Synthesis and Mechanical Testing of Calcium Aluminosilicoferrite Crystals with High Alumina Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of SFCA Crystals
- heating from room temperature to 1450 °C (1500 °C for PM-A) with 5 °C min−1;
- holding at maximum temperature for 120 min;
- cooling from peak temperature to 1200 °C with 1 °C min−1;
- cooling from 1200 °C to 700 °C with 5 °C min−1;
- cooling from 700 °C to room temperature through passive cooling inside the furnace after the power was switched off.
2.3. Mechanical Testing
2.4. Analytical Methods
2.4.1. Powder X-ray Diffraction (XRD)
2.4.2. Electron Probe Microanalysis (EPMA)
2.4.3. Forescattered Electron Imaging (FSE)
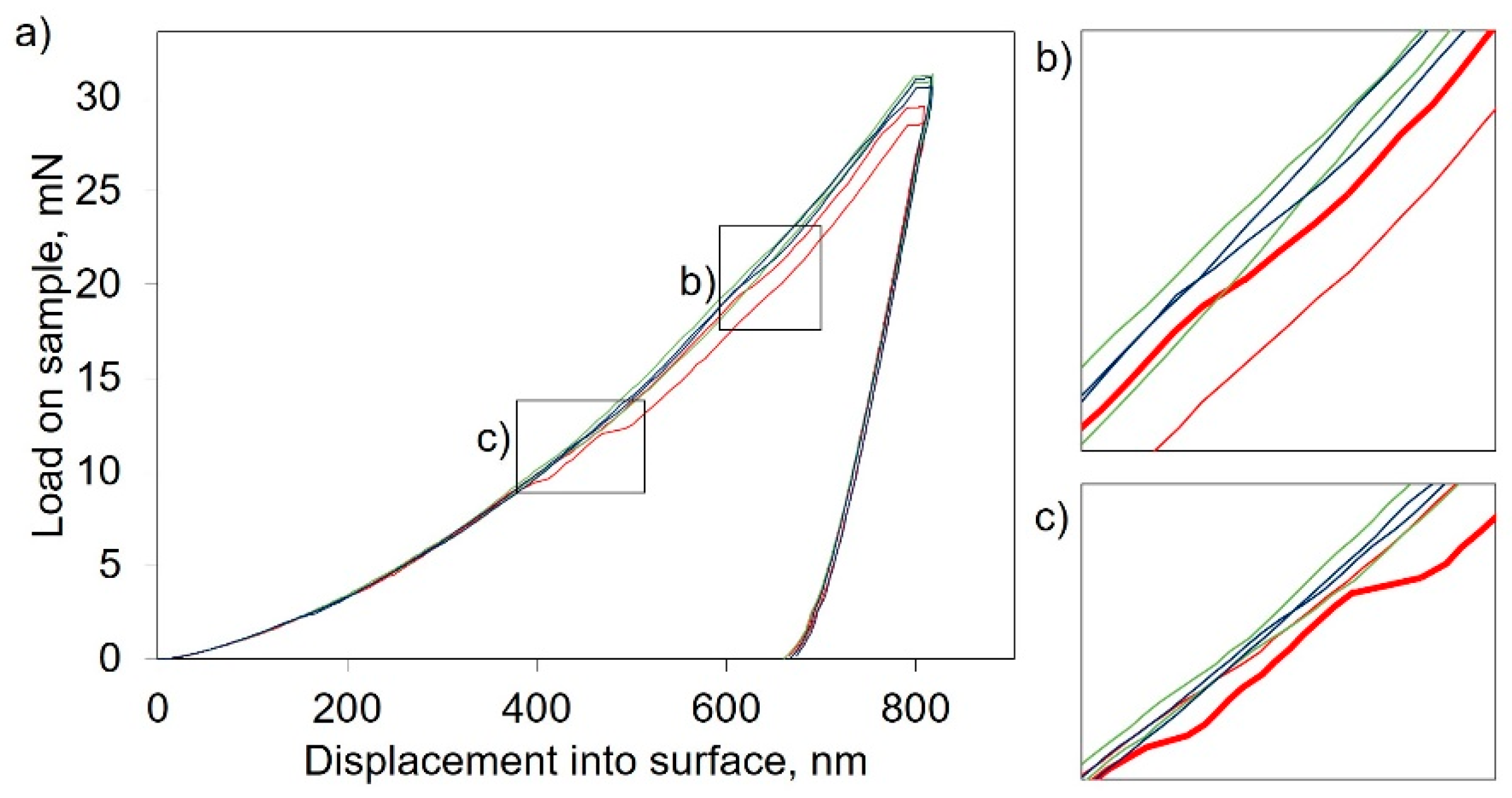
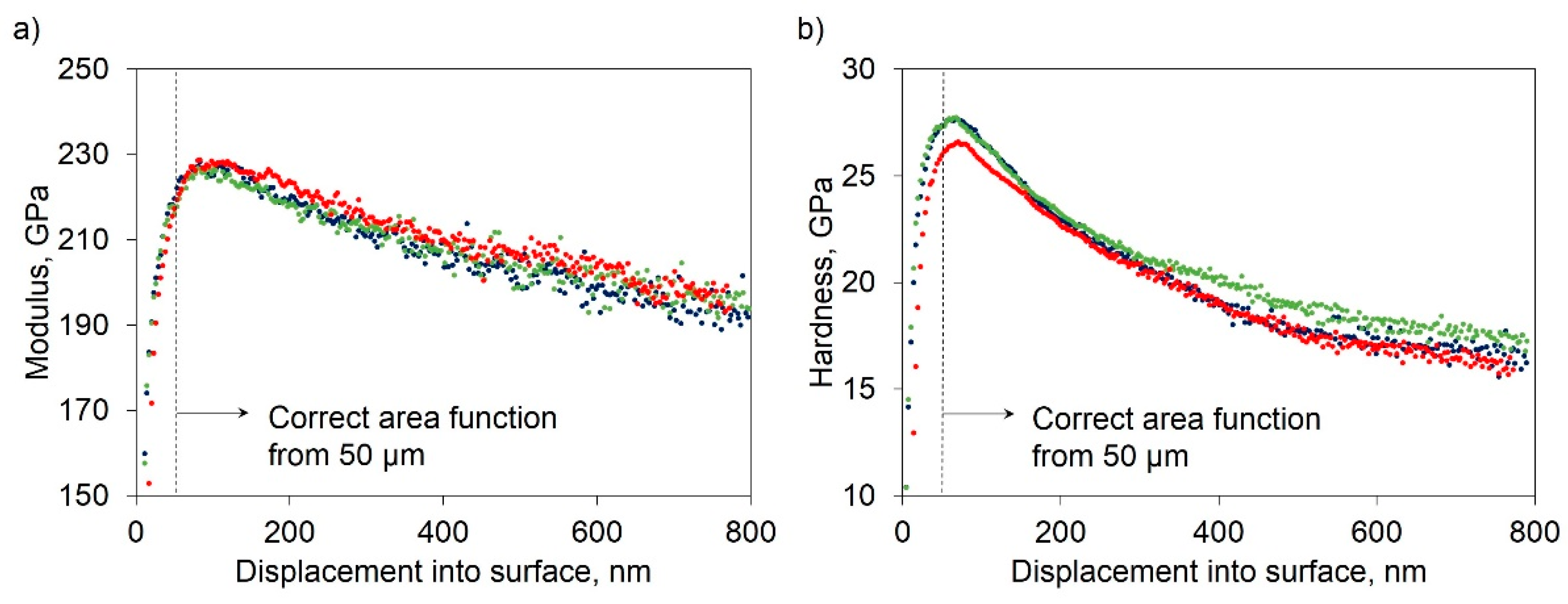
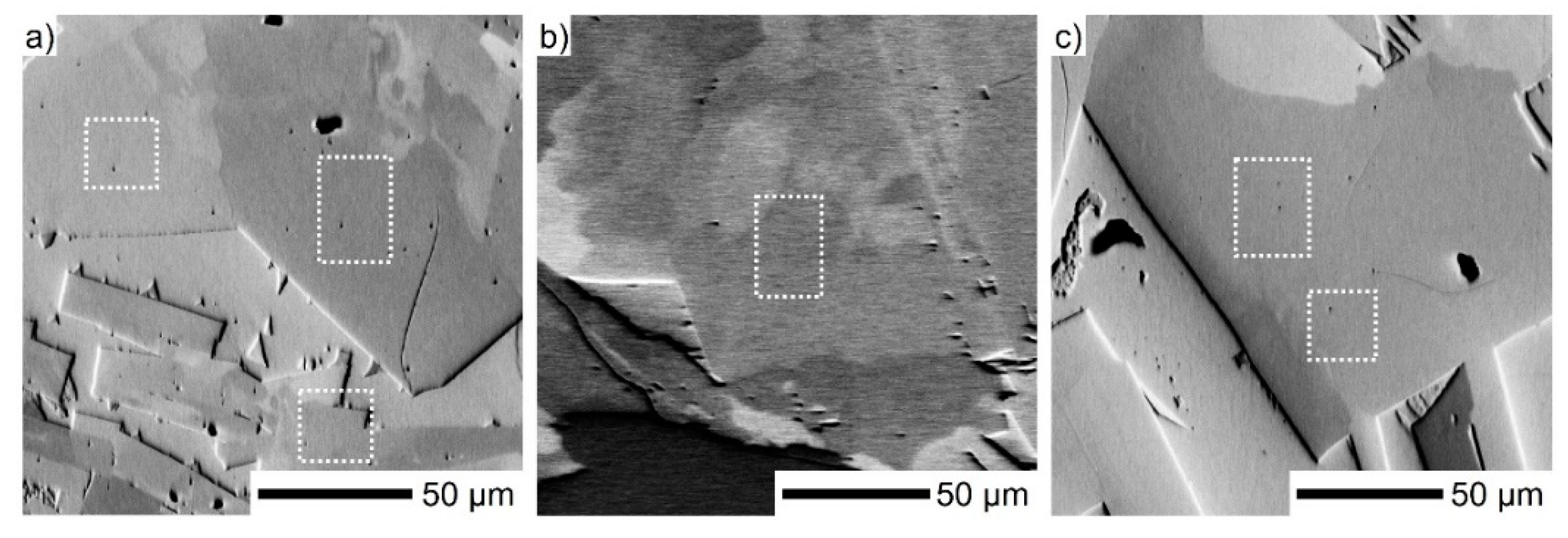
3. Results
3.1. Mineralogical Characterization
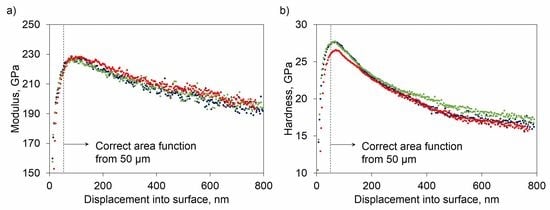
3.2. Mechanical Characterization
4. Discussion
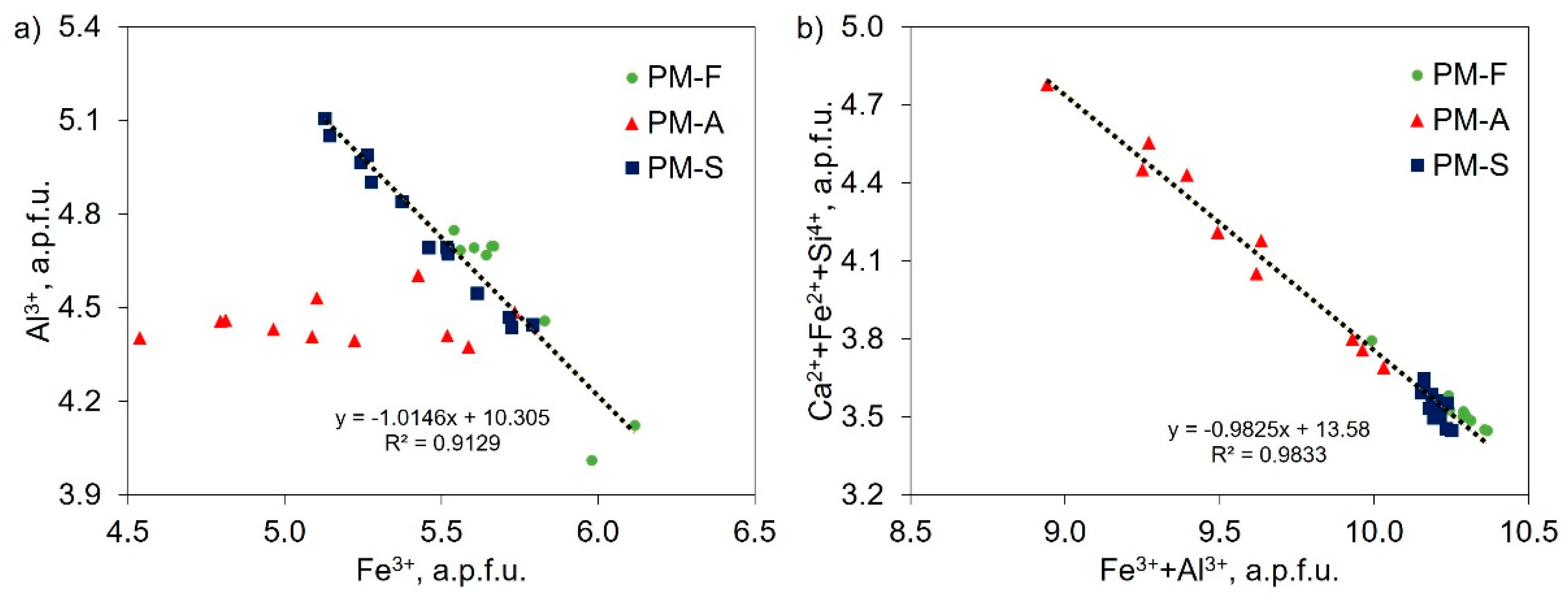
4.1. Chemical Composition of SFCA
4.2. Factors Influencing the Mechanical Properties
4.3. Correlation with Industrial Sintering
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalenga, M.K.; Garbers-Craig, A.M. Investigation into how the magnesia, silica, and alumina contents of iron ore sinter influence its mineralogy and properties. J. South. Afr. Inst. Min. Metall. 2010, 110, 447–456. [Google Scholar]
- Mežibrický, R.; Fröhlichová, M. Comparison of iron ore sinter mineralogy quantification. Acta Metall. Slov. 2017, 23, 283–289. [Google Scholar] [CrossRef]
