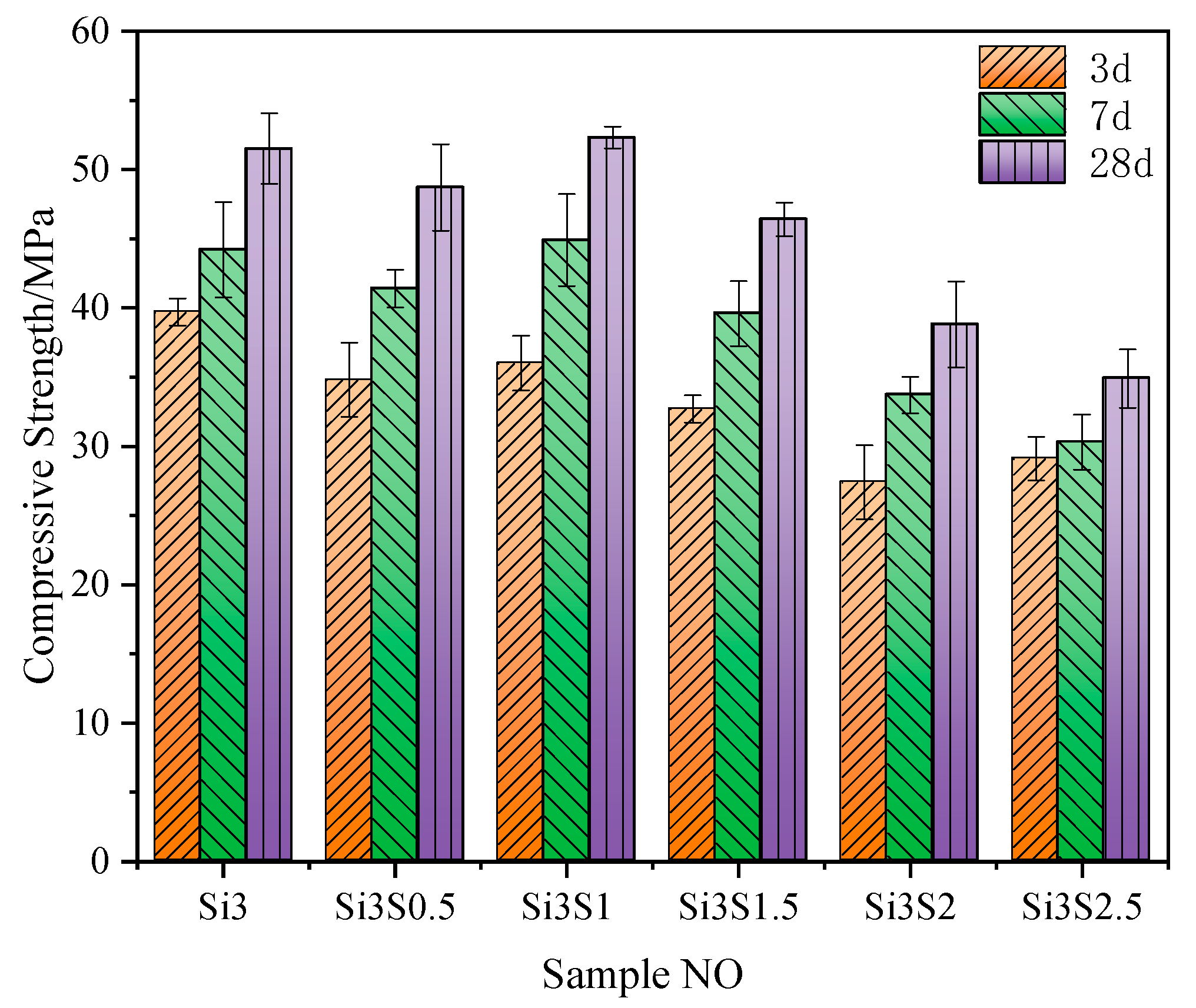
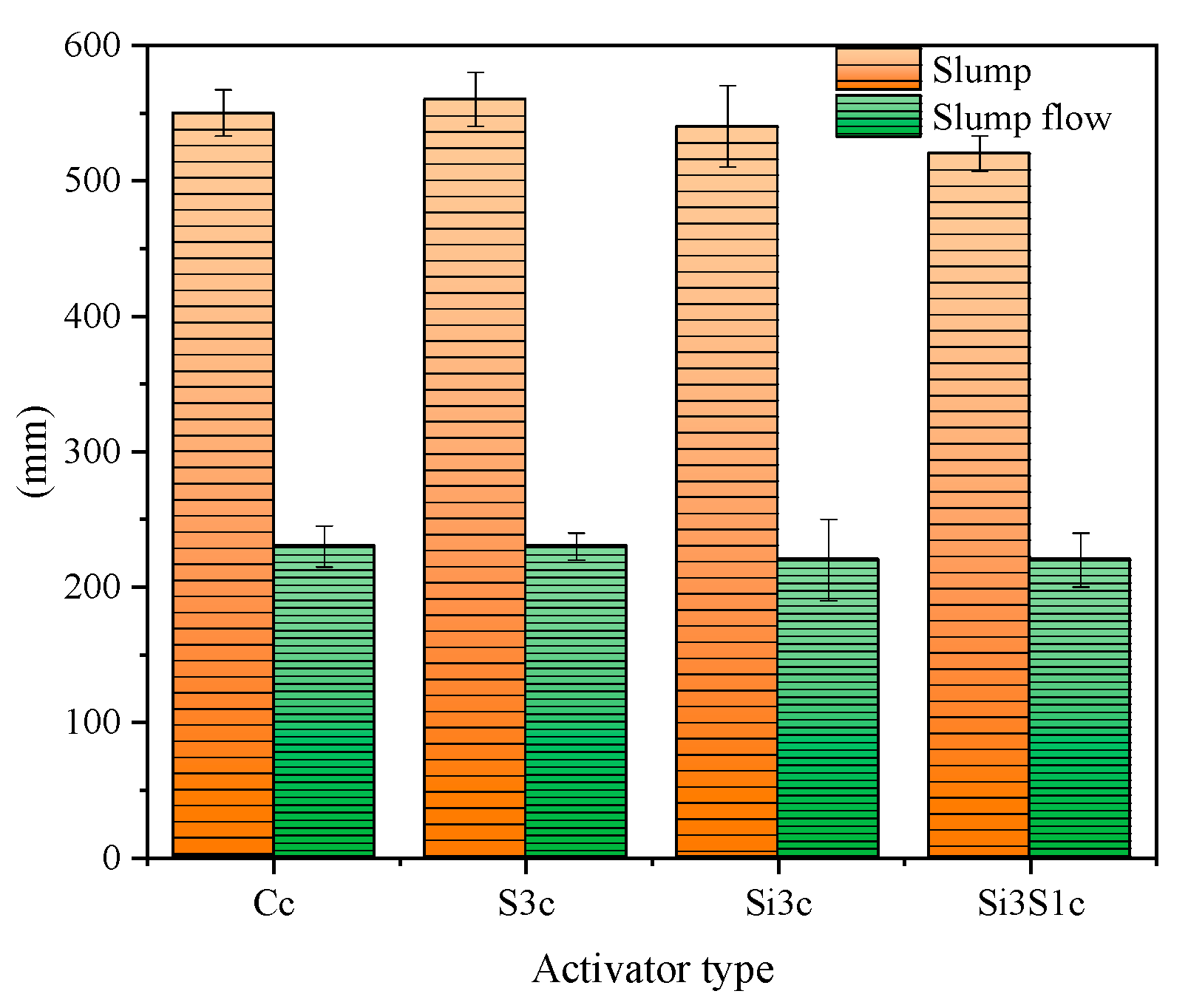
3.3.1. Workability and Compressive Strength
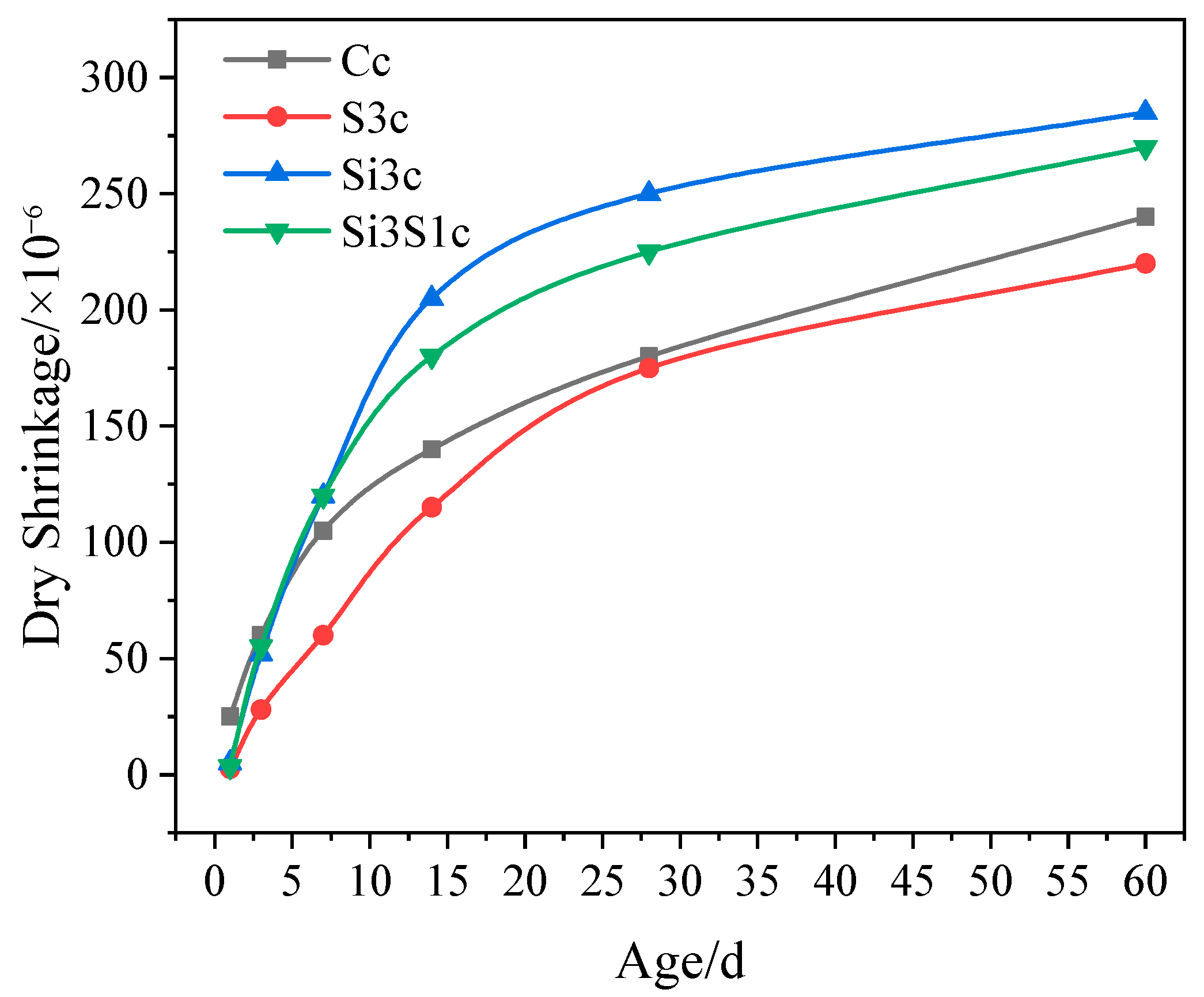
3.3.2. Dry Shrinkage
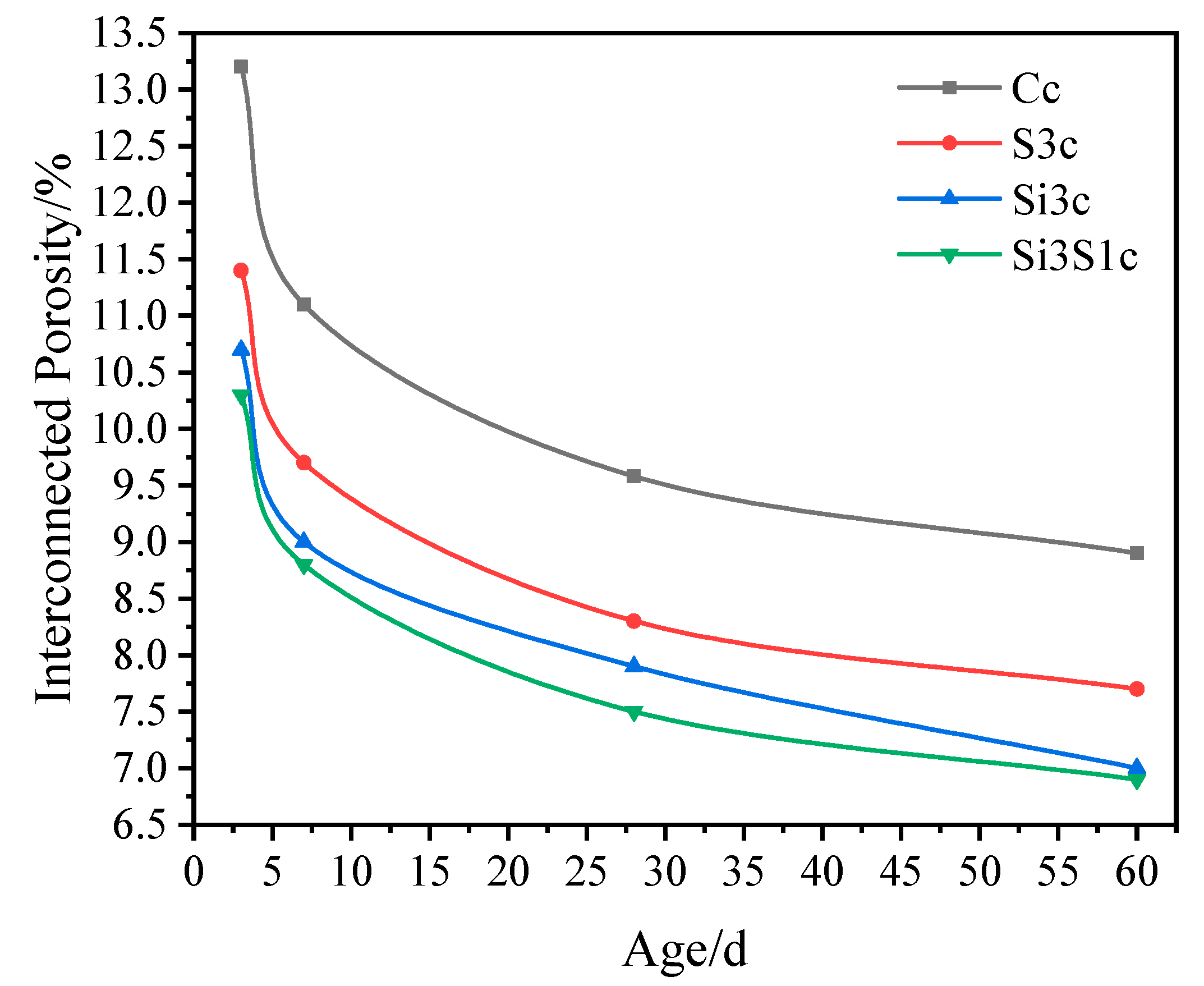
3.3.3. Interconnected Porosity
