Mechanical Behavior and Frost-Resistance of Alkali-Activated Cement Concrete with Blended Binder at Ambient Curing Condition
Abstract
1. Introduction
2. Experimental Program
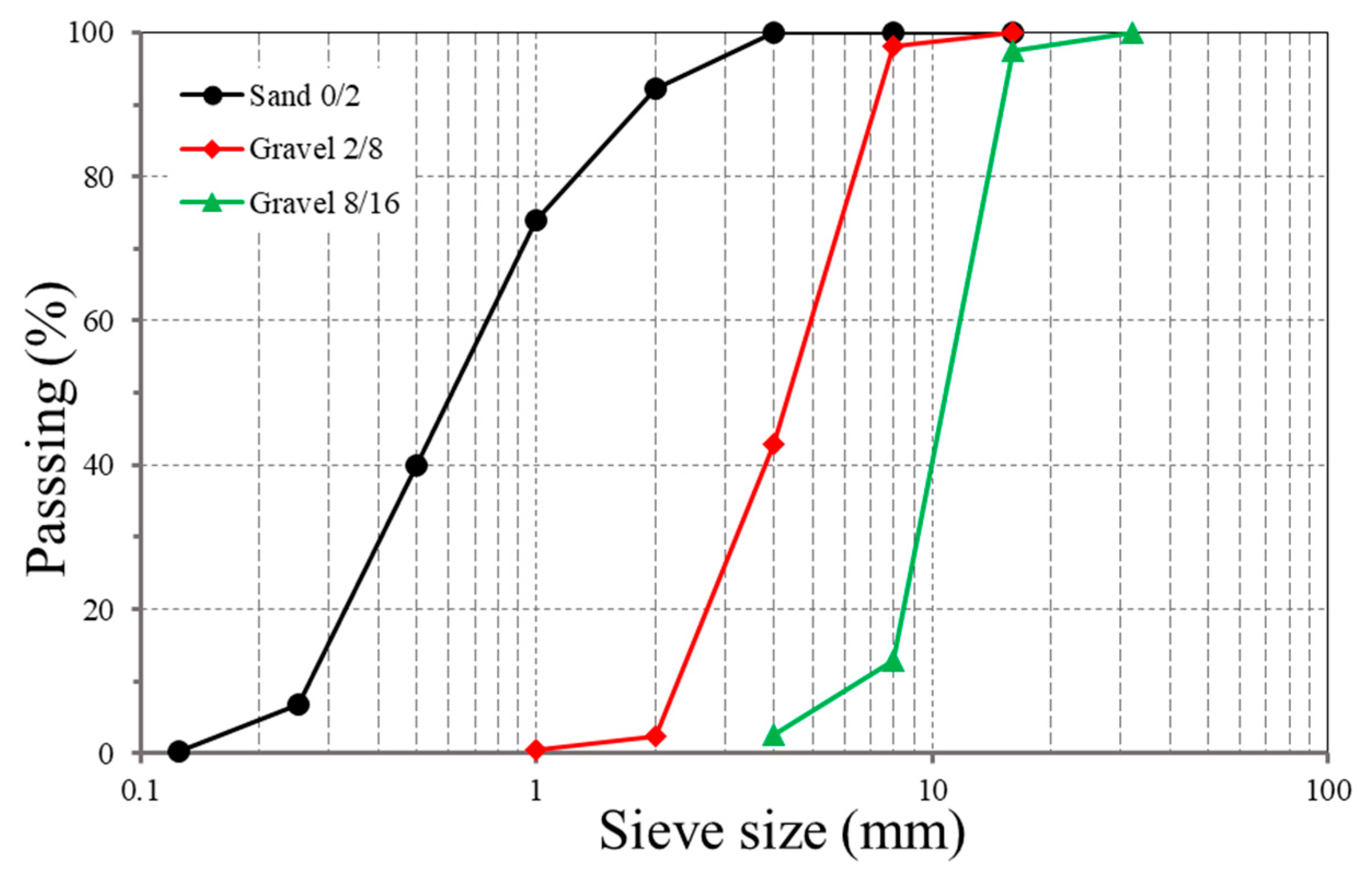
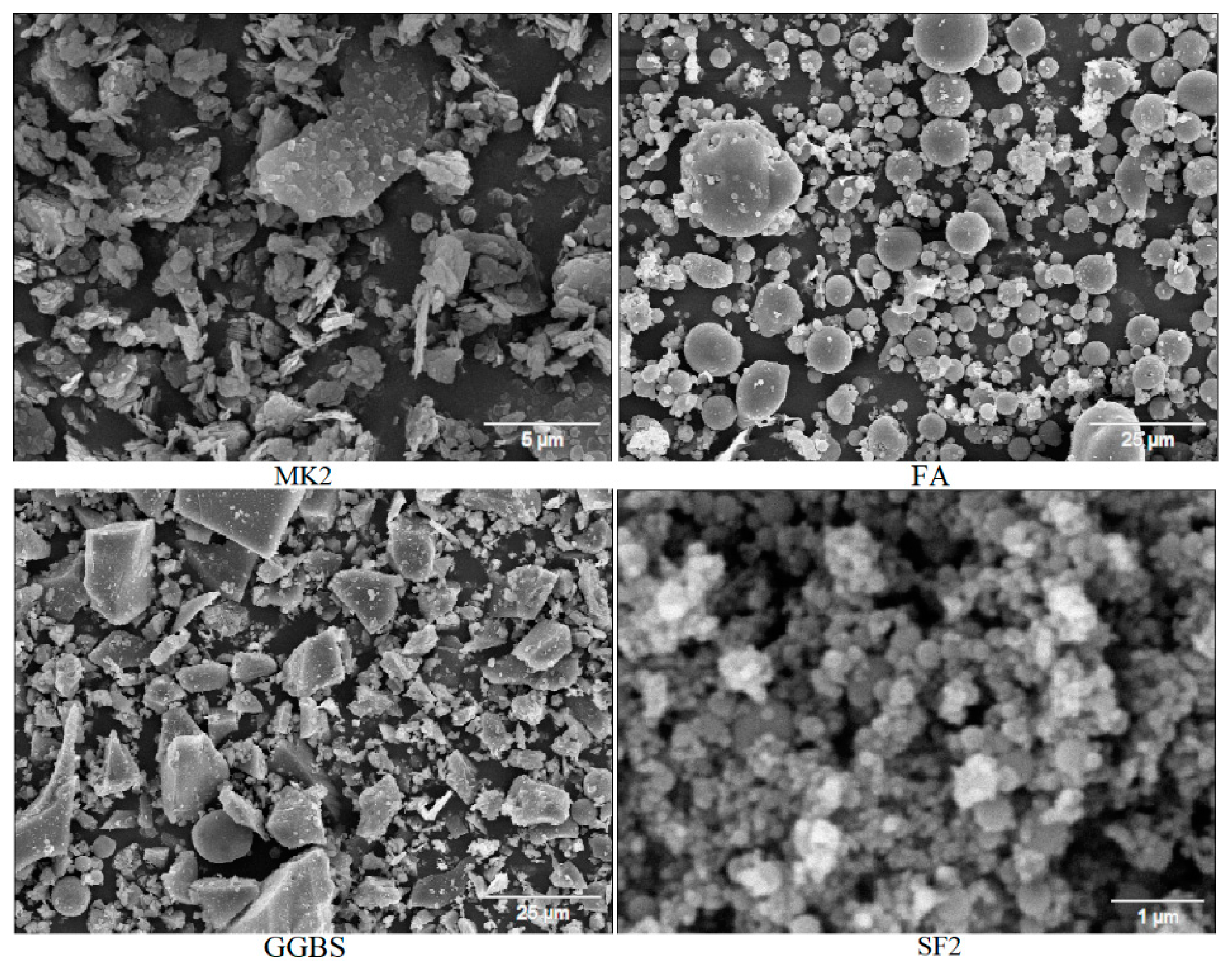
2.1. Materials
2.2. Specimen Preparations and Test Methods
2.3. Mix Proportions
3. Results and Discussions
3.1. Effect of Parameters
3.1.1. Effect of Source Material
3.1.2. Effect of Alkaline Solution
3.2. Strength Development
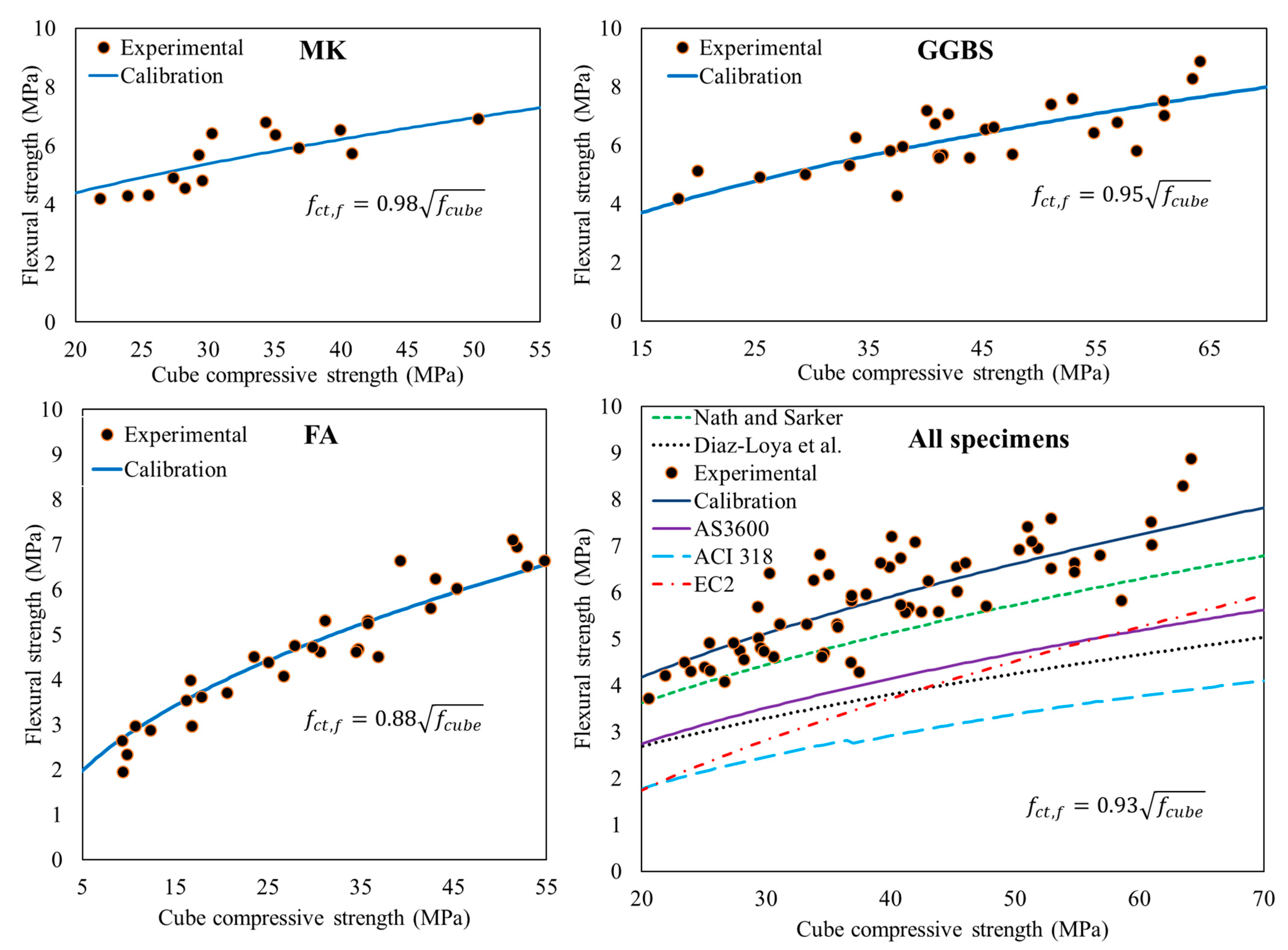
3.3. Flexural Strength
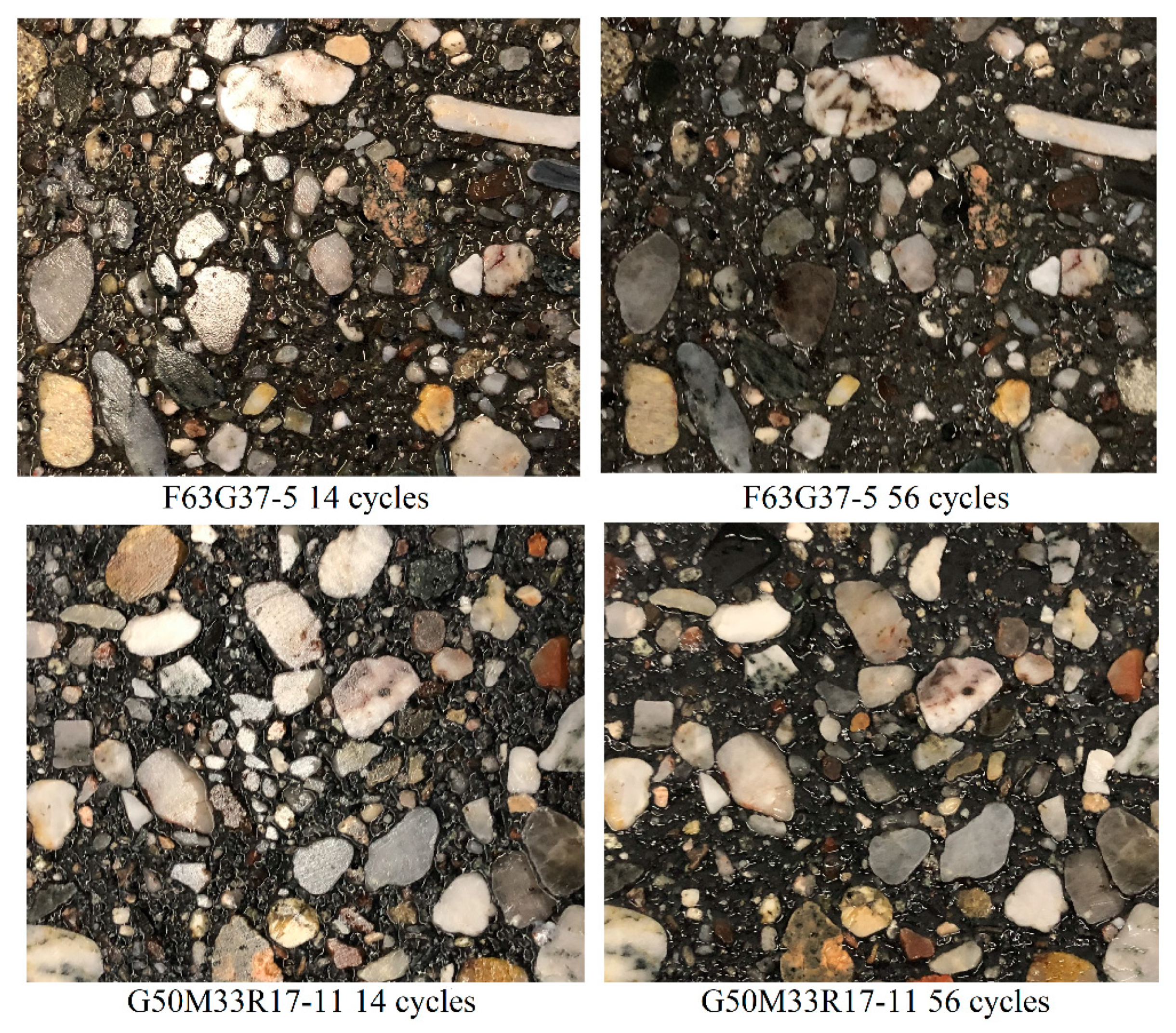
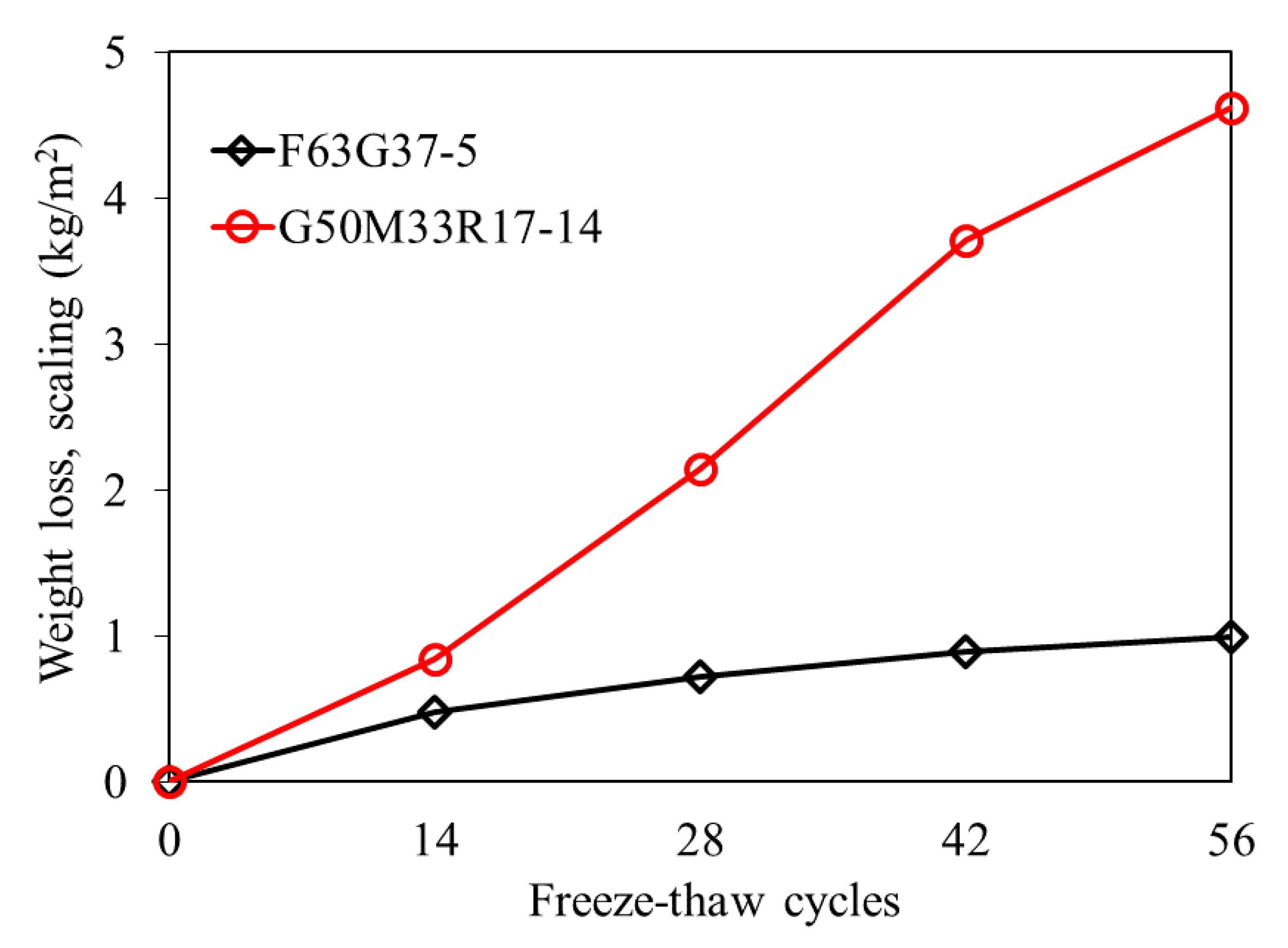
3.4. Freeze-Thaw Resistance
4. Conclusions
- The performance of AAC concrete depends on both the relative proportion of the source materials and the type of source material used. Mixes with high CaO and Al2O3 content result in higher strength.
- The effect of the alkaline solid to binder ratio depends on the source material used and the value of the ratio. Strength increases until an optimum ratio is reached, and then it decreases.
- Mixes with high GGBS and MK levels resulted in high early-age strength, while mixes with high FA developed their strength slower.
- The flexural strength of AAC concrete showed a good correlation with the square root of the compressive strength. MK-based mixes showed the highest flexural to compressive strength ratio.
- The freeze-thaw test results showed that it is possible to design a frost resistance AAC concrete mixture.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. (Eds.) Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781782422761. [Google Scholar]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix design and mechanical properties of geopolymer and alkali activated concrete: Review of the state-of-the-art and the development of a new unified approach. Constr. Build. Mater. 2020, 256, 119380. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef]
- John, L.P.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers. Structure, Processing, Properties and Industrial Applications; Provis, J.L., van Deventer, J.S.J., Eds.; CRC: Oxford, UK; Woodhead: Boca Raton, FL, USA, 2009; ISBN 9781845694494. [Google Scholar]
- Davidovits, J. Geopolymer. Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020; ISBN 9782954453118. [Google Scholar]
- Torres-Carrasco, M.; Puertas, F. Alkaline activation of different aluminosilicates as an alternative to Portland cement: Alkali activated cements or geopolymers. Rev. Ing. Constr. 2017, 32, 5–12. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; MacKenzie, K.J.D. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, B.-J.; Saraswathy, V.; Kwon, S.-J.; Wu, Y. Strength and Durability Performance of Alkali-Activated Rice Husk Ash Geopolymer Mortar. Sci. World J. 2014, 2014, 209584. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Hadi, M.N.S.; Farhan, N.A.; Sheikh, M.N. Design of geopolymer concrete with GGBFS at ambient curing condition using Taguchi method. Constr. Build. Mater. 2017, 140, 424–431. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Rajamma, R.; Labrincha, J.A.; Ferreira, V.M. Alkali activation of biomass fly ash–metakaolin blends. Fuel 2012, 98, 265–271. [Google Scholar] [CrossRef]