- Da Costa, E.; Coheur, J.P. Association of calcium ferrites in iron ore sinter with aenigmatites. Ironmak. Steelmak. 1995, 22, 223–226. [Google Scholar]
- Hapugoda, S.; Lu, L.; Donskoi, E.; Manuel, J. Mineralogical quantification of iron ore sinter. Miner. Process. Extr. Metall. 2016, 125, 156–164. [Google Scholar] [CrossRef]
- Sugiyama, K.; Monkawa, A.; Sugiyama, T. Crystal structure of the SFCAM phase Ca2(Ca,Fe,Mg,Al)6(Fe,Al,Si)6O20. ISIJ Int. 2005, 45, 560–568. [Google Scholar] [CrossRef]
- Mumme, W.G.; Clout, J.M.F.; Gable, R.W. The crystal structure of SFCA-I, Ca3.18Fe3+14.66Al1.34Fe2+0.82O28, a homologue of the aenigmatite structure type, and new crystal structure refinements of ß-CFF, Ca2.99Fe3+14.30Fe2+0.55O25 and Mg-free SFCA, Ca2.45Fe3+9.04Al1.74Fe2+0.16Si0.6O20. N. Jb. Mineral. Abh. 1998, 173, 93–117. [Google Scholar]
- Mumme, W.G. The crystal structure of SFCA-II, Ca5.1Al9.3Fe3+18.7Fe2+0.9O48 a new homologue of the aenigmatite structure-type, and structure refinement of SFCA-type, Ca2Al5Fe7O20. Implications for the nature of the “ternary-phase solid-solution” previously reported in the CaO-Al2O3-iron oxide system. N. Jb. Mineral. Abh. (J. Mineral. Geochem.) 2003, 178, 307–335. [Google Scholar]
- Patrick, T.R.; Pownceby, M.I. Stability of silico-ferrite of calcium and aluminum (SFCA) in air-solid solution limits between 1240° C and 1390° C and phase relationships within the Fe2O3-CaO-Al2O3-SiO2 (FCAS) system. Metall. Mater. Trans. B 2002, 33, 79–89. [Google Scholar] [CrossRef]
