Effect of Different Additives on Reaction Characteristics of Fluorapatite During Coal-Based Reduction of Iron Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Equipment
3. Results and Discussion
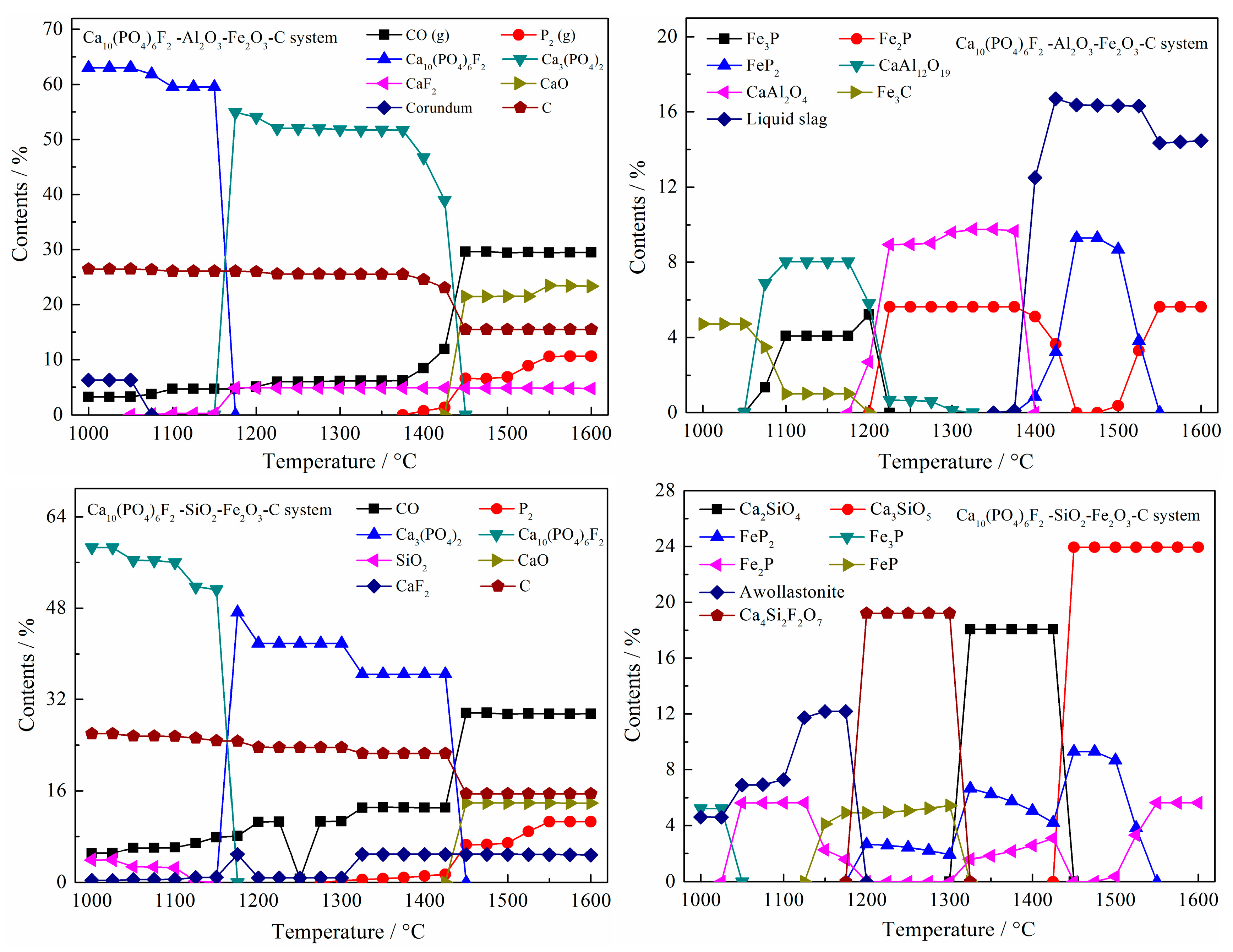