- Samson, G.; Cyr, M.; Gao, X.X. Formulation and characterization of blended alkali-activated materials based on flash-calcined metakaolin, fly ash and GGBS. Constr. Build. Mater. 2017, 144, 50–64. [Google Scholar] [CrossRef]
- Ludwig, H.M.; Egersdörfer, A.; Trümer, A. Leistungsfähigkeit von Metapor@ als Kompositmaterial im Zement; Zement- und Betoneigenschaften, F. A. Finger Institut für Baustoffkunde, Bauhaus-Universität-FIB: Weimar, Germany, 2013. [Google Scholar]
- DIN EN 196-1:2016-11. Methods of Testing Cement—Part 1: Determination of Strength, German version EN 196-1:2016; CEN: Brussels, Belgium, 2016. [Google Scholar]
- DIN CEN/TS 12390-9:2017-05. Freeze-Thaw Resistance with De-Icing Salts-Scaling, German version CEN/TS 12390-9:2016; CEN: Brussels, Belgium, 2016. [Google Scholar]
- DIN EN 206:2017-01. Concrete—Specification, Performance, Production and Conformity; German Version EN 206:2013+A1:2016; CEN: Brussels, Belgium, 2017. [Google Scholar]
- Herrmann, A.; Koenig, A.; Dehn, F. Proposal for the classification of alkali-activated binders and Geopolymer binders: Vorschlag zur Klassifizierung von alkalisch-aktivierten Bindemitteln und Geopolymeren. Cem. Int. 2015, 13, 62–69. [Google Scholar]
- Herrmann, A.; Koenig, A.; Dehn, F. Structural concrete based on alkali-activated binders: Terminology, reaction mechanisms, mix designs and performance. Struct. Concr. 2018, 19, 918–929. [Google Scholar] [CrossRef]
- Buchwald, A. The Influence of Calcium on the Condensation of (Alumino-) Silicates in Alkali-Activated Binders. In German: Der Einfluss des Kalziums auf die Kondensation von (Alumo-)Silikaten in Alkali-Aktivierten Bindern. Habilitationsschrift, der Bauhaus-Universität Weimar, Weimar, Germany, 2012. [Google Scholar]