- Xiang, S.; Lv, X.; Yu, B.; Xu, J.; Yin, J. The Dissolution Kinetics of Al2O3 into Molten CaO-Al2O3-Fe2O3 Slag. Metall. Mater. Trans. B 2014, 45, 2106–2117. [Google Scholar] [CrossRef]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of alumina on sintering performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef]
- Pimenta, H.P.; Seshadri, V. Influence of Al2O3 and TiO2 Degradation Behaviour of Sinter and Hematite at Low Temperatures on Reduction. Ironmak. Steelmak. 2002, 29, 175–179. [Google Scholar] [CrossRef]
- Umadevi, T.; Mahapatra, P.C.; Prabhu, M. Influence of MgO addition on microstructure and properties of low and high silica iron ore sinter. Miner. Process. Extr. Metall. 2013, 122, 238–248. [Google Scholar] [CrossRef]
- Mitra, D.; Sinha, S. Five decades of sinter-making at TATA Steel, a continuous improvement journey. In Proceedings of the 8th ICSTI Conference Proceedings, Vienna, Austria, 25–28 September 2018; pp. 262–267. [Google Scholar]
- Okazaki, J.; Higuchi, K. Marra Mamba ore, its mineralogical properties and evaluation for utilization. ISIJ Int. 2005, 45, 427–435. [Google Scholar] [CrossRef]
- De Magalhães, M.S.; Brandão, P.R.G.; Tavares, R.P. Types of goethite from Quadrilátero Ferrífero’s iron ores and their implications in the sintering process. Miner. Process. Extr. Metall. 2007, 116, 54–64. [Google Scholar] [CrossRef]
- Sinha, M.; Nistala, S.H.; Chandra, S.; Mankhand, T.R. Mineralogy of Iron Ores of Different Alumina Levels from Singhbhum Belt and Their Implication on Sintering Process. J. Miner. Mater. Charact. Eng. 2015, 3, 180–193. [Google Scholar] [CrossRef]
- Haga, T.; Ohshio, A.; Nakamura, K.; Kozono, T.; Uekawa, K. Control Technique of the Melting Reaction in Sintering Process by the Fine Part Selective Granulation of Clayish Iron Ores. ISIJ Int. 1997, 83, 103–108. [Google Scholar]