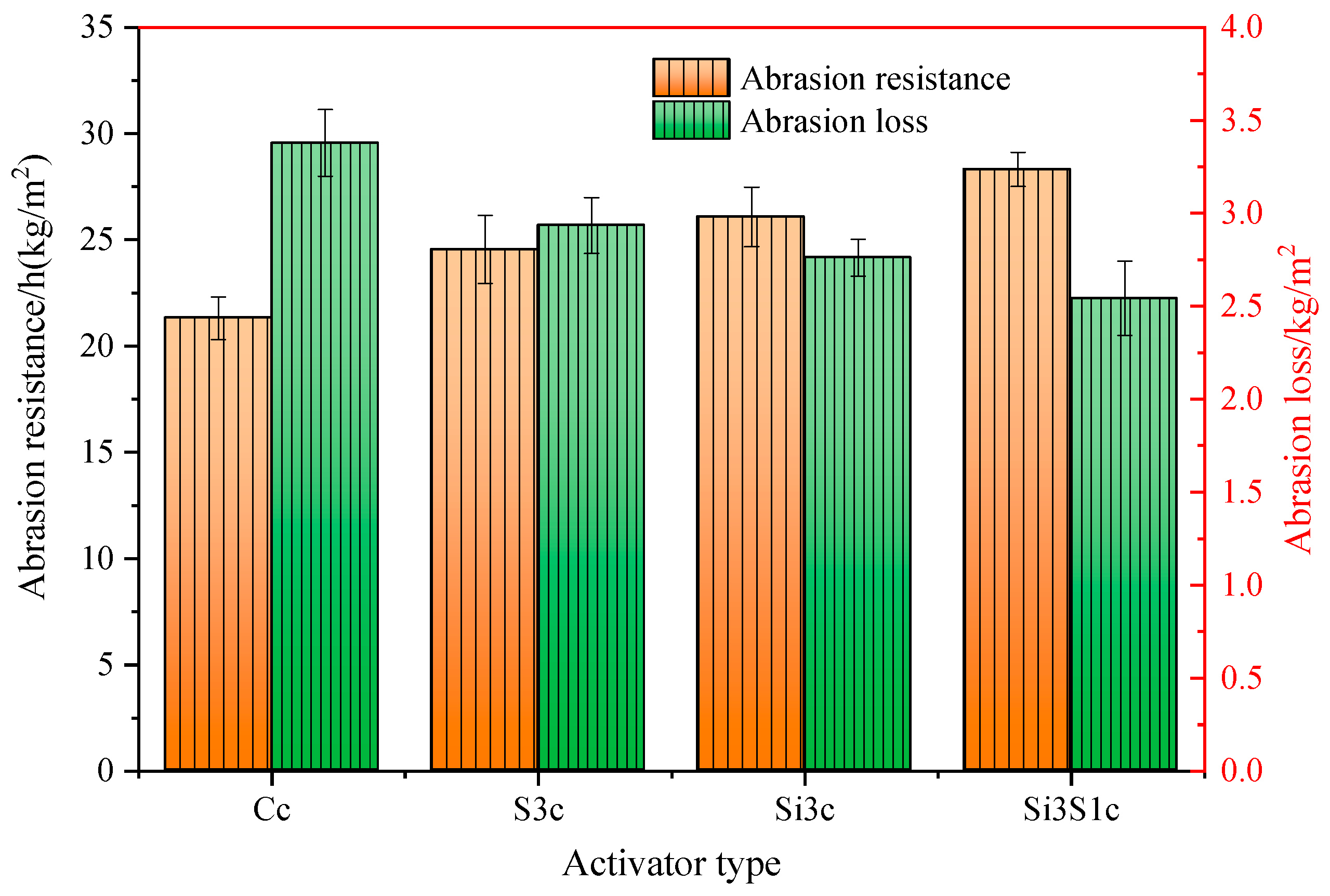
3.3.4. Abrasion Performance
3.3.5. Freeze–Thaw Resistance
4. Conclusions
- (1)
- Both Na2SO4 and Na2SiO3 can promote the strength development of the GBFS-SS composite. The composite paste with 3% of Na2SO4 addition has a 28 d compressive strength of 47.8 MPa, which is 9.4% higher than the control group. The composite paste with 3% of Na2SiO3 addition has a compressive strength of 50.6 MPa, which is 15.8% higher than the control group. Na2CO3 shows a limited effect on the strength development of GBFS-SS composite paste. At the optimal Na2CO3 content of 4%, the 28 d compressive strength of the composite paste is only 44.6 MPa, which is 2.1% higher than the control group.