- Kouamo, H.T.; Elimbi, A.; Mbey, J.A.; Sabouang, C.J.N.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Alanazi, H.; Hu, J.; Kim, Y.-R. Effect of slag, silica fume, and metakaolin on properties and performance of alkali-activated fly ash cured at ambient temperature. Constr. Build. Mater. 2019, 197, 747–756. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P.L. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.-X.; Zhang, B.; Poon, C.-S. Rheology behavior of one-part alkali activated slag/glass powder (AASG) pastes. Constr. Build. Mater. 2020, 258, 120381. [Google Scholar] [CrossRef]
- Atiş, C.D.; Görür, E.B.; Karahan, O.; Bilim, C.; İlkentapar, S.; Luga, E. Very high strength (120MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- ACI 318-19. Building Code Requirements for Structural Concrete and Commentary; American Concrete Institute: Farmington Hills, MI, USA, 2019. [Google Scholar]
- AS 3600. Concrete Structures; Standards Australia: Sidney, Australia, 2018. [Google Scholar]
- EC 2. EN 1992-1-1: Design of Concrete Structures—Part 1-1: General Rules and Rules for Buildings; CEN: Brussels, Belgium, 2004. [Google Scholar]
- Diaz-Loya, E.I.; Allouche, E.N.; Vaidya, S. Mechanical Properties of Fly-Ash-Based Geopolymer Concrete. ACI Mater. J. 2011, 108. [Google Scholar] [CrossRef]
- Gomaa, E.; Sargon, S.; Kashosi, C.; Gheni, A.; ElGawady, M.A. Mechanical Properties of High Early Strength Class C Fly Ash-Based Alkali Activated Concrete. Transp. Res. Rec. 2020, 2674, 430–443. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef]
- Yi, S.-T.; Yang, E.-I.; Choi, J.-C. Effect of specimen sizes, specimen shapes, and placement directions on compressive strength of concrete. Nucl. Eng. Des. 2006, 236, 115–127. [Google Scholar] [CrossRef]