3.1. Thermodynamic Analysis
3.2. Reduction Degree Analysis
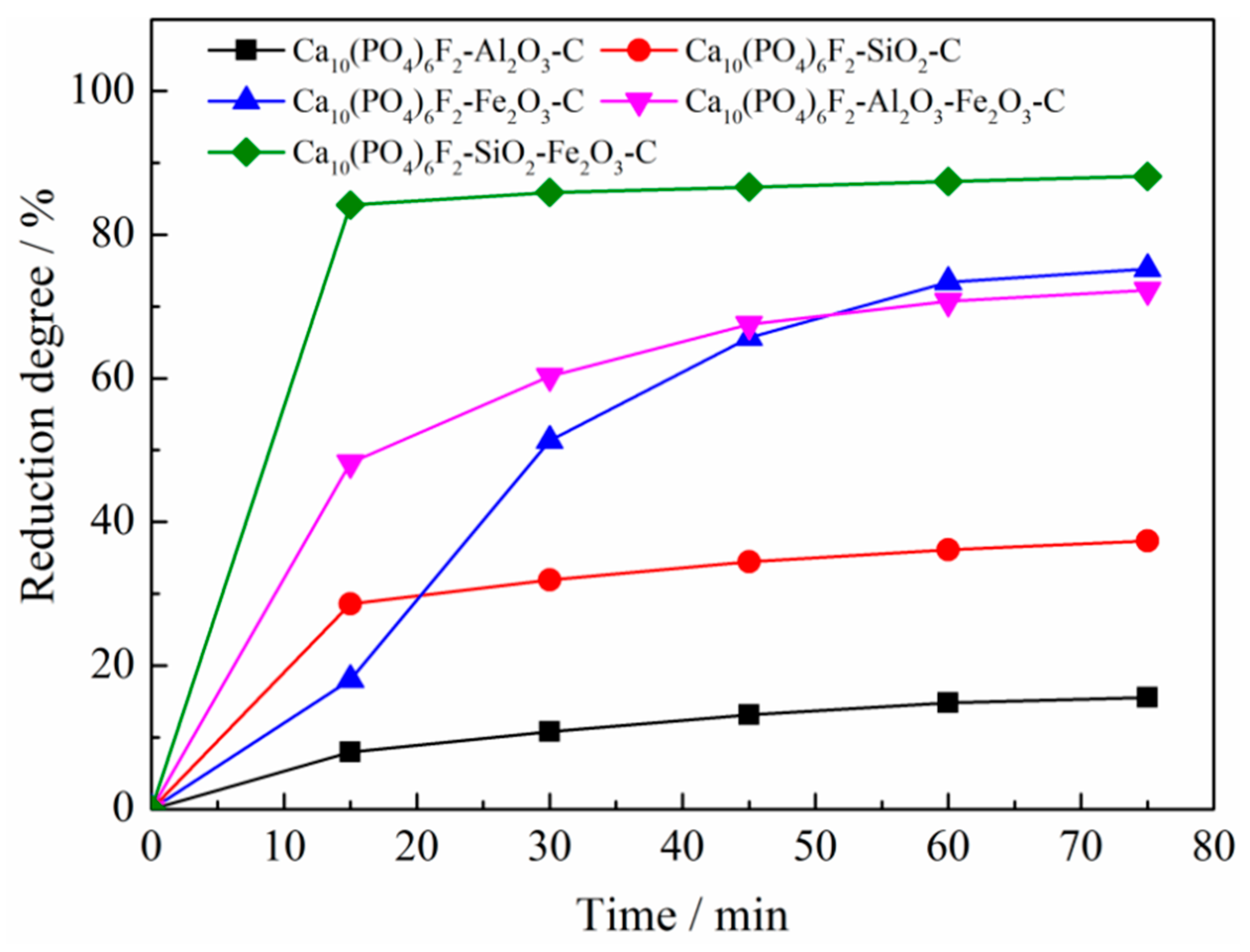
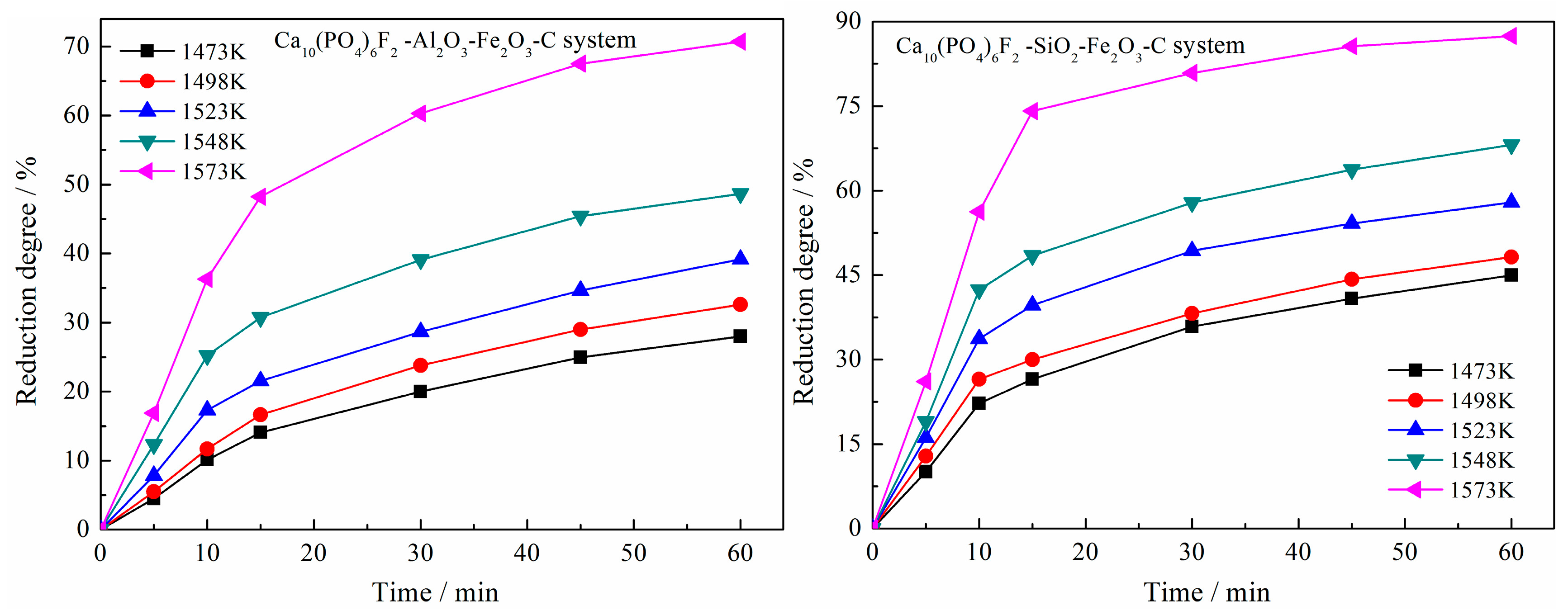
3.2.1. Effects of Reduction Time
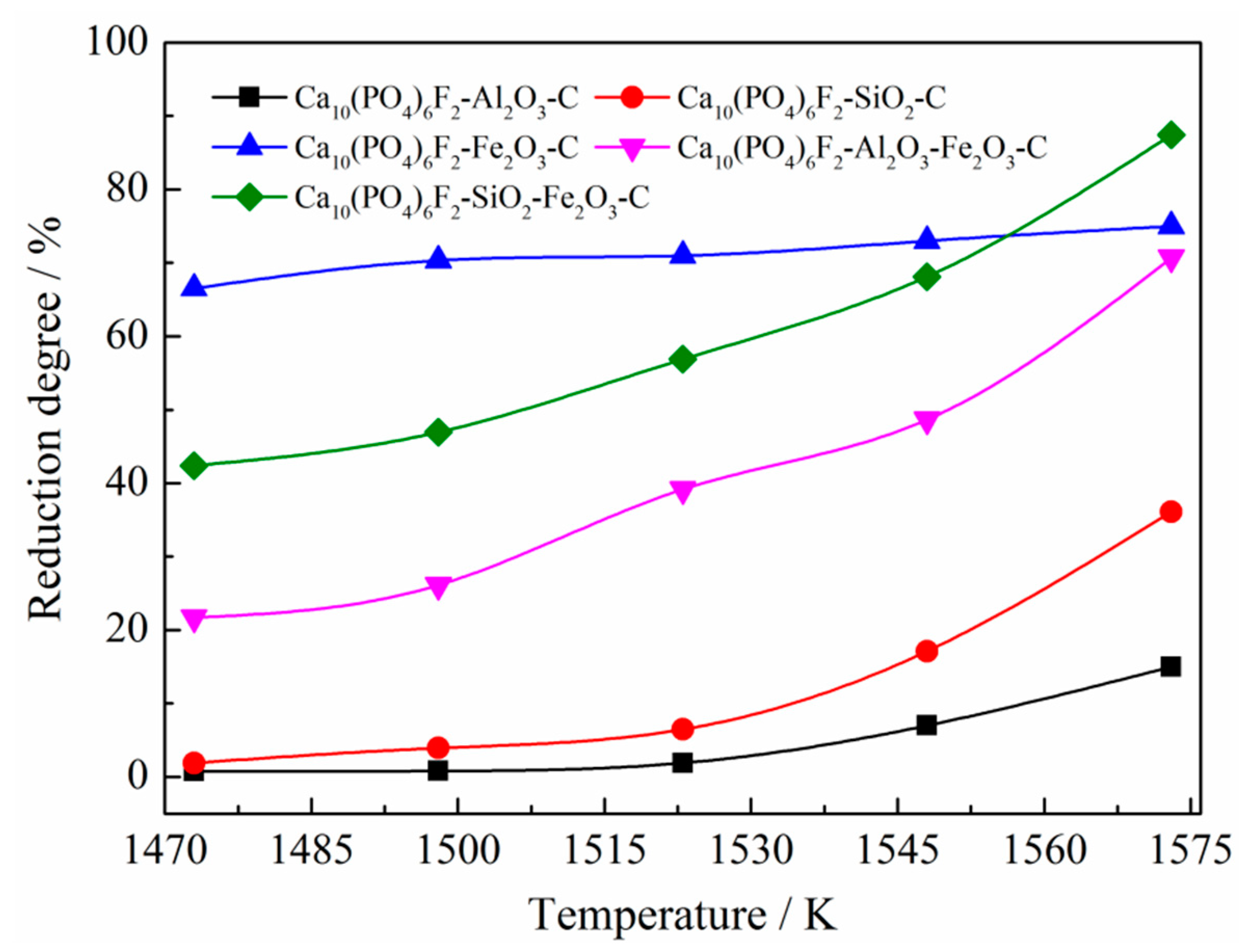
3.2.2. Effects of Reduction Temperature