- (2)
- As compared with Na2SiO3 or Na2SO4 single activation, the combined activation of Na2SiO3 and Na2SO4 shows a better improving effect on the strength development of the composite paste. The GBFS-SS composite concrete with 3% of Na2SiO3 and 1% of Na2SO4 addition has a 60 d compressive strength of 73.5 MPa, a 60 d drying shrinkage of 270 × 10−6, a 60 d interconnected porosity of 6.85%, and the 28 d abrasion resistance of 28.32 h/kg/m2.
- (3)
- All the GBFS-SS composite paste contains the hydration products of ettringite, portlandite and amorphous C-A-S-H gel. The Na2SiO3 and Na2SO4 activated paste has an extra hydration product of zoisite. SO42− can accelerate the depolymerization of the aluminosilicate network in GBFS and SS. SiO32− not only facilitates the pozzolanic reaction of GBFS and SS, but also participates in the hydration to form more C-A-S-H gel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feiz, R.; Ammenberg, J.; Baas, L.; Eklund, M.; Helgstrand, A.; Marshall, R. Improving the CO2, performance of cement, part i: Utilizing life-cycle assessment and key performance indicators to assess development within the cement industry. J. Clean. Prod. 2015, 98, 272–281. [Google Scholar] [CrossRef]
- Nassar, R.; Soroushian, P.; Ghebrab, T. Field investigation of high-volume fly ash pavement concrete. Resour. Conserv. Recycl. 2013, 73, 78–85. [Google Scholar] [CrossRef]
- Kenzhebek, A.; Nazerke, B.; Ina, P. The Effect of Mechanical Activation of Fly Ash on Cement-Based Materials Hydration and Hardened State Properties. Materials 2023, 16, 082959. [Google Scholar]
- Han, Y.; Lin, R.S.; Wang, X.Y. Performance and sustainability of quaternary composite paste comprising limestone, calcined Hwangtoh clay, and granulated blast furnace slag. J. Build. Eng. 2021, 43, 102655. [Google Scholar] [CrossRef]
- Marathe, S.; Mithanthaya, I.R.; Shenoy, R.Y. Durability and microstructure studies on Slag-Fly Ash-Glass powder based alkali activated pavement quality concrete mixes. Constr. Build. Mater. 2021, 287, 123047. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S. Study on processability, compressive strength, drying shrinkage and evolution mechanisms of microstructures of alkali-activated slag-glass powder cementitious material. Constr. Build. Mater. 2022, 344, 128196. [Google Scholar] [CrossRef]
- Sha, F.; Liu, P.; Ding, Y. Application investigation of high-phosphorus steel slag in cementitious material and ordinary concrete. J. Mater. Res. Technol. 2021, 11, 2074–2091s. [Google Scholar] [CrossRef]
- Lian, C.; Wang, Y.; Liu, S. Experimental study on dynamic mechanical properties of fly ash and slag based alkali-activated concrete. Constr. Build. Mater. 2023, 364, 129912. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, S.; Ni, W. Performance optimization and hydration mechanism of a clinker-free ultra-high performance concrete with solid waste based binder and steel slag aggregate. J. Build. Eng. 2023, 63, 105479. [Google Scholar] [CrossRef]
- Han, G.X.; Zhang, J.B.; Sun, H.J. Application of Iron Ore Tailings and Phosphogypsum to Create Artificial Rockfills Used in Rock-Filled Concrete. Buildings 2022, 12, 555. [Google Scholar] [CrossRef]
- Zhu, L. Influence of waste residue treatment in metallurgical iron and steel plant on environmental protection. China Metal Bull. 2020, 1, 191–192, In Chinese. [Google Scholar]
- Wang, Q.; Yan, P.Y. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Furlani, E.; Tonello, G.; Maschio, S. Recycling of steel slag and glass cullet from energy saving lamps by fast firing production of ceramics. Waste Manag. 2010, 30, 1714–1719. [Google Scholar] [CrossRef]
- Wang, J.; Fu, H.; Yan, X.; Wang, L.; Wang, P. Research status of comprehensive utilization of steel slag. China Nonferrous Metall. 2021, 50, 77–82. [Google Scholar]
- Zhang, T.S.; Yu, Q.J.; Wei, J.X.; Li, J.X. Investigation on mechanical properties, durability and micro-structural development of steel slag blended cements. J. Therm. Anal. Calorim. 2012, 110, 633–639. [Google Scholar] [CrossRef]