- Cyr, M.; Pouhet, R. The frost resistance of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 293–318. ISBN 978-1-78242-276-1. [Google Scholar]
- Bilek, V.; Hurta, J.; Done, P.; Zidek, L. Development of alkali-activated concrete for structures—Mechanical properties and durability. Perspect. Sci. 2016, 7, 190–194. [Google Scholar] [CrossRef][Green Version]
- Eslam, Y.; Ahmed, G.; Gheni, A.; ElGawady, M.A. Durability of Class C Fly Ash-Based Alkali Activated Concrete. ACI Symp. Publ. 2019, 334, 185–204. [Google Scholar]
- Fu, Y.; Cai, L.; Yonggen, W. Freeze–thaw cycle test and damage mechanics models of alkali-activated slag concrete. Constr. Build. Mater. 2011, 25, 3144–3148. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. The Durability of One-Part Alkali-Activated Slag-Based Mortars in Different Environments. Sustainability 2020, 12, 3561. [Google Scholar] [CrossRef]
- Bilek, V.; Hurta, J.; Zidek, L.; Mec, P. Searching for Durable and Friendly Alkali Activated Concrete for Building Elements Constructions. In Engineering Researches and Decisions in Materials Science; Trans Tech Publications Ltd.: Zurich, Switzerland, 2015; pp. 126–131. [Google Scholar]
- Gifford, P.M.; Gillott, J.E. Freeze-Thaw Durability of Activated Blast Furnace Slag Cement Concrete. ACI Mater. J. 1996, 93. [Google Scholar] [CrossRef]
- Cai, L.; Wang, H.; Fu, Y. Freeze–thaw resistance of alkali–slag concrete based on response surface methodology. Constr. Build. Mater. 2013, 49, 70–76. [Google Scholar] [CrossRef]