- Sakamoto, N.; Fukuyo, H.; Iwata, Y.; Miyashita, T. Mineralogical Study on Degradation of Sinter Structure at Low Temperature Reduction. Tetsu-to-Hagané 1984, 70, 512–519. [Google Scholar] [CrossRef]
- Shigaki, I.; Sawada, M.; Gennai, N. Increase in Low-temperature Reduction of Iron Ore Sinter due to Hematite-alumina Solid Solution and Columnar Calcium Ferite. Trans. Iron Steel Inst. Jpn. 1986, 26, 503–511. [Google Scholar] [CrossRef]
- Ying, Z.W.; Jiang, M.F.; Xu, L.X. Effects of mineral composition and microstructure on crack resistance of sintered ore. J. Iron Steel Res., Int. 2006, 13, 9–12. [Google Scholar] [CrossRef]
- Sinha, M.; Nistala, S.H.; Chandra, S.; Mankhand, T.R.; Ghose, A.K. Correlating mechanical properties of sinter phases with their chemistry and its effect on sinter quality. Ironmak. Steelmak. 2017, 44, 100–107. [Google Scholar] [CrossRef]
- Yang, D.; Wang, W.; Xu, R.; Li, J.; Song, M. Effect of SiO2 on the Mechanical Property and Reduction of Calcium Ferrite. Metals 2019, 9, 152. [Google Scholar] [CrossRef]
- De Magalhães, M.S.; Brandão, P.R.G. Microstructures of industrial sinters from Quadrilatero Ferrifero’s iron ores, Minas Gerais State, Brazil. Miner. Eng. 2003, 16, 1251–1256. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Wu, X.; Ren, X.; Bing, W.; Wu, L. Influence of Al2O3 on equilibrium sinter phase in N2 atmosphere. ISIJ Int. 2010, 50, 327–329. [Google Scholar] [CrossRef][Green Version]