- Gao, W.H.; Zhou, W.T.; Lyu, X.J. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Soni, A.; Das, P.K.; Hashmi, A.W. Challenges and opportunities of utilizing municipal solid waste as alternative building materials for sustainable development goals: A review. Sustain. Chem. Pharm. 2022, 27, 100706. [Google Scholar] [CrossRef]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags: A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Martins, A.C.P.; De Carvalho, J.M.F.; Costa, L.C.B. Steel slags in cement-based composites: An ultimate review on characterization, applications and performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- Hu, S.G.; Wang, H.X.; Zhang, G.Z.; Ding, Q.J. Bonding and abrasion resistance of geopolymeric repair material made with steel slag. Cem. Concr. Compos. 2008, 30, 239–244. [Google Scholar] [CrossRef]
- Sun, J.W.; Zhang, Z.Q.; Zhuang, S.Y.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, D.M.; Yan, P.Y.; Zhang, D.W.; Wang, H. Self-cementitious property of steel slag powder blended with gypsum. Constr. Build. Mater. 2016, 113, 835–842. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, D. Hydration and mechanical properties of cement-steel slag system incorporating different activators. Constr. Build. Mater. 2023, 363, 129981. [Google Scholar] [CrossRef]
- Wang, G.X.; Xu, K.J.; Zhu, M.Q. Experimental Study on Alkali Stimulating-Steel Slag Cementitious Materials. Int. Conf. Civ. Eng. Build. Mater. 2011, 261–263, 551–554. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D. Influence of steel slag-silica fume composite mineral admixture on the properties of concrete. Powder Technol. 2017, 320, 230–238. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Liu, Q. Steel slag for roadway construction: A review of material characteristics and application mechanisms. J. Mater. Civ. Eng. 2022, 34, 3122001. [Google Scholar] [CrossRef]
- Aziz, M.M.A.; Hainin, M.R.; Yaacob, H.; Ali, Z.; Chang, F.L.; Adnan, A.M. Characterisation and utilisation of steel slag for the construction of roads and highways. Mater. Res. Innov. 2014, 18, 255–259. [Google Scholar] [CrossRef]
- Xu, O.; Han, S.; Liu, Y. Experimental investigation surface abrasion resistance and surface frost resistance of concrete pavement incorporating fly ash and slag. Int. J. Pavement Eng. 2020, 22, 1858–1866. [Google Scholar] [CrossRef]
- Cao, W.; Yang, Q. Properties of a carbonated steel slag-slaked lime mixture. J. Mater. Civil Eng. 2015, 27, 04014115. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z.; Wang, D.; Yan, P. Hydration heat evolution and kinetics of blended cement containing steel slag at different temperatures. Thermochim. Acta 2015, 605, 43–51. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA-GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Chang, T.P.; Thymotie, A. Enhancement of early engineering characteristics of modified slag cement paste with alkali silicate and sulfate. Constr. Build. Mater. 2020, 230, 117013. [Google Scholar] [CrossRef]
- Adesina, A.; Kaze, C.R. Physico-mechanical and microstructural properties of sodium sulfate activated materials: A review. Constr. Build. Mater. 2021, 295, 123668. [Google Scholar] [CrossRef]
- GB/T 18046-2017; cement, mortar, concrete ground granular blast furnace slag. Standard of the People’s Republic of China: Beijing, China, 2017; In Chinese.
- ASTM C131; Standard Test Method for Resistance to Degradation of Small-Size Coarse Aggregate by Abrasion and Impact in the Los Angeles Machine. ASTM International: West Conshohocken, PA, USA, 1961.
- Li, P.Q.; Chen, D.Y.; Jia, Z.R.; Li, Y.L.; Li, S.J.; Yu, B. Effects of Borax, Sucrose, and Citric Acid on the Setting Time and Mechanical Properties of Alkali-Activated Slag. Materials 2023, 16, 3010. [Google Scholar] [CrossRef]
- TG E30-2005; Test Regulations for Cement and Cement Concrete for Highway Engineering. Standard of the People’s Republic of China: Beijing, China, 2005; In Chinese.