3.2.3. Effects of Carbon Content
3.2.4. Effects of Additive Contents
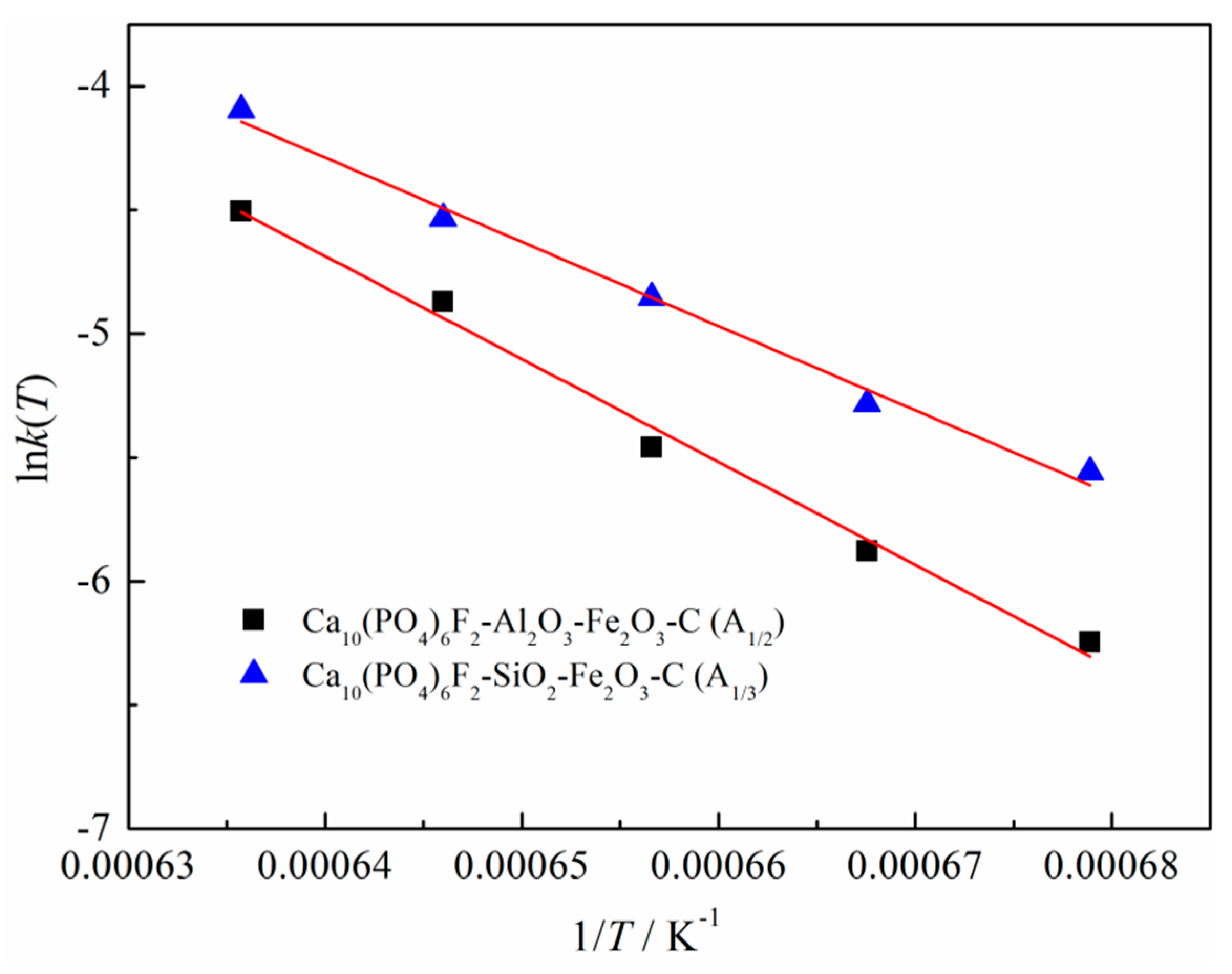
3.3. Kinetic Analysis
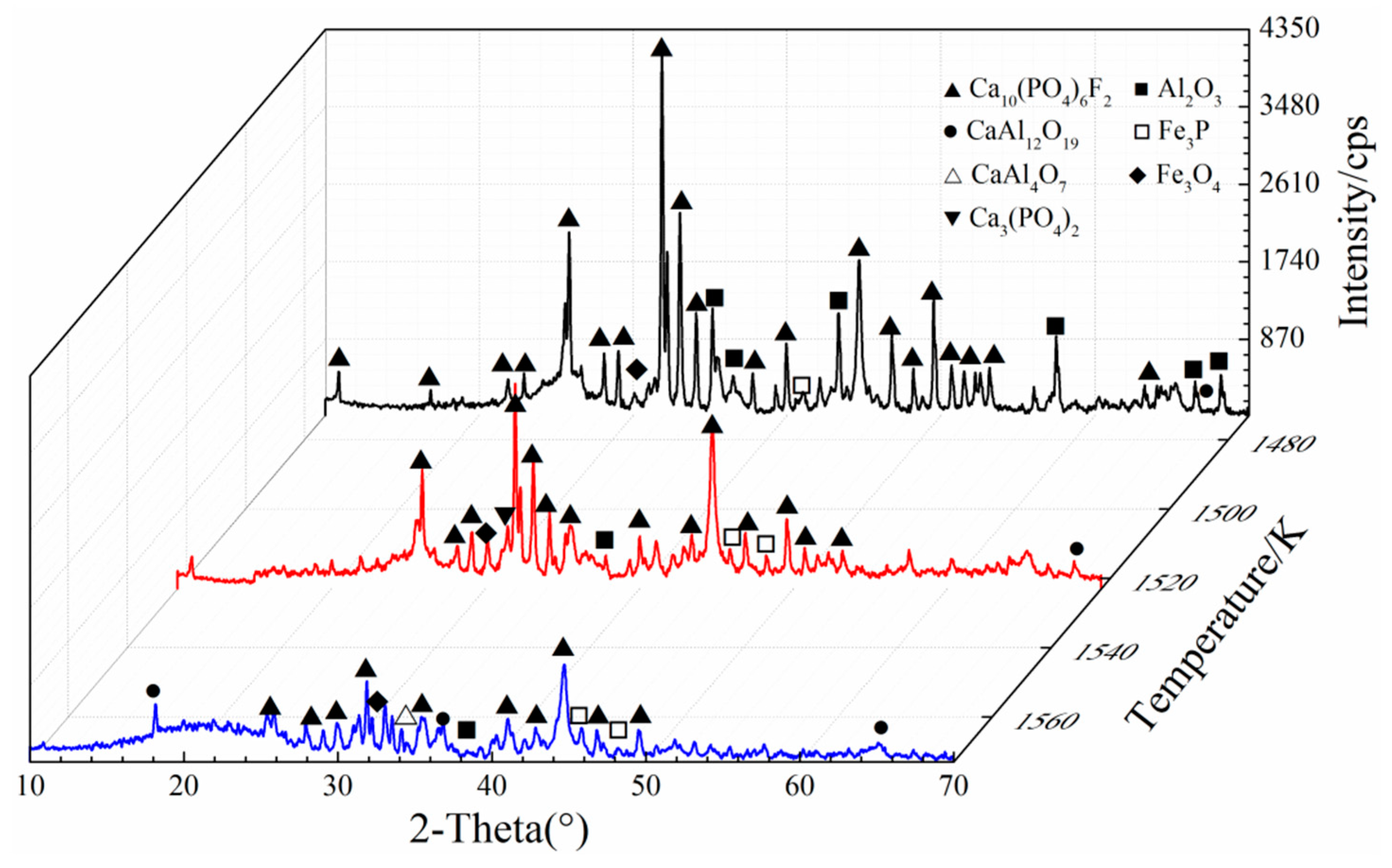
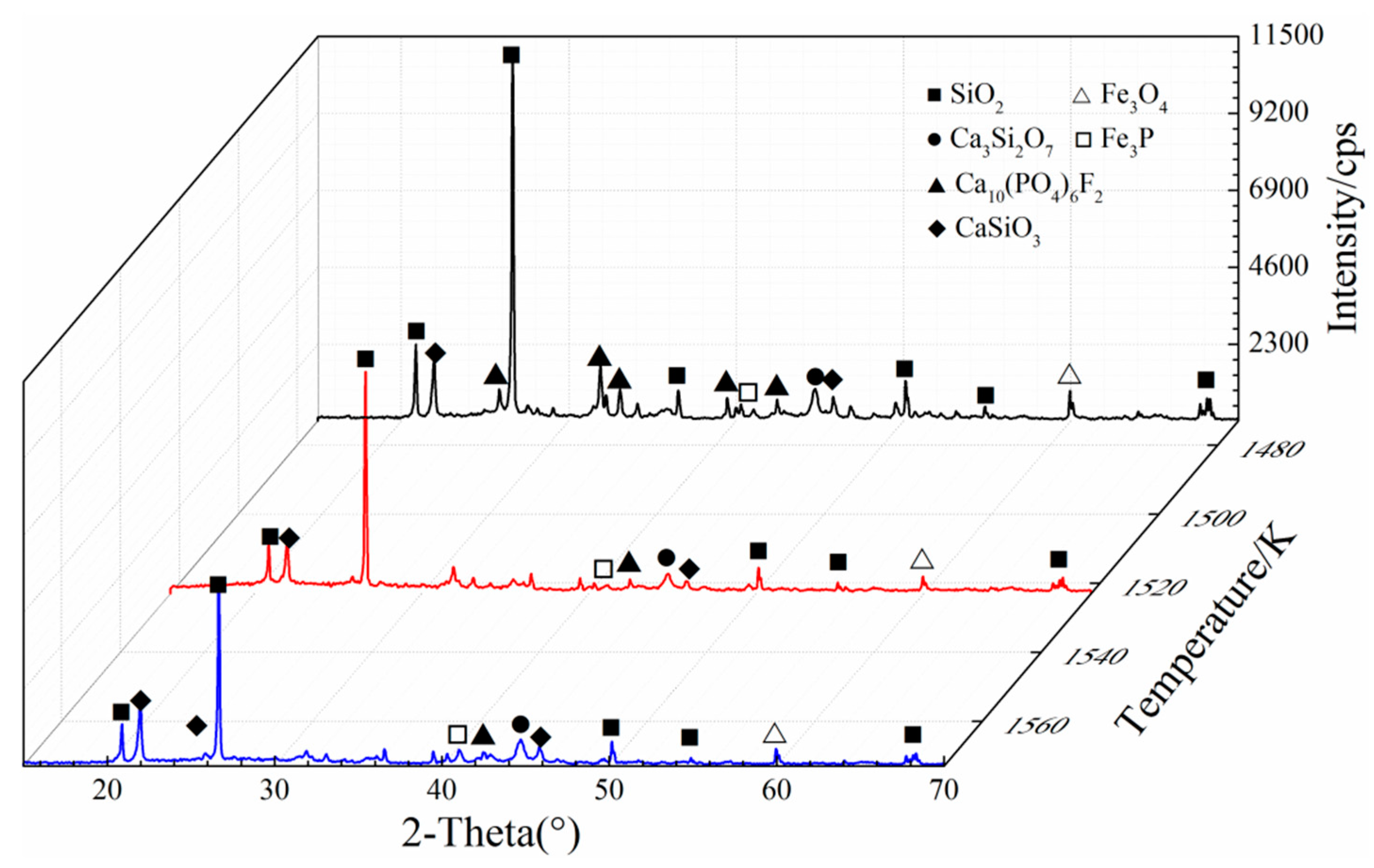
3.4. Phase Transformation