- Mežibrický, R.; Fröhlichová, M. Mineral phases in iron ore sinters produced with oak sawdust substitute fuel. J. Min. Metall. Sect. B-Metall. 2018, 54, 9–20. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Silico-ferrite of calcium and aluminum (SFCA) iron ore sinter bonding phases: New insights into their formation during heating and cooling. Metall. Mater. Trans. B 2012, 43, 1344–1357. [Google Scholar] [CrossRef]
- Mežibrický, R.; Fröhlichová, M. Silico-ferrite of Calcium and Aluminum Characterization by Crystal Morphology in Iron Ore Sinter Microstructure. ISIJ Int. 2016, 56, 1111–1113. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Li, X.; Bhushan, B. A review of nanoindentation continuous stiffness measurement technique and its applications. Mater. Charact. 2002, 48, 11–36. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Gercek, H. Poisson’s ratio values for rocks. Int. J. Rock Mech. Min. Sc. 2007, 44, 1–13. [Google Scholar] [CrossRef]
- Hamilton, J.D.G.; Hoskins, B.F.; Mumme, W.G.; Borbidge, W.E.; Montague, M.A. The crystal structure and crystal chemistry of Ca2.3Mg0.8Al1.5Si1.1Fe8.3O20 (SFCA): Solid solution limits and selected phase relationships of SFCA in the SiO2 – Fe2O3 – CaO(– Al2O3) system. N. Jb. Mineral. Abh. 1989, 161, 1–26. [Google Scholar]
- Murao, R.; Harano, T.; Kimura, M.; Jung, I.H. Thermodynamic Modeling of the SFCA Phase Ca2(Fe,Ca)6(Fe,Al,Si)6O20. ISIJ Int. 2018, 58, 259–266. [Google Scholar] [CrossRef]
- Morris, D.J.; Cook, R.F. In situ cube-corner indentation of soda-lime glass and fused silica. J. Am. Ceram. Soc. 2004, 87, 1494–1501. [Google Scholar] [CrossRef]