- Shahrajabian, F.; Behfarnia, K. The effects of nano particles on freeze and thaw resistance of alkali-activated slag concrete. Constr. Build. Mater. 2018, 176, 172–178. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. Durability Properties of Alkali Activated Slag Composites: Short Overview. Silicon 2020, 12, 987–996. [Google Scholar] [CrossRef]
- Winnefeld, F.; Gluth, G.J.G.; Bernal, S.A.; Bignozzi, M.C.; Carabba, L.; Chithiraputhiran, S.; Dehghan, A.; Dolenec, S.; Dombrowski-Daube, K.; Dubey, A.; et al. RILEM TC 247-DTA round robin test: Sulfate resistance, alkali-silica reaction and freeze–thaw resistance of alkali-activated concretes. Mater. Struct. 2020, 53, 140. [Google Scholar] [CrossRef]
- Setzer, M.J.; Auberg, A. Freeze-thaw and deicing salt resistance of concrete testing by the CDF method CDF resistance limit and evaluation of precision. Mater. Struct. 1995, 28, 16–31. [Google Scholar] [CrossRef]

| Component (%) | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Na2O | SO3 | Average Particle Size (µm) |
|---|---|---|---|---|---|---|---|---|---|
| FA | 49.79 | 26.71 | 8.57 | 2.47 | 4.34 | 3.36 | 1.28 | 1.49 | - |
| GGBS | 34.48 | 11.48 | - | 7.08 | 42.43 | 0.66 | 0.56 | 2.17 | - |
| MK1 [20] | 54.4 | 27.1 | 1 | 0.7 | 4.5 | 2.3 | 7.9 | 0.3 | 14.9 |
| MK2 | 53.16 | 44.51 | 0.54 | - | - | - | - | - | 1.3 * |
| SF1 * | 93.81 | 0.48 | 1.49 | 0.46 | 0.3 | 0.77 | 0.42 | 0.2 | - |
| SF2 | 96.05 | 1.11 | - | 0.46 | 0.72 | 1.2 | 0.46 | - | 0.15 * |
| RHA * | 92 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 51.1 |
| Mix ID | AS/B | TW/TS | Flexural (N/mm2) | Compressive (N/mm2) | ||
|---|---|---|---|---|---|---|
| 7-Day | 28-Day | 7-Day | 28-Day | |||
| F63G37-1 | 0.21 | 0.43 | 4.69 | 6.64 | 34.65 | 54.74 |