- GB/T 50082-2009; Standard for Long-Term Performance and Durability of Ordinary Concrete. Standard of the People’s Republic of China: Beijing, China, 2009; In Chinese.
- Qian, H.; Hua, S.D.; Gao, Y.A.; Qian, L.Y.; Ren, X.J. Synergistic effect of EVA copolymer and sodium desulfurization ash on the printing performance of high volume blast furnace slag mixtures. Addit. Manuf. 2021, 46, 102183. [Google Scholar] [CrossRef]
- Angulo-Ramirez, D.E.; De-Gutierrez, R.M.; Puertas, F. Alkali-activated Portland blast-furnace slag cement: Mechanical properties and hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Xue, L.L.; Zhang, Z.H.; Wang, H. Hydration mechanisms and durability of hybrid alkaline cements (HACs): A review. Constr. Build. Mater. 2021, 266, 121039. [Google Scholar] [CrossRef]
- Li, W.T.; Wei, Q.; Chen, Q.; Jiang, Z.W. Effect of CO32− and Ca2+ on self-healing of cementitious materials due to “build-in” carbonation. J. Build. Eng. 2022, 56, 104781. [Google Scholar] [CrossRef]
- Xu, Z.K.; Yue, J.C.; Pang, G.H.; Li, R.X.; Zhang, P.; Xu, S.T. Influence of the Activator Concentration and Solid/Liquid Ratio on the Strength and Shrinkage Characteristics of Alkali-Activated Slag Geopolymer Pastes. Adv. Civ. Eng. 2021, 11, 6631316. [Google Scholar] [CrossRef]
- Li, W.W.; Jiang, Z.L.; Lu, M.Y.; Long, W.J.; Xing, F.; Liu, J. Effects of Seawater, NaCl, and Na2SO4 Solution Mixing on Hydration Process of Cement Paste. J. Mater. Civ. Eng. 2021, 33, 0899–1561. [Google Scholar] [CrossRef]
- Yan, J.J.; Wu, S.P.; Yang, C.; Zhao, Z.G.; Xie, J. Influencing mechanisms of RO phase on the cementitious properties of steel slag powder. Constr. Build. Mater. 2022, 350, 128926. [Google Scholar] [CrossRef]
- Zhou, X.F.; Zheng, K.R.; Chen, L.; Prateek, G.; Yuan, Q. An approach to improve the reactivity of basic oxygen furnace slag: Accelerated carbonation and the combined use of metakaolin. Constr. Build. Mater. 2023, 379, 131218. [Google Scholar] [CrossRef]
- Atis, C.D.; Bilim, C.; Celik, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Ding, Q.J.; Deng, C.; Yang, J.; Zhang, G.Z.; Hou, D.S. Preparation of Heavyweight Ultra-high Performance Concrete Using Barite Sand and Titanium-rich Heavy Slag Sand. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2021, 36, 644–651. [Google Scholar] [CrossRef]


| Raw Material | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | MnO | P2O5 | SO3 | Other | Loss of Ignition |
|---|---|---|---|---|---|---|---|---|---|---|
| SS | 14.74 | 6.51 | 36.13 | 15.18 | 9.21 | 2.73 | 1.16 | 1.05 | 8.42 | 4.87 |
| GBFS | 31.03 | 14.92 | 40.85 | 0.27 | 8.03 | 0.47 | 0.01 | 2.48 | 1.60 | 0.34 |
| Cement | 20.24 | 4.11 | 62.92 | 3.06 | 2.54 | 0.07 | 0.14 | 2.31 | 1.43 | 3.18 |
| Aperture Size/mm | 0~2.36 | 2.36~4.75 | 4.75~9.50 | 9.50~13.2 |
|---|---|---|---|---|
| Grading/% | 0.4 | 6.1 | 92.0 | 1.5 |
| Aperture Size/mm | 0~2.36 | 2.36~4.75 | 4.75~9.50 | 9.50~13.2 | 13.2~16.0 | 16.0~19.0 |
|---|---|---|---|---|---|---|
| Grading/% | 0.2 | 0.2 | 7.9 | 83.9 | 4.6 | 3.2 |
| Aperture Size/mm | <0.15 | 0.15~0.3 | 0.3~0.6 | 0.6~1.18 | 1.18~2.36 | 2.36~4.75 | ≥4.75 |
|---|---|---|---|---|---|---|---|
| Grading/% | 1.7 | 4.5 | 23.3 | 25.7 | 28.2 | 16.6 | 0 |