- Cuadrado, N.; Seuba, J.; Casellas, D.; Anglada, M.; Jiménez-Piqué, E. Geometry of nanoindentation cube-corner cracks observed by FIB tomography: Implication for fracture resistance estimation. J. Eur. Ceram. Soc. 2015, 35, 2949–2955. [Google Scholar] [CrossRef]
- Csanádi, T.; Németh, D.; Lofaj, F. Mechanical properties of hard W-C coating on steel substrate deduced from nanoindentation and finite element modeling. Exp. Mech. 2017, 57, 1057–1069. [Google Scholar] [CrossRef]
- Nabarro, F.R.N.; Shrivastava, S.; Luyckx, S.B. The size effect in microindentation. Philos. Mag. 2006, 86, 4173–4180. [Google Scholar] [CrossRef]
- Nix, W.D.; Gao, H. Indentation size effects in crystalline materials: A law for strain gradient plasticity. J. Mech. Phys. Solids. 1998, 46, 411–425. [Google Scholar] [CrossRef]
- Lawn, B.R.; Evans, A.G.; Marshall, D.B. Elastic/plastic indentation damage in ceramics: The median/radial crack system. J. Am. Ceram. Soc. 1980, 63, 574–581. [Google Scholar] [CrossRef]
- Webster, N.A.S.; O’dea, D.P.; Ellis, B.G.; Pownceby, M.I. Effects of gibbsite, kaolinite and Al-rich goethite as alumina sources on silico-ferrite of calcium and aluminium (SFCA) and SFCA-I iron ore sinter bonding phase formation. ISIJ Int. 2017, 57, 41–47. [Google Scholar] [CrossRef]

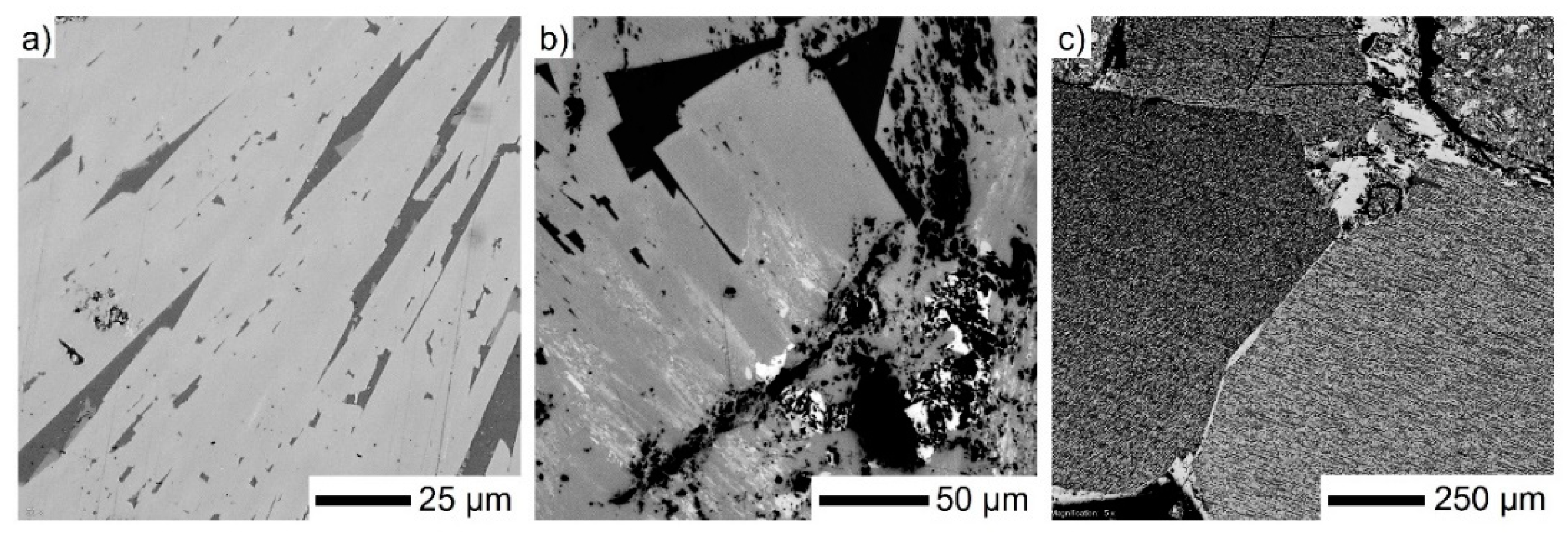
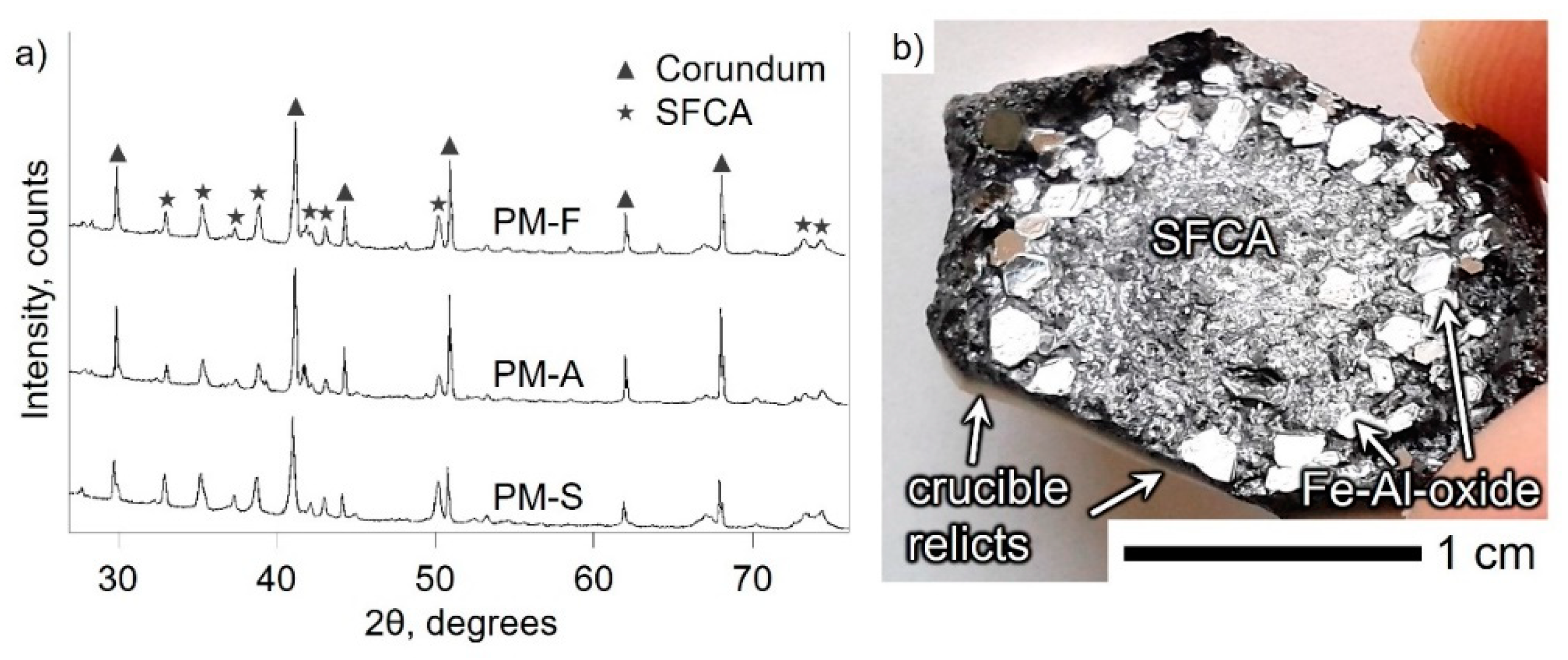

| Material | Fetot | FeO | Fe2O3 | CaO | SiO2 | Al2O3 | MgO |
|---|---|---|---|---|---|---|---|
| Carajás ore | 68.74 | 0.26 | 92.86 | 0.13 | 1.31 | 0.98 | 0.25 |
| Hematite pellets | 65.78 | 0.26 | 93.76 | 1.80 | 3.72 | 0.21 | 0.22 |
| Krivbass ore | 62.72 | 0.63 | 88.99 | 0.23 | 7.82 | 1.65 | 0.24 |