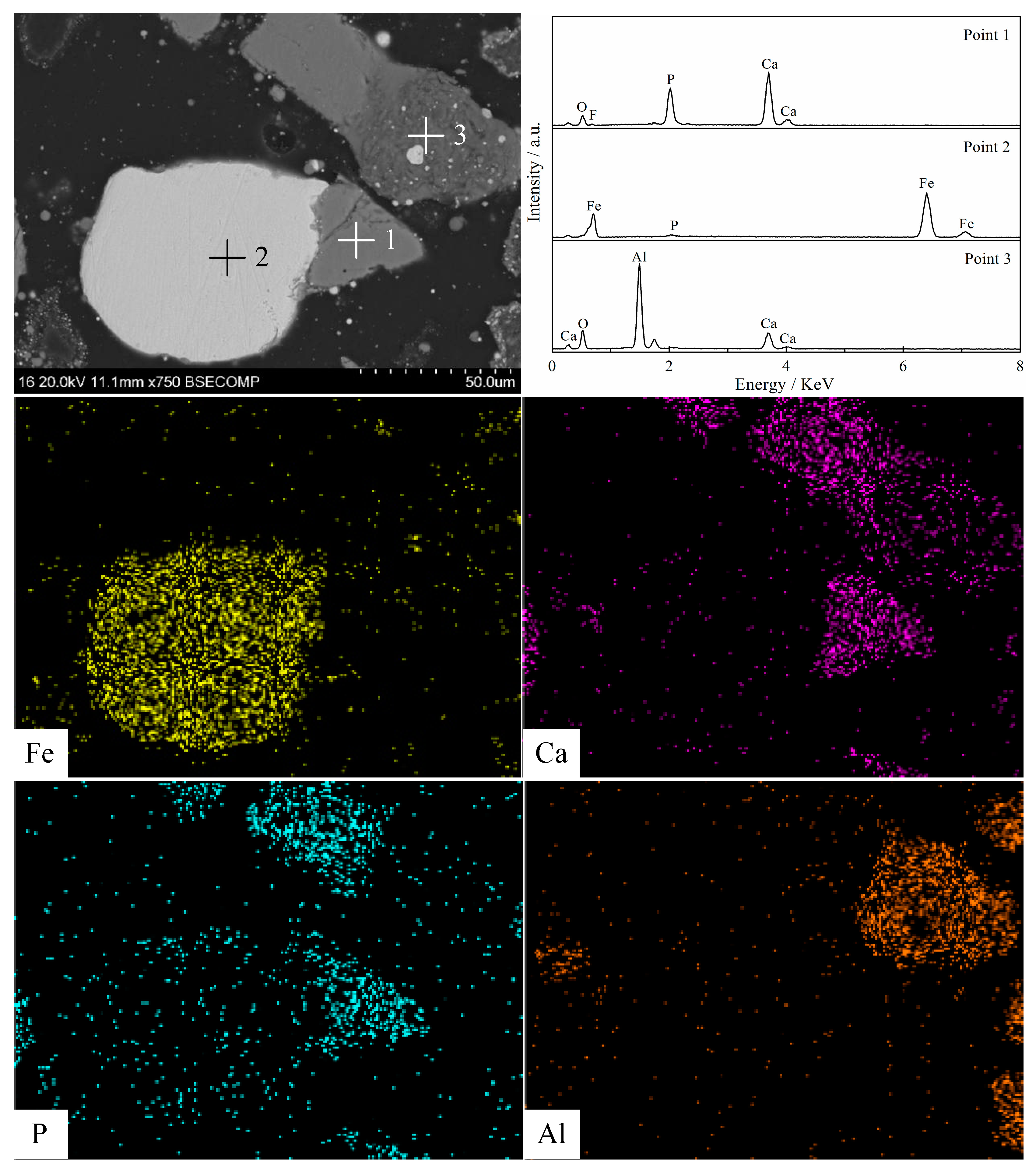
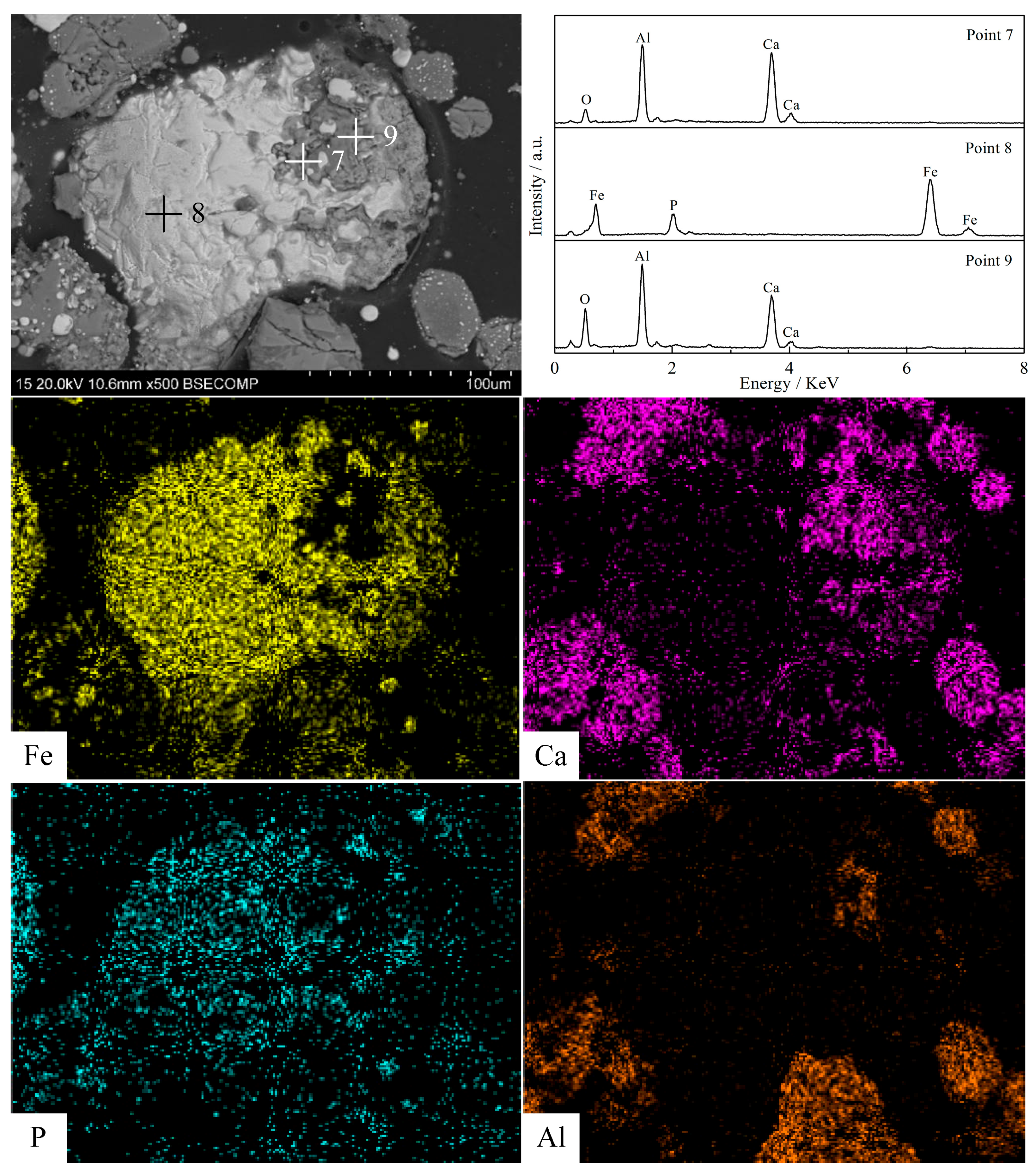
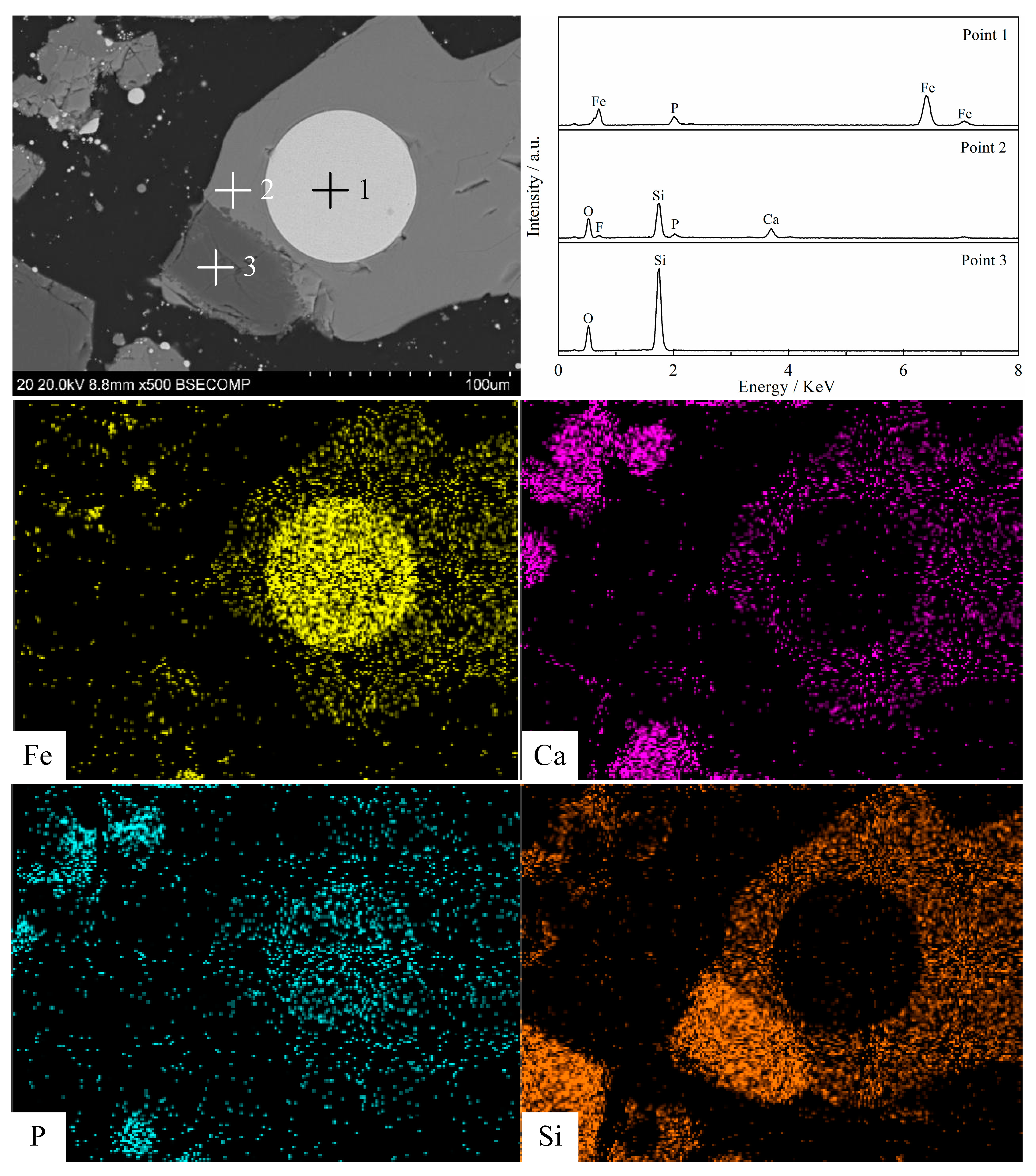
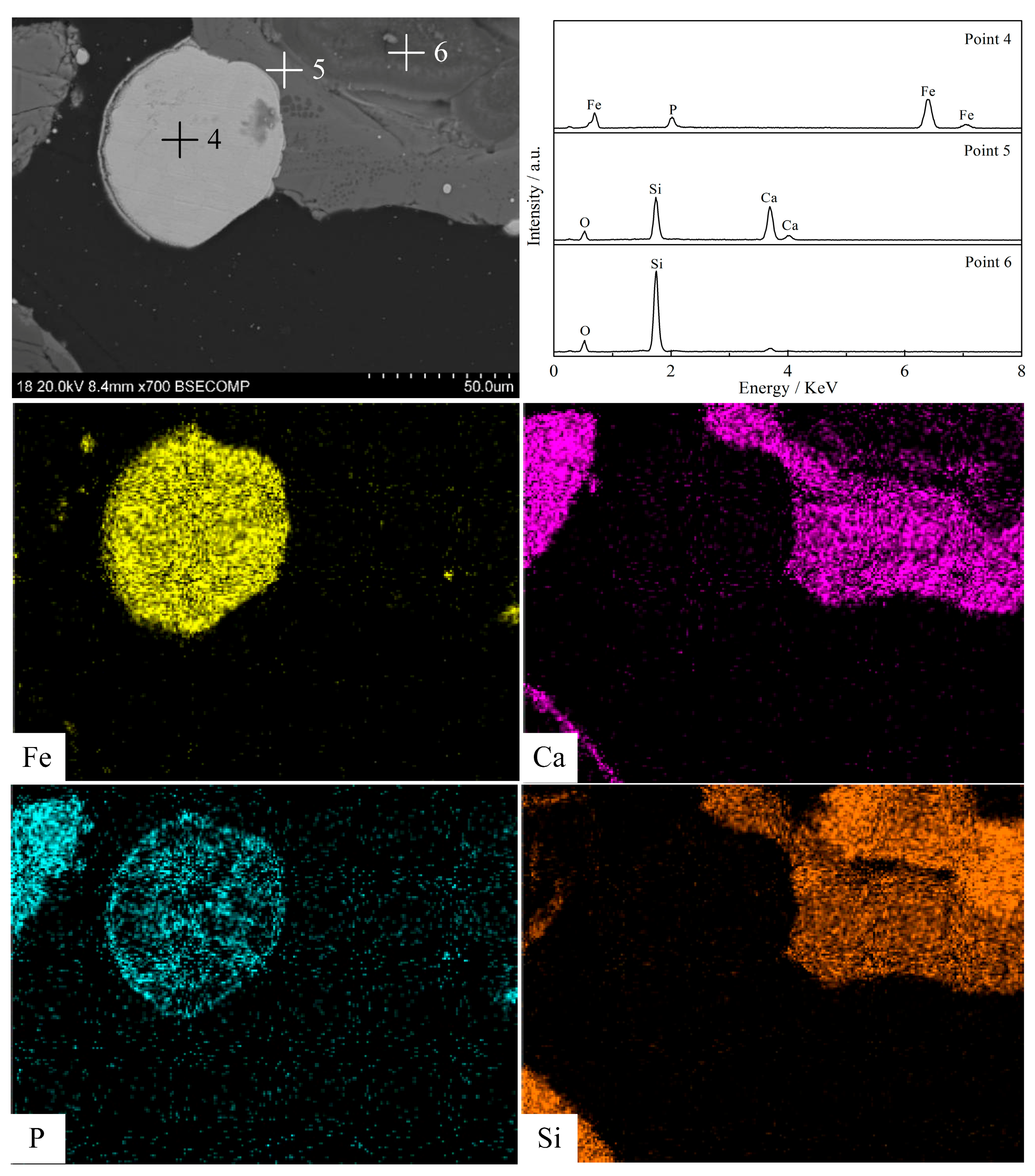
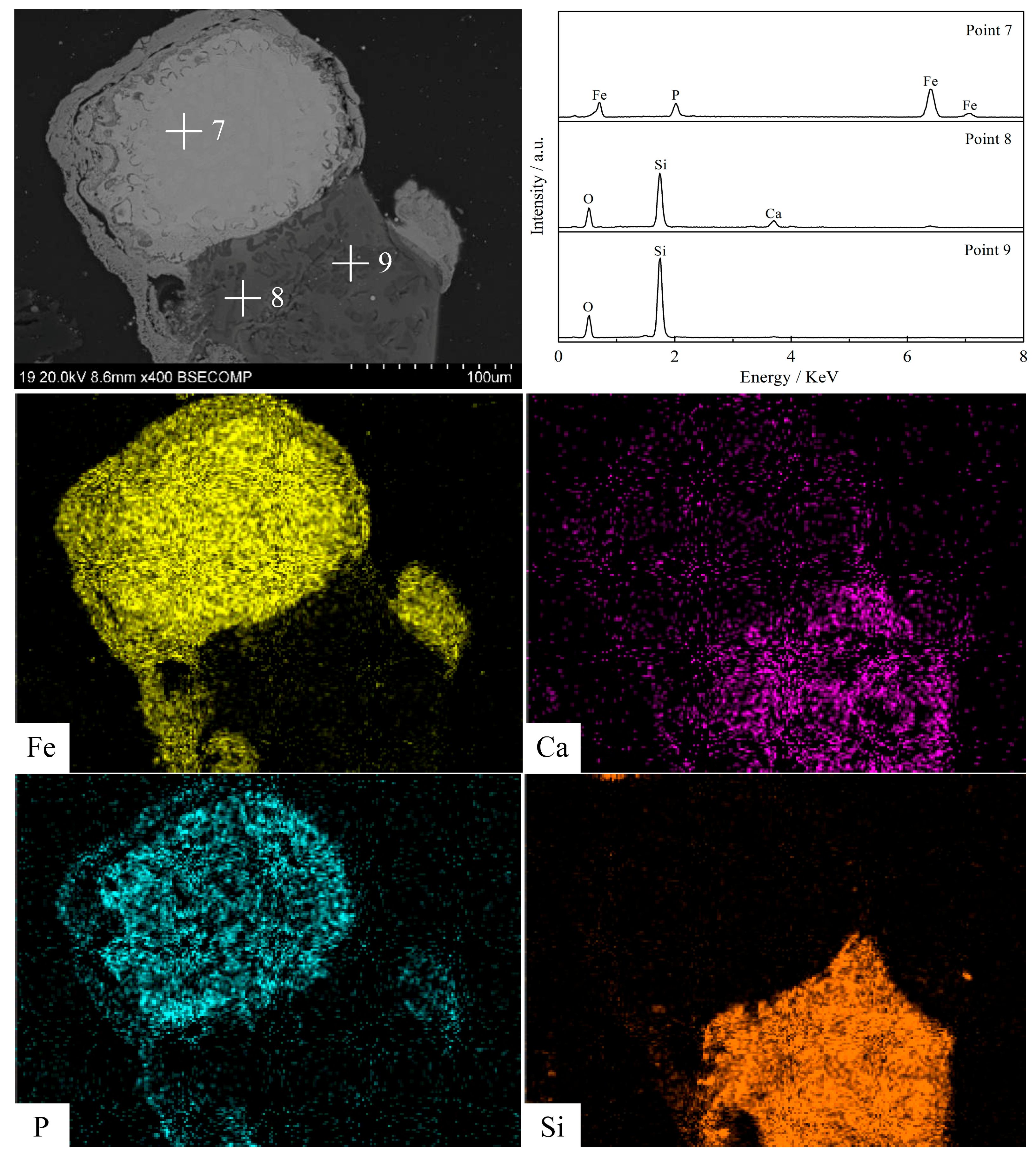
3.5. Morphology Structure Characterization
4. Conclusions
- (1)
- The effect of SiO2 and Fe2O3 on reducing the initial reduction temperature of fluorapatite was stronger than that of Al2O3 and Fe2O3; the initial reduction temperature of fluorapatite was reduced to 985 °C and 1059 °C, respectively. When the mass ratios of Al2O3, SiO2, and Fe2O3 to fluorapatite were less than 0.6, 1.8, and 1.8, respectively, the reduction reaction of fluorapatite could be promoted by increasing the dosage of silica, iron oxide, carbon, reduction time, and reduction temperature.