| F63G37-2 | 0.26 | 0.43 | 4.62 | 6.52 | 34.48 | 52.90 |
| F63G37-3 | 0.21 | 0.50 | 4.51 | - | 23.43 | 31.61 |
| F63G37-4 | 0.21 | 0.44 | 4.51 | - | 36.82 | - |
| F63G37-5 * | 0.21 | 0.26 | 3.21d | 3.74d | 46.19 | 64.32 |
| F80G20-6 | 0.21 | 0.35 | 3.72 | 6.64 | 20.54 | 39.19 |
| F80G20-7 | 0.25 | 0.35 | 4.40 | 6.25 | 25.04 | 43.03 |
| F80G20-8 | 0.28 | 0.35 | 5.32 | 6.95 | 35.70 | 51.81 |
| F37G37M26-9 | 0.26 | 0.46 | 4.09 | 5.59 | 26.66 | 42.46 |
| F35G35M30-10 | 0.26 | 0.46 | 4.76 | 7.10 | 27.86 | 51.32 |
| F35G35M25S5-11 | 0.25 | 0.42 | 4.63 | 6.03 | 30.57 | 45.35 |
| Mix ID | AS/B | TW/TS | Flexural (N/mm2) | Compressive (N/mm2) | ||
|---|---|---|---|---|---|---|
| 7-Day | 28-Day | 7-Day | 28-Day | |||
| G52M30S18-1 | 0.33 | 0.39 | 4.29 | 7.08 | 37.46 | 41.96 |
| G52M30S18-2 | 0.29 | 0.39 | 5.66 | 6.80 | 41.14 | 56.84 |
| G52M30S18-3 | 0.25 | 0.39 | 4.92 | 6.75 | 25.41 | 40.80 |
| G52M30S18-4 a | 0.33 | 0.39 | 5.71 | - | 47.64 | - |
| G52M30S18-5 b | 0.33 | 0.39 | 5.83 | 7.03 | 58.52 | 60.96 |
| G52M30S18-6 b | 0.26 | 0.43 | 5.83 | 7.41 | 36.86 | 50.99 |
| G52M30R18-7 | 0.32 | 0.47 | 6.55 | 8.29 | 45.26 | 63.48 |
| G52M30R18-8 | 0.26 | 0.49 | 5.68 | 8.88 | 41.46 | 64.14 |
| G52M30R18-9 | 0.26 | 0.64 | 6.27 | - | 33.82 | - |
| G52M30R18-10 | 0.19 | 0.54 | - | - | 15.07 | - |
| G50M33R17-11 * | 0.21 | 0.39 | 2.25 d | 3.51 d | 34.21 | 54.11 |
| Mix ID | AS/B | TW/TS | Flexural (N/mm2) | Compressive (N/mm2) | ||
|---|---|---|---|---|---|---|
| 7-Day | 28-Day | 7-Day | 28-Day | |||
| M75S25-1 | 0.39 | 0.43 | 4.82 | 6.38 | 29.53 | 35.04 |
| M75S25-2 | 0.34 | 0.43 | 4.92 | 6.81 | 27.35 | 34.30 |
| M75S25-3 | 0.31 | 0.47 | 4.33 | 5.70 | 25.47 | 29.30 |