| Zaporozhskiy ore | 62.41 | 1.15 | 87.96 | 0.69 | 8.54 | 1.09 | 0.32 |
| Sukha Balka ore | 61.94 | 0.30 | 88.22 | 0.10 | 10.28 | 0.77 | 0.10 |
| SB + 8 mm ore * | 48.41 | 0.00 | 69.22 | 0.07 | 17.40 | 0.75 | 0.73 |
| limestone ** | NA | NA | NA | 46.37 | 2.51 | 1.30 | 4.28 |
| Mixture | Carajás | Hematite Pellets | Krivbass | Zaporozhskiy | Sukha Balka | SB+8 | Al2O3 | Lime-Stone |
|---|---|---|---|---|---|---|---|---|
| PM-F | 5 | 29 | 15 | 10 | 15 | - | - | 26 |
| PM-A | 11 | 15 | 20 | 15 | 10 | - | 4 | 25 |
| PM-S | - | - | 16 | 25 | 15 | 6 | - | 38 |
| Mixture | Fetot | FeO | Fe2O3 | CaO | SiO2 | Al2O3 | MgO | CaO/SiO2 |
|---|---|---|---|---|---|---|---|---|
| PM-F | 52.93 | 0.38 | 75.26 | 14.28 | 6.03 | 1.03 | 1.43 | 2.37 |
| PM-A | 50.39 | 0.44 | 71.55 | 13.47 | 5.82 | 5.59 | 1.38 | 2.31 |
| PM-S | 45.11 | 0.52 | 63.93 | 21.28 | 8.26 | 1.42 | 2.15 | 2.58 |
| SFCA | Fe2O3 | CaO | SiO2 | Al2O3 | MgO | TiO2 | MnO | Total | ||||||
| PM-F | 54.40 (1.45) | 13.49 (0.13) | 5.70 (0.33) | 25.36 (1.71) | 0.86 (0.07) | 0.01 (0.02) | 0.03 (0.01) | 99.85 (0.32) | ||||||
| PM-A | 50.51 (1.91) | 13.70 (0.80) | 7.74 (1.30) | 24.57 (0.39) | 1.09 (0.23) | 0.02 (0.02) | 0.06 (0.01) | 97.69 (0.24) | ||||||
| PM-S | 51.75 (1.40) | 14.23 (0.22) | 6.01 (0.09) | 26.99 (1.55) | 1.14 (0.19) | 0.02 (0.01) | 0.04 (0.01) | 100.18 (0.23) | ||||||
| Glass | CaO | SiO2 | Al2O3 | FeO | MgO | Na2O | K2O | TiO2 | MnO | BaO | Total | |||
| PM-F | 34.77 | 38.83 | 16.22 | 6.75 | 0.00 | 1.58 | 0.26 | 0.13 | 0.01 | 0.07 | 98.61 | |||
| PM-A | 28.02 | 34.51 | 26.63 | 6.17 | 0.00 | 3.58 | 0.26 | 0.19 | 0.03 | 0.05 | 99.45 | |||