- (2)
- The best kinetic mechanism functions of the SiO2–Fe2O3–C system and the Al2O3–Fe2O3–C system were A1/3 = 1/3(1 − α)[−ln(1 − α)]−2 and A1/2 = 1/2(1 − α)[−ln(1 − α)]−1, respectively. Among them, the pre-exponential factor of the kinetics equation of fluorapatite reduction with the Al2O3 system was 3.26789 × 109 min−1, the activation energy was 345.479 kJ/mol, the pre-exponential factor of the kinetics equation with the SiO2 system was 3.89033 × 107 min−1, and the activation energy was 282.748 kJ/mol. The activation energy and pre-exponential factor of the reduction kinetics equation in the system containing silica were significantly lower than those in the system containing alumina, which explained that the catalytic effect of silica on the reduction of calcium fluorophosphate was far greater than that of alumina.
- (3)
- Silica (or alumina) promoted the reduction of fluorapatite by forming calcium silicate (or calcium aluminate) at the interface between fluorapatite and silica (or alumina); CaSiO3 (or CaAl12O19) was mainly formed in the early stage, while Ca3Si2O7(or CaAl4O7) was mainly produced and defluorinated in the later stage. Increasing the reduction temperature and prolonging the reduction time were beneficial to the formation and growth of iron particles and the reduction of fluorapatite.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.S.; Zhu, X.R.; Han, Y.X.; Li, Y.J. Green magnetization roasting technology for refractory iron ore using siderite as a reductant. J. Clean. Prod. 2019, 206, 40–50. [Google Scholar] [CrossRef]
- Tang, H.Q.; Guo, Z.C.; Zhao, Z.L. Phosphorus removal of high phosphorus iron ore by gas-based reduction and melt separation. J. Iron Steel Res. Int. 2010, 17, 1–6. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhang, Q.; Han, Y.X.; Gao, P.; Li, G.F. Comprehensive utilization of iron and phosphorus from high-phosphorus refractory iron ore. JOM 2018, 70, 144–149. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Zhang, Y.R.; Sun, T.C. Dephosphorization behavior of high-phosphorus oolitic hematite-solid waste containing carbon briquettes during the process of direct reduction magnetic separation. Metals 2018, 8, 897. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, G.Q.; Zhao, D. Dephosphorization of high phosphorus oolitic hematite by acid leaching and the leaching kinetics. Hydrometallurgy 2017, 171, 61–68. [Google Scholar] [CrossRef]
- Zhang, X.L.; Han, Y.X.; Sun, Y.S.; Li, Y.J. Innovative utilization of refractory iron ore via suspension magnetization roasting: A pilot-scale study. Powder Technol. 2019, 352, 16–24. [Google Scholar] [CrossRef]
- Tang, H.Q.; Qin, Y.Q.; Qi, T.F. Application of wood char in processing oolitic high-phosphorus Hematite for phosphorus removal. J. Iron. Steel. Res. Int. 2016, 2, 109–115. [Google Scholar] [CrossRef]
- Mamdouh, O.; Timo, F.; Riku, M. Thermally assisted liberation of high phosphorus oolitic iron ore: A comparison between microwave and conventional furnaces. Powder Technol. 2015, 269, 7–14. [Google Scholar]
- Zhang, H.Q.; Zhang, Z.Q.; Luo, L.Q.; Yu, H. Behavior of Fe and P during reduction magnetic roasting-separation of phosphorus-rich oolitic hematite. Energy Sources Part A 2019, 1, 47–64. [Google Scholar] [CrossRef]
- Kang, Y. Desiliconisation and dephosphorisation behaviours of various oxygen sources in uot metal pre-treatment. Metals 2019, 9, 251. [Google Scholar] [CrossRef]
- Ionkov, K.; Gaydardzhiev, S. Amenability for processing of oolitic iron ore concentrate for phosphorus removal. Miner. Eng. 2013, 46–47, 119–127. [Google Scholar] [CrossRef]
- Salama, W. Paleoenvironmental significance of aluminum phosphate-sulfate minerals in the upper Cretaceous ooidal ironstones, E-NE Aswan area, southern Egypt. Int. J. Earth Sci. 2014, 103, 1621–1639. [Google Scholar] [CrossRef]
- Mamdouh, O.; Timo, F.; Riku, M. Effect of microwave pre-treatment on the magnetic properties of iron ore and its implications on magnetic separation. Sep. Purif. Technol. 2014, 136, 223–232. [Google Scholar]
- Mamdouh, O.; Timo, F.; Riku, M. Influence of microwave radiation on the magnetic properties of molybdenite and arsenopyrite. Powder Technol. 2017, 15, 276–281. [Google Scholar]
- Xia, W.T.; Ren, Z.D.; Gao, Y.F. Removal of phosphorus from high phosphorus iron ores by selective HCl leaching method. J. Iron Steel Res. Int. 2011, 18, 1–4. [Google Scholar] [CrossRef]
- Wang, J.C.; Shen, S.B.; Kang, J.H.; Li, H.X.; Guo, Z.C. Effect of ore solid concentration on the bioleaching of phosphorus from high-phosphorus iron ores using indigenous sulfur-oxidizing bacteria from municipal wastewater. Process. Biochem. 2010, 45, 1624–1631. [Google Scholar] [CrossRef]
- Delvasto, P.; Ballester, A.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Igual, J.M.; Valverde, A.; García-Balboa, C. Mobilization of phosphorus from iron ore by the bacterium Burkholderia caribensis FeGL03. Miner. Eng. 2009, 22, 1–9. [Google Scholar] [CrossRef]
- Gao, P.; Li, G.F.; Han, Y.X.; Sun, Y.S. Reaction behavior of phosphorus in coal-based reduction of an oolitic hematite ore and pre-dephosphorization of reduced iron. Metals 2016, 6, 82. [Google Scholar] [CrossRef]
- Li, K.Q.; Ni, W.; Zhu, M. Iron extraction from oolitic iron ore by a deep reduction process. J. Iron Steel. Res. Int. 2011, 8, 9–13. [Google Scholar] [CrossRef]