| M75S25-4 b | 0.39 | 0.43 | 6.55 | 6.92 | 39.91 | 50.30 |
| M75S25-5 a | 0.39 | 0.43 | - | - | 2.60 | 6.31 |
| M49G30R13S8-6 a | 0.28 | 0.53 | 4.31 | 5.93 | 23.92 | 36.83 |
| M44G44S12-7 a | 0.28 | 0.48 | 4.56 | 5.74 | 28.22 | 40.79 |
| M63G25S12-8 a | 0.28 | 0.48 | 4.22 | 6.42 | 21.86 | 30.26 |
| Mixture | Cycles | Average (kg/m2) | Standard Deviation |
|---|---|---|---|
| F63G37-5 | 14 | 0.476 | 0.119 |
| 28 | 0.726 | 0.104 | |
| 42 | 0.892 | 0.089 | |
| 56 | 0.999 | 0.073 | |
| G50M33R17-11 | 14 | 0.839 | 0.368 |
| 28 | 2.145 | 0.467 | |
| 42 | 3.715 | 0.166 | |
| 56 | 4.618 | 0.262 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekle, B.H.; Holschemacher, K.; Löber, P.; Heiden, B. Mechanical Behavior and Frost-Resistance of Alkali-Activated Cement Concrete with Blended Binder at Ambient Curing Condition. Buildings 2021, 11, 52. https://doi.org/10.3390/buildings11020052
Tekle BH, Holschemacher K, Löber P, Heiden B. Mechanical Behavior and Frost-Resistance of Alkali-Activated Cement Concrete with Blended Binder at Ambient Curing Condition. Buildings. 2021; 11(2):52. https://doi.org/10.3390/buildings11020052
Chicago/Turabian StyleTekle, Biruk Hailu, Klaus Holschemacher, Philipp Löber, and Björn Heiden. 2021. "Mechanical Behavior and Frost-Resistance of Alkali-Activated Cement Concrete with Blended Binder at Ambient Curing Condition" Buildings 11, no. 2: 52. https://doi.org/10.3390/buildings11020052
APA StyleTekle, B. H., Holschemacher, K., Löber, P., & Heiden, B. (2021). Mechanical Behavior and Frost-Resistance of Alkali-Activated Cement Concrete with Blended Binder at Ambient Curing Condition. Buildings, 11(2), 52. https://doi.org/10.3390/buildings11020052