| No. | SS | GBFS | Cement | Na2SO4 | Na2CO3 | Na2SiO3 | Borax | w/b Ratio | Superplasticizer |
|---|---|---|---|---|---|---|---|---|---|
| C | 36 | 54 | 10 | - | - | - | - | 0.25 | 3.2 |
| S1 | 36 | 54 | 10 | 1 | - | - | 0.2 | 0.28 | 3.2 |
| S2 | 36 | 54 | 10 | 2 | - | - | 0.2 | 0.28 | 3.2 |
| S3 | 36 | 54 | 10 | 3 | - | - | 0.2 | 0.28 | 3.2 |
| S4 | 36 | 54 | 10 | 4 | - | - | 0.2 | 0.28 | 3.2 |
| S5 | 36 | 54 | 10 | 5 | - | - | 0.2 | 0.28 | 3.2 |
| C1 | 36 | 54 | 10 | - | 1 | - | 0.2 | 0.28 | 3.2 |
| C2 | 36 | 54 | 10 | - | 2 | - | 0.2 | 0.28 | 3.2 |
| C3 | 36 | 54 | 10 | - | 3 | - | 0.2 | 0.28 | 3.2 |
| C4 | 36 | 54 | 10 | - | 4 | - | 0.2 | 0.28 | 3.2 |
| C5 | 36 | 54 | 10 | - | 5 | - | 0.2 | 0.28 | 3.2 |
| Si1 | 36 | 54 | 10 | - | - | 1 | 0.2 | 0.28 | 3.2 |
| Si2 | 36 | 54 | 10 | - | - | 2 | 0.2 | 0.28 | 3.2 |
| Si3 | 36 | 54 | 10 | - | - | 3 | 0.2 | 0.28 | 3.2 |
| Si4 | 36 | 54 | 10 | - | - | 4 | 0.2 | 0.28 | 3.2 |
| Si5 | 36 | 54 | 10 | - | - | 5 | 0.2 | 0.28 | 3.2 |
| No. | SS | GBFS | Cement | Na2SiO3 | Na2SO4 | Borax |
|---|---|---|---|---|---|---|
| Si3S0.5 | 36 | 54 | 10 | 3 | 0.5 | 0.2 |
| Si3S1 | 36 | 54 | 10 | 3 | 1.0 | 0.2 |
| Si3S1.5 | 36 | 54 | 10 | 3 | 1.5 | 0.2 |
| Si3S2 | 36 | 54 | 10 | 3 | 2.0 | 0.2 |
| Si3S2.5 | 36 | 54 | 10 | 3 | 2.5 | 0.2 |
| No. | GBFS | SS | Cement | Basalt Stone | Sand | Water | Superplasticizer /% | Borax /% | Activators and Content/% |
|---|---|---|---|---|---|---|---|---|---|
| Cc | 324 | 216 | 60 | 956 | 692 | 156 | 3.6 | - | - |
| S3c | 324 | 216 | 60 | 940 | 708 | 162 | 2.4 | 0.2 | 3% Na2SO4 |
| Si3c | 3.2 | 3% Na2SiO3 | |||||||
| Si3S1c | 3.8 | 3% Na2SiO3 +1% Na2SO4 |
| No. | Mass Loss Ratio/% | Compressive Strength Loss Ratio/% | Classification | ||||
|---|---|---|---|---|---|---|---|
| 25 Times | 50 Times | 100 Times | 25 Times | 50 Times | 100 Times | ||
| Cc | 0.52 | 0.71 | 1.01 | 5.24 | 8.01 | 11.87 | >D100 |
| S3 | 0.37 | 0.54 | 0.80 | 3.86 | 6.12 | 9.51 | >D100 |
| Si3 | 0.38 | 0.50 | 0.84 | 3.72 | 6.26 | 9.38 | >D100 |
| Si3S1 | 0.34 | 0.47 | 0.73 | 3.36 | 5.92 | 8.74 | >D100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Liu, L.; Zhang, G.; Ding, Q.; Sun, X. The Preparation of Ground Blast Furnace Slag-Steel Slag Pavement Concrete Using Different Activators and Its Performance Investigation. Buildings 2023, 13, 1590. https://doi.org/10.3390/buildings13071590
Yang J, Liu L, Zhang G, Ding Q, Sun X. The Preparation of Ground Blast Furnace Slag-Steel Slag Pavement Concrete Using Different Activators and Its Performance Investigation. Buildings. 2023; 13(7):1590. https://doi.org/10.3390/buildings13071590
Chicago/Turabian StyleYang, Jun, Li Liu, Gaozhan Zhang, Qingjun Ding, and Xiaoping Sun. 2023. "The Preparation of Ground Blast Furnace Slag-Steel Slag Pavement Concrete Using Different Activators and Its Performance Investigation" Buildings 13, no. 7: 1590. https://doi.org/10.3390/buildings13071590
APA StyleYang, J., Liu, L., Zhang, G., Ding, Q., & Sun, X. (2023). The Preparation of Ground Blast Furnace Slag-Steel Slag Pavement Concrete Using Different Activators and Its Performance Investigation. Buildings, 13(7), 1590. https://doi.org/10.3390/buildings13071590