- Han, H.; Duan, D.; Yuan, P. Recovery of metallic iron from high phosphorus oolitic hematite by carbothermic reduction and magnetic separation. Ironmak. Steelmak. 2014, 7, 542–547. [Google Scholar] [CrossRef]
- Grabke, H.J. Grain boundary segregation of impurities in iron and effects on steel properties. Mater. Eng. ISIJ. 1999, 13, 143–192. [Google Scholar]
- Sun, Y.S.; Han, Y.X.; Gao, P.; Wang, Z.H.; Ren, D.Z. Recovery of iron from high phosphorus oolitic iron ore using coal-based reduction followed by magnetic separation. Int. J. Miner. Metall. Mater. 2013, 20, 411–419. [Google Scholar] [CrossRef]
- Xu, C.Y.; Sun, T.C.; Kou, J.; Li, Y.L.; Mo, X.L.; Tang, L.G. Mechanism of phosphorus removal in beneficiation of high phosphorous oolitic hematite by direct reduction roasting with dephosphorization agent. Trans. Nonferrous Met. Soc. China. 2012, 22, 2806–2812. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.C.; Kou, J.; Wei, Y.X.; Xu, C.Y.; Liu, Z.Z. The function of Ca(OH)2 and Na2CO3 as additive on the reduction of high-phosphorus oolitic hematite-coal mixed pellets. ISIJ Int. 2013, 53, 427–433. [Google Scholar] [CrossRef]




| Fixed Carbon | Volatile | Ash | Water | S |
|---|---|---|---|---|
| 85.44 | 1.26 | 12.46 | 0.22 | 0.62 |
| Reagent Name | Chemical Formula | Specifications |
|---|---|---|
| Silicon dioxide | SiO2 | Analytical Purity |
| Alumina | Al2O3 | Analytical Purity |
| Ferric oxide | Fe2O3 | Analytical Purity |
| Code | Reaction Model | Differential f(α) | Integral G(α) |
|---|---|---|---|
| Am | Avrami–Erofeev | m(1 − α)[−ln(1 − α)](m − 1)/m | [−ln(1 − α)]1/m |
| A1 | m = 1 | 1 − α | −ln(1 − α) |
| A2 | m = 2 | 2(1 − α)[−ln(1 − α)]1/2 | [−ln(1 − α)]1/2 |
| A3 | m = 3 | 3(1 − α)[−ln(1 − α)]2/3 | [−ln(1 − α)]1/3 |
| A4 | m = 4 | 4(1 − α)[−ln(1 − α)]3/4 | [−ln(1 − α)]1/4 |
| A3/2 | m = 3/2 | 3/2(1 − α)[−ln(1 − α)]1/3 | [−ln(1 − α)]2/3 |
| A1/4 | m = 1/4 | 1/4(1 − α)[−ln(1 − α)]−3 | [−ln(1 − α)]4 |
| A1/3 | m = 1/3 | 1/3(1 − α)[−ln(1 − α)]−2 | [−ln(1 − α)]3 |
| A1/2 | m = 1/2 | 1/2(1 − α)[−ln(1 − α)]−1 | [−ln(1 − α)]2 |
| Dm | Diffusion | ||
| D1 | One-dimensional diffusion | 1/2α−1 | α2 |
| D2 | 2-D diffusion (Valensi) | [−ln(1 − α)]−1 | α + (1 − α)ln(1 − α) |
| D3 | 2-D diffusion (Jander) | (1 − α)1/2[1 − (1 − α)1/2]−1 | [1 − (1 − α)1/2]2 |
| D4 | 3-D diffusion (anti-Jander) | 3/2(1 + α)2/3[(1 + α)1/3 − 1]−1 | [(1 + α)1/3 − 1]2 |
| D5 | 3-D diffusion (Z-L-T) | 3/2(1 − α)4/3[(1 − α)−1/3 −1]−1 | [(1 − α)−1/3 − 1]2 |
| D6 | 3-D diffusion (Jander, n = 2) | 3/2(1 − α)2/3[1 − (1 − α)1/3]−1 | [1 − (1 − α)1/3]2 |
| D7 | 3-D diffusion (Jander, n = 1/2) | 6(1 − α)2/3[1 − (1 − α)1/3]1/2 | [1 − (1 − α)1/3]1/2 |
| D8 | 3-D diffusion (G-B) | 3/2[(1 − α)]−1/3 − 1]−1 | 1 − 2/3α − (1 − α)2/3 |
| Function Code | Experimental Conditions/K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca10(PO4)6F2–Al2O3–Fe2O3–C | Ca10(PO4)6F2–SiO2–Fe2O3–C | |||||||||
| 1473 | 1498 | 1523 | 1548 | 1573 | 1473 | 1498 | 1523 | 1548 | 1573 | |
| Correlation Coefficients R2, ×10−4 | ||||||||||
| Am | Avrami–Erofeev Equation, f(x) = m(1 − x)[−ln(1 − x)](m − 1)/m | |||||||||
| A1 | 9433 | 9436 | 9367 | 8944 | 8863 | 9030 | 8993 | 8529 | 8508 | 7879 |
| A2 | 8590 | 8627 | 8474 | 8029 | 7885 | 8084 | 8071 | 7522 | 7368 | 6765 |
| A3 | 8208 | 8263 | 8080 | 7656 | 7479 | 7691 | 7695 | 7130 | 6926 | 6342 |
| A4 | 7998 | 8064 | 7867 | 7458 | 7263 | 7482 | 7497 | 6927 | 6698 | 6124 |
| A3/2 | 8923 | 8944 | 8821 | 8370 | 8253 | 8440 | 8415 | 7888 | 7782 | 7165 |
| A1/4 | 9331 | 9334 | 9208 | 9754 | 9789 | 9659 | 9646 | 9904 | 9754 | 9910 |
| A1/3 | 9777 | 9795 | 9731 | 9962 | 9950 | 9953 | 9937 | 9993 | 9976 | 9805 |
| A1/2 | 9984 | 9991 | 9977 | 9848 | 9802 | 9906 | 9884 | 9681 | 9750 | 9263 |
| Dm | Diffusion | |||||||||
| D1 | 9943 | 9927 | 9891 | 9482 | 8954 | 9623 | 9545 | 8971 | 8708 | 6820 |
| D2 | 9963 | 9957 | 9936 | 9626 | 9294 | 9736 | 9683 | 9241 | 9125 | 7657 |
| D3 | 9971 | 9969 | 9953 | 9695 | 9466 | 9789 | 9747 | 9376 | 9334 | 8186 |
| D4 | 9902 | 9870 | 9808 | 9290 | 8635 | 9461 | 9355 | 8670 | 8304 | 6363 |
| D5 | 9986 | 9995 | 9972 | 9916 | 9924 | 9957 | 9940 | 9827 | 9904 | 9705 |
| D6 | 9977 | 9979 | 9966 | 9753 | 9598 | 9834 | 9800 | 9489 | 9497 | 8591 |
| D7 | 8508 | 8531 | 8340 | 7840 | 7528 | 7913 | 7881 | 7256 | 6982 | 6112 |
| D8 | 9968 | 9966 | 9948 | 9673 | 9409 | 9772 | 9727 | 9331 | 9265 | 8004 |
| Mechanism Function | k(T) | R2 |
|---|---|---|
| A1/2 (Ca10(PO4)6F2–Al2O3–Fe2O3–C) | 0.9892 | |
| A1/3 (Ca10(PO4)6F2–SiO2–Fe2O3–C) | 0.9904 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhou, W.; Han, Y.; Li, Y. Effect of Different Additives on Reaction Characteristics of Fluorapatite During Coal-Based Reduction of Iron Ore. Metals 2019, 9, 923. https://doi.org/10.3390/met9090923
Sun Y, Zhou W, Han Y, Li Y. Effect of Different Additives on Reaction Characteristics of Fluorapatite During Coal-Based Reduction of Iron Ore. Metals. 2019; 9(9):923. https://doi.org/10.3390/met9090923
Chicago/Turabian StyleSun, Yongsheng, Wentao Zhou, Yuexin Han, and Yanjun Li. 2019. "Effect of Different Additives on Reaction Characteristics of Fluorapatite During Coal-Based Reduction of Iron Ore" Metals 9, no. 9: 923. https://doi.org/10.3390/met9090923
APA StyleSun, Y., Zhou, W., Han, Y., & Li, Y. (2019). Effect of Different Additives on Reaction Characteristics of Fluorapatite During Coal-Based Reduction of Iron Ore. Metals, 9(9), 923. https://doi.org/10.3390/met9090923