| PM-S | 40.06 | 33.13 | 17.97 | 7.35 | 0.02 | 0.87 | 0.08 | 0.12 | 0.01 | 0.00 | 99.60 | |||
| Sample | Average Composition of SFCA | Modulus, GPa | Hardness, GPa | ||
|---|---|---|---|---|---|
| avg | stdv | avg | stdv | ||
| PM-F | Ca2(Ca0.19Mg0.20Fe2+0.48Fe3+5.13)(Fe3+0.60Si0.86Al4.53)O20 | 224.7 | 6.1 | 26.9 | 1.4 |
| PM-A | Ca2(Ca0.26Mg0.25Fe2+0.68Fe3+4.80)(Fe3+0.36Si1.19Al4.45)O20 | 221.3 | 11.5 | 27.2 | 2.9 |
| PM-S | Ca2(Ca0.28Mg0.25Fe2+0.37Fe3+5.10)(Fe3+0.35Si0.90Al4.75)O20 | 227.9 | 5.0 | 27.4 | 1.2 |
| Average | 225.1 | 8.1 | 27.2 | 1.9 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mežibrický, R.; Csanádi, T.; Habler, G.; Fröhlichová, M.; Dusza, J.; Abart, R. Synthesis and Mechanical Testing of Calcium Aluminosilicoferrite Crystals with High Alumina Content. Metals 2019, 9, 906. https://doi.org/10.3390/met9080906
Mežibrický R, Csanádi T, Habler G, Fröhlichová M, Dusza J, Abart R. Synthesis and Mechanical Testing of Calcium Aluminosilicoferrite Crystals with High Alumina Content. Metals. 2019; 9(8):906. https://doi.org/10.3390/met9080906
Chicago/Turabian StyleMežibrický, Roland, Tamás Csanádi, Gerlinde Habler, Mária Fröhlichová, Ján Dusza, and Rainer Abart. 2019. "Synthesis and Mechanical Testing of Calcium Aluminosilicoferrite Crystals with High Alumina Content" Metals 9, no. 8: 906. https://doi.org/10.3390/met9080906
APA StyleMežibrický, R., Csanádi, T., Habler, G., Fröhlichová, M., Dusza, J., & Abart, R. (2019). Synthesis and Mechanical Testing of Calcium Aluminosilicoferrite Crystals with High Alumina Content. Metals, 9(8), 906. https://doi.org/10.3390/met9080906